How High-Entropy Alloys Enhance Photocatalytