How Ethylene Vinyl Acetate Can Advance Healthcare Solutions?
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
EVA in Healthcare: Background and Objectives
Ethylene Vinyl Acetate (EVA) has emerged as a versatile material with significant potential in advancing healthcare solutions. The evolution of EVA in the medical field can be traced back to the 1960s when its unique properties were first recognized for biomedical applications. Since then, EVA has undergone continuous development, with researchers and manufacturers exploring its capabilities in various healthcare contexts.
The primary objective of utilizing EVA in healthcare is to enhance patient care, improve medical device performance, and contribute to the development of innovative therapeutic solutions. EVA's flexibility, biocompatibility, and customizable properties make it an attractive material for a wide range of medical applications, from drug delivery systems to prosthetics and medical packaging.
In recent years, the healthcare industry has witnessed a growing demand for advanced materials that can meet the complex requirements of modern medical technologies. EVA has positioned itself as a promising candidate to address these needs, offering a combination of durability, chemical resistance, and ease of processing that is highly valued in medical product development.
The technological trajectory of EVA in healthcare has been marked by several key milestones. These include its incorporation into controlled release drug delivery systems, the development of EVA-based foam for orthopedic applications, and its use in creating soft, skin-friendly medical devices. Each of these advancements has contributed to expanding the scope of EVA's applications in the medical field.
As the healthcare sector continues to evolve, driven by factors such as an aging population, the rise of personalized medicine, and the need for cost-effective solutions, EVA is expected to play an increasingly important role. The material's adaptability allows for its integration into cutting-edge technologies, such as 3D-printed medical devices and smart drug delivery systems.
The ongoing research and development efforts in EVA technology aim to further enhance its properties and explore new applications. These objectives include improving its barrier properties for better drug preservation, enhancing its compatibility with a broader range of pharmaceutical compounds, and developing EVA-based materials with antimicrobial properties for infection control in healthcare settings.
Understanding the background and objectives of EVA in healthcare is crucial for appreciating its current applications and envisioning its future potential. As researchers and manufacturers continue to innovate, EVA is poised to contribute significantly to the advancement of healthcare solutions, potentially revolutionizing patient care and medical device technology in the coming years.
The primary objective of utilizing EVA in healthcare is to enhance patient care, improve medical device performance, and contribute to the development of innovative therapeutic solutions. EVA's flexibility, biocompatibility, and customizable properties make it an attractive material for a wide range of medical applications, from drug delivery systems to prosthetics and medical packaging.
In recent years, the healthcare industry has witnessed a growing demand for advanced materials that can meet the complex requirements of modern medical technologies. EVA has positioned itself as a promising candidate to address these needs, offering a combination of durability, chemical resistance, and ease of processing that is highly valued in medical product development.
The technological trajectory of EVA in healthcare has been marked by several key milestones. These include its incorporation into controlled release drug delivery systems, the development of EVA-based foam for orthopedic applications, and its use in creating soft, skin-friendly medical devices. Each of these advancements has contributed to expanding the scope of EVA's applications in the medical field.
As the healthcare sector continues to evolve, driven by factors such as an aging population, the rise of personalized medicine, and the need for cost-effective solutions, EVA is expected to play an increasingly important role. The material's adaptability allows for its integration into cutting-edge technologies, such as 3D-printed medical devices and smart drug delivery systems.
The ongoing research and development efforts in EVA technology aim to further enhance its properties and explore new applications. These objectives include improving its barrier properties for better drug preservation, enhancing its compatibility with a broader range of pharmaceutical compounds, and developing EVA-based materials with antimicrobial properties for infection control in healthcare settings.
Understanding the background and objectives of EVA in healthcare is crucial for appreciating its current applications and envisioning its future potential. As researchers and manufacturers continue to innovate, EVA is poised to contribute significantly to the advancement of healthcare solutions, potentially revolutionizing patient care and medical device technology in the coming years.
Market Analysis for EVA-based Medical Products
The market for EVA-based medical products has shown significant growth and potential in recent years, driven by the material's unique properties and versatility in healthcare applications. EVA's biocompatibility, flexibility, and durability make it an ideal choice for various medical devices and healthcare solutions.
In the pharmaceutical sector, EVA is widely used in drug delivery systems, particularly in transdermal patches and controlled-release formulations. The global transdermal drug delivery market, where EVA plays a crucial role, is experiencing robust growth. This growth is attributed to the increasing prevalence of chronic diseases, the rising demand for non-invasive drug administration, and the advantages of controlled drug release.
The medical device industry represents another substantial market for EVA-based products. EVA is commonly used in the production of catheters, tubing, and other disposable medical devices. The global medical tubing market, which heavily relies on EVA, is expanding due to the growing aging population and the increasing number of surgical procedures worldwide.
In the orthopedic sector, EVA-based products are gaining traction, particularly in the manufacturing of orthopedic insoles and cushioning materials. The rising incidence of foot-related disorders and the growing awareness of the importance of proper foot care are driving the demand for EVA-based orthopedic solutions.
The wound care market is another area where EVA-based products are making significant inroads. EVA's properties make it suitable for advanced wound dressings, contributing to the growth of the global advanced wound care market. The increasing prevalence of chronic wounds, such as diabetic foot ulcers and pressure ulcers, is a key factor driving this market segment.
Geographically, North America and Europe currently dominate the market for EVA-based medical products, owing to advanced healthcare infrastructure and higher healthcare expenditure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing disposable income, and a large patient pool.
The COVID-19 pandemic has further highlighted the importance of EVA in healthcare, particularly in the production of personal protective equipment (PPE) such as face shields and disposable gloves. This has led to an increased demand for EVA in the medical sector, a trend that is likely to continue in the post-pandemic era.
Looking ahead, the market for EVA-based medical products is poised for continued growth. Factors such as technological advancements in material science, increasing healthcare expenditure, and the growing focus on patient comfort and safety are expected to drive further innovation and adoption of EVA-based solutions in the healthcare industry.
In the pharmaceutical sector, EVA is widely used in drug delivery systems, particularly in transdermal patches and controlled-release formulations. The global transdermal drug delivery market, where EVA plays a crucial role, is experiencing robust growth. This growth is attributed to the increasing prevalence of chronic diseases, the rising demand for non-invasive drug administration, and the advantages of controlled drug release.
The medical device industry represents another substantial market for EVA-based products. EVA is commonly used in the production of catheters, tubing, and other disposable medical devices. The global medical tubing market, which heavily relies on EVA, is expanding due to the growing aging population and the increasing number of surgical procedures worldwide.
In the orthopedic sector, EVA-based products are gaining traction, particularly in the manufacturing of orthopedic insoles and cushioning materials. The rising incidence of foot-related disorders and the growing awareness of the importance of proper foot care are driving the demand for EVA-based orthopedic solutions.
The wound care market is another area where EVA-based products are making significant inroads. EVA's properties make it suitable for advanced wound dressings, contributing to the growth of the global advanced wound care market. The increasing prevalence of chronic wounds, such as diabetic foot ulcers and pressure ulcers, is a key factor driving this market segment.
Geographically, North America and Europe currently dominate the market for EVA-based medical products, owing to advanced healthcare infrastructure and higher healthcare expenditure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing disposable income, and a large patient pool.
The COVID-19 pandemic has further highlighted the importance of EVA in healthcare, particularly in the production of personal protective equipment (PPE) such as face shields and disposable gloves. This has led to an increased demand for EVA in the medical sector, a trend that is likely to continue in the post-pandemic era.
Looking ahead, the market for EVA-based medical products is poised for continued growth. Factors such as technological advancements in material science, increasing healthcare expenditure, and the growing focus on patient comfort and safety are expected to drive further innovation and adoption of EVA-based solutions in the healthcare industry.
Current EVA Applications and Challenges in Healthcare
Ethylene Vinyl Acetate (EVA) has emerged as a versatile material in healthcare applications, offering unique properties that address various medical needs. Currently, EVA is widely used in medical devices, drug delivery systems, and healthcare packaging. In medical devices, EVA is utilized for manufacturing catheters, tubing, and prosthetic components due to its flexibility, biocompatibility, and resistance to chemical degradation. These properties ensure patient comfort and device longevity in various medical procedures.
In drug delivery systems, EVA plays a crucial role in controlled release formulations. It is commonly used in transdermal patches, implants, and vaginal rings, allowing for sustained and targeted drug release. The polymer's ability to be formulated with different drug loadings and release rates makes it an ideal candidate for personalized medicine applications.
Healthcare packaging benefits from EVA's excellent barrier properties and compatibility with sterilization methods. It is used in blister packs, IV bags, and medical equipment packaging, ensuring product integrity and sterility throughout the supply chain.
Despite its widespread use, EVA faces several challenges in healthcare applications. One significant challenge is achieving precise control over drug release rates in delivery systems. While EVA allows for controlled release, fine-tuning the release profile for specific therapeutic needs remains complex. Researchers are working on developing advanced EVA formulations and manufacturing techniques to address this issue.
Another challenge lies in enhancing the material's antimicrobial properties. Although EVA exhibits good chemical resistance, improving its ability to prevent microbial growth on medical devices and packaging is an ongoing area of research. Incorporating antimicrobial agents into EVA without compromising its other beneficial properties presents a technical hurdle.
The biocompatibility of EVA, while generally good, still requires further improvement for certain applications. Long-term implantable devices, in particular, demand materials with exceptional biocompatibility to minimize adverse reactions and ensure patient safety. Researchers are exploring surface modifications and composite formulations to enhance EVA's biocompatibility for these critical applications.
Additionally, the recyclability and environmental impact of EVA in healthcare settings pose challenges. As sustainability becomes increasingly important in the medical field, developing eco-friendly EVA formulations and effective recycling processes for medical-grade EVA products is gaining attention.
Addressing these challenges will be crucial for expanding EVA's role in healthcare solutions. Ongoing research and development efforts focus on overcoming these limitations, paving the way for innovative applications that can significantly advance healthcare technologies and patient outcomes.
In drug delivery systems, EVA plays a crucial role in controlled release formulations. It is commonly used in transdermal patches, implants, and vaginal rings, allowing for sustained and targeted drug release. The polymer's ability to be formulated with different drug loadings and release rates makes it an ideal candidate for personalized medicine applications.
Healthcare packaging benefits from EVA's excellent barrier properties and compatibility with sterilization methods. It is used in blister packs, IV bags, and medical equipment packaging, ensuring product integrity and sterility throughout the supply chain.
Despite its widespread use, EVA faces several challenges in healthcare applications. One significant challenge is achieving precise control over drug release rates in delivery systems. While EVA allows for controlled release, fine-tuning the release profile for specific therapeutic needs remains complex. Researchers are working on developing advanced EVA formulations and manufacturing techniques to address this issue.
Another challenge lies in enhancing the material's antimicrobial properties. Although EVA exhibits good chemical resistance, improving its ability to prevent microbial growth on medical devices and packaging is an ongoing area of research. Incorporating antimicrobial agents into EVA without compromising its other beneficial properties presents a technical hurdle.
The biocompatibility of EVA, while generally good, still requires further improvement for certain applications. Long-term implantable devices, in particular, demand materials with exceptional biocompatibility to minimize adverse reactions and ensure patient safety. Researchers are exploring surface modifications and composite formulations to enhance EVA's biocompatibility for these critical applications.
Additionally, the recyclability and environmental impact of EVA in healthcare settings pose challenges. As sustainability becomes increasingly important in the medical field, developing eco-friendly EVA formulations and effective recycling processes for medical-grade EVA products is gaining attention.
Addressing these challenges will be crucial for expanding EVA's role in healthcare solutions. Ongoing research and development efforts focus on overcoming these limitations, paving the way for innovative applications that can significantly advance healthcare technologies and patient outcomes.
Existing EVA-based Medical Devices and Materials
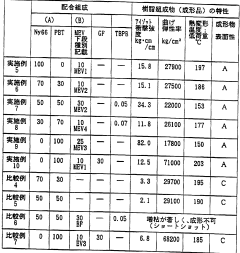
01 Composition and synthesis of EVA copolymers
Ethylene Vinyl Acetate (EVA) copolymers are synthesized through the copolymerization of ethylene and vinyl acetate monomers. The composition and properties of EVA can be tailored by adjusting the ratio of these monomers and the polymerization conditions. This versatility allows for the production of EVA with varying degrees of flexibility, toughness, and adhesion properties.- Composition and synthesis of EVA copolymers: Ethylene Vinyl Acetate (EVA) copolymers are synthesized through the copolymerization of ethylene and vinyl acetate monomers. The composition and properties of EVA can be adjusted by varying the ratio of these monomers, resulting in materials with different characteristics suitable for various applications.
- EVA blends and modifications: EVA copolymers can be blended with other polymers or modified with additives to enhance their properties. These modifications can improve characteristics such as thermal stability, mechanical strength, and processability, making EVA suitable for a wider range of applications.
- EVA in adhesive applications: EVA copolymers are widely used in adhesive formulations due to their excellent adhesion properties, flexibility, and compatibility with various substrates. They can be used in hot melt adhesives, pressure-sensitive adhesives, and other bonding applications across multiple industries.
- EVA in foam and encapsulation applications: EVA copolymers are utilized in the production of foams and encapsulation materials. Their low-temperature flexibility, good barrier properties, and ability to be crosslinked make them suitable for applications such as shoe soles, solar panel encapsulation, and packaging materials.
- EVA processing and manufacturing techniques: Various processing and manufacturing techniques are employed for EVA copolymers, including extrusion, injection molding, and film blowing. These methods allow for the production of EVA products in different forms such as sheets, films, and molded parts, catering to diverse industrial needs.
02 EVA in adhesive applications
EVA copolymers are widely used in adhesive formulations due to their excellent adhesion properties and compatibility with various substrates. They can be used as hot melt adhesives, pressure-sensitive adhesives, and in lamination applications. The adhesive properties of EVA can be further enhanced by blending with other polymers or additives.Expand Specific Solutions03 EVA foam and cellular materials
EVA can be processed into foam or cellular materials with unique properties such as low density, high flexibility, and excellent shock absorption. These foams find applications in footwear, sports equipment, and packaging. The foaming process often involves the use of chemical blowing agents and crosslinking techniques to achieve the desired cellular structure.Expand Specific Solutions04 Modification and blending of EVA
EVA copolymers can be modified or blended with other polymers and additives to enhance their properties or create new materials with unique characteristics. This includes grafting, crosslinking, and the incorporation of fillers or reinforcing agents. Such modifications can improve properties like heat resistance, mechanical strength, and weatherability.Expand Specific Solutions05 EVA in film and packaging applications
EVA copolymers are extensively used in the production of flexible films and packaging materials. Their properties such as clarity, flexibility, and sealability make them suitable for food packaging, agricultural films, and shrink wrap applications. EVA can be processed using various film extrusion techniques and can be co-extruded with other polymers to create multi-layer films with enhanced properties.Expand Specific Solutions
Key Players in EVA Healthcare Solutions
The ethylene vinyl acetate (EVA) market in healthcare is in a growth phase, driven by increasing demand for advanced medical devices and drug delivery systems. The global market size is expanding, with major players like Celanese, Bayer, and Eastman Chemical leading innovation. These companies are leveraging EVA's versatility to develop novel healthcare solutions. The technology's maturity varies across applications, with established uses in medical packaging and emerging potential in drug delivery and tissue engineering. Companies like Grünenthal and bioMérieux are exploring EVA's capabilities in pharmaceutical and diagnostic applications, indicating a trend towards more sophisticated healthcare products utilizing this versatile polymer.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced EVA copolymers for healthcare applications. Their research focuses on improving the properties of EVA for medical devices and drug delivery systems. Sinopec's EVA formulations offer enhanced biocompatibility and controlled release characteristics[1]. They have engineered EVA materials with varying vinyl acetate content (18-40%) to optimize flexibility and drug permeability for transdermal patches and implants[3]. Sinopec has also developed EVA-based nanocomposites incorporating antimicrobial agents for infection control in medical devices[5].
Strengths: Large-scale production capabilities, extensive R&D resources. Weaknesses: Potential regulatory hurdles in international markets, competition from specialized healthcare materials companies.
Celanese International Corp.
Technical Solution: Celanese has developed a range of medical-grade EVA copolymers under their VitalDose® platform. These materials are designed specifically for controlled release drug delivery applications. Celanese's EVA formulations offer tunable release profiles by adjusting the vinyl acetate content (15-40%) and molecular weight[2]. They have engineered EVA matrices for long-acting implants and intravaginal rings, capable of sustained drug release for several months[4]. Celanese has also developed EVA-based hot-melt extrusion formulations for oral dosage forms, improving bioavailability of poorly soluble drugs[6].
Strengths: Specialized in medical-grade polymers, strong intellectual property portfolio. Weaknesses: Limited to drug delivery applications, potential supply chain dependencies.
Innovative EVA Formulations for Medical Use
Implantable Medical Device for the Delivery of a Nucleic Acid
PatentPendingUS20250101417A1
Innovation
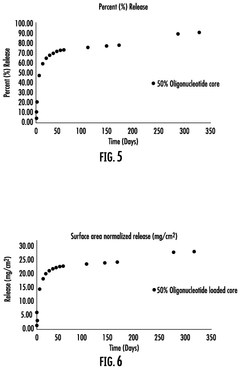
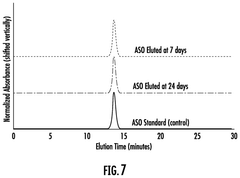
- An implantable medical device with a core polymer matrix containing ethylene vinyl acetate copolymer, where at least 10% of the internucleoside linkages are chemically modified, allowing for the controlled release of antisense oligonucleotides over a period of about seven days, with a melting temperature and melt flow index optimized to minimize nucleic acid degradation.
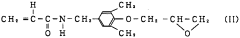
Graft ethylene-vinyl acetate copolymer and resin composition containing the same
PatentWO1997002302A1
Innovation
- A graft-modified ethylene-vinyl acetate copolymer is developed by incorporating a glycidyl group-containing compound and unsaturated glycidyl ester, along with a radical initiator, to enhance thermal stability and adhesion, and is blended with polyamide or polyester resins to improve mechanical and thermal properties.
Regulatory Framework for EVA in Medical Devices
The regulatory framework for Ethylene Vinyl Acetate (EVA) in medical devices is a critical aspect of its application in healthcare solutions. As a biocompatible and versatile material, EVA must adhere to stringent regulations to ensure patient safety and product efficacy.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices containing EVA. These devices are typically classified under Class II or Class III, depending on their intended use and risk level. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain premarket approval or clearance through the 510(k) process before introducing their products to the market.
The European Union regulates EVA-based medical devices under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations require manufacturers to obtain CE marking, demonstrating compliance with essential safety and performance requirements. The MDR also emphasizes post-market surveillance and clinical evaluation throughout the product lifecycle.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices containing EVA. Manufacturers must obtain approval through the PMDA's review process, which includes evaluating the device's safety, efficacy, and quality.
International standards play a crucial role in the regulatory framework for EVA in medical devices. ISO 10993 series, particularly ISO 10993-1, provides guidelines for evaluating the biocompatibility of medical devices. Manufacturers must conduct appropriate tests to assess cytotoxicity, sensitization, and other potential biological risks associated with EVA-based products.
The regulatory landscape for EVA in medical devices also addresses specific applications. For instance, EVA-based drug delivery systems must comply with additional regulations related to pharmaceutical products. This includes demonstrating the stability of the drug formulation within the EVA matrix and ensuring consistent release profiles.
Environmental considerations are increasingly important in the regulatory framework. Regulations such as the European Union's Restriction of Hazardous Substances (RoHS) directive impact the use of certain additives in EVA formulations for medical devices. Manufacturers must ensure their products comply with these environmental regulations while maintaining the required performance characteristics.
As the healthcare industry continues to innovate, regulatory bodies are adapting their frameworks to address emerging technologies. This includes the development of guidance documents for 3D-printed medical devices, which may incorporate EVA materials. Manufacturers must stay informed about these evolving regulations to ensure compliance and market access for their EVA-based healthcare solutions.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices containing EVA. These devices are typically classified under Class II or Class III, depending on their intended use and risk level. Manufacturers must comply with the FDA's Quality System Regulation (QSR) and obtain premarket approval or clearance through the 510(k) process before introducing their products to the market.
The European Union regulates EVA-based medical devices under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations require manufacturers to obtain CE marking, demonstrating compliance with essential safety and performance requirements. The MDR also emphasizes post-market surveillance and clinical evaluation throughout the product lifecycle.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices containing EVA. Manufacturers must obtain approval through the PMDA's review process, which includes evaluating the device's safety, efficacy, and quality.
International standards play a crucial role in the regulatory framework for EVA in medical devices. ISO 10993 series, particularly ISO 10993-1, provides guidelines for evaluating the biocompatibility of medical devices. Manufacturers must conduct appropriate tests to assess cytotoxicity, sensitization, and other potential biological risks associated with EVA-based products.
The regulatory landscape for EVA in medical devices also addresses specific applications. For instance, EVA-based drug delivery systems must comply with additional regulations related to pharmaceutical products. This includes demonstrating the stability of the drug formulation within the EVA matrix and ensuring consistent release profiles.
Environmental considerations are increasingly important in the regulatory framework. Regulations such as the European Union's Restriction of Hazardous Substances (RoHS) directive impact the use of certain additives in EVA formulations for medical devices. Manufacturers must ensure their products comply with these environmental regulations while maintaining the required performance characteristics.
As the healthcare industry continues to innovate, regulatory bodies are adapting their frameworks to address emerging technologies. This includes the development of guidance documents for 3D-printed medical devices, which may incorporate EVA materials. Manufacturers must stay informed about these evolving regulations to ensure compliance and market access for their EVA-based healthcare solutions.
Biocompatibility and Safety Considerations of EVA
Ethylene Vinyl Acetate (EVA) has emerged as a promising material in healthcare applications due to its unique properties and biocompatibility. The safety and compatibility of EVA with biological systems are crucial factors in its adoption for medical devices and drug delivery systems. Extensive research has been conducted to evaluate the biocompatibility of EVA, focusing on its interactions with living tissues and potential adverse effects.
One of the primary advantages of EVA in healthcare is its low toxicity and minimal inflammatory response when in contact with biological tissues. Studies have shown that EVA exhibits excellent biocompatibility, with minimal tissue irritation and foreign body reactions. This characteristic makes it suitable for various medical applications, including implantable devices, wound dressings, and drug-eluting systems.
The safety profile of EVA is further enhanced by its resistance to degradation in biological environments. Unlike some polymers that may break down and release potentially harmful byproducts, EVA maintains its structural integrity over extended periods. This stability ensures consistent performance and reduces the risk of unintended consequences associated with material degradation.
In terms of sterilization, EVA demonstrates compatibility with various sterilization methods commonly used in healthcare settings. It can withstand gamma radiation, ethylene oxide treatment, and steam sterilization without significant alterations to its physical or chemical properties. This versatility in sterilization options contributes to its safety profile and ease of integration into existing medical manufacturing processes.
However, it is essential to consider the potential for leaching of additives or residual monomers from EVA-based medical devices. Rigorous testing protocols have been developed to assess the release of such compounds and their potential biological effects. Regulatory bodies, such as the FDA, have established guidelines for evaluating the safety of EVA in medical applications, ensuring that products meet stringent safety standards before approval.
The biocompatibility of EVA extends to its use in drug delivery systems. Its ability to control drug release rates and maintain drug stability has been extensively studied. Research has shown that EVA-based drug delivery systems can provide sustained release of various therapeutic agents without compromising their efficacy or causing local tissue reactions.
While EVA demonstrates excellent biocompatibility in many applications, it is crucial to note that its safety profile may vary depending on the specific formulation and intended use. Factors such as the grade of EVA, the presence of additives, and the manufacturing process can influence its biocompatibility. Therefore, comprehensive testing and evaluation are necessary for each specific application to ensure safety and efficacy.
One of the primary advantages of EVA in healthcare is its low toxicity and minimal inflammatory response when in contact with biological tissues. Studies have shown that EVA exhibits excellent biocompatibility, with minimal tissue irritation and foreign body reactions. This characteristic makes it suitable for various medical applications, including implantable devices, wound dressings, and drug-eluting systems.
The safety profile of EVA is further enhanced by its resistance to degradation in biological environments. Unlike some polymers that may break down and release potentially harmful byproducts, EVA maintains its structural integrity over extended periods. This stability ensures consistent performance and reduces the risk of unintended consequences associated with material degradation.
In terms of sterilization, EVA demonstrates compatibility with various sterilization methods commonly used in healthcare settings. It can withstand gamma radiation, ethylene oxide treatment, and steam sterilization without significant alterations to its physical or chemical properties. This versatility in sterilization options contributes to its safety profile and ease of integration into existing medical manufacturing processes.
However, it is essential to consider the potential for leaching of additives or residual monomers from EVA-based medical devices. Rigorous testing protocols have been developed to assess the release of such compounds and their potential biological effects. Regulatory bodies, such as the FDA, have established guidelines for evaluating the safety of EVA in medical applications, ensuring that products meet stringent safety standards before approval.
The biocompatibility of EVA extends to its use in drug delivery systems. Its ability to control drug release rates and maintain drug stability has been extensively studied. Research has shown that EVA-based drug delivery systems can provide sustained release of various therapeutic agents without compromising their efficacy or causing local tissue reactions.
While EVA demonstrates excellent biocompatibility in many applications, it is crucial to note that its safety profile may vary depending on the specific formulation and intended use. Factors such as the grade of EVA, the presence of additives, and the manufacturing process can influence its biocompatibility. Therefore, comprehensive testing and evaluation are necessary for each specific application to ensure safety and efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!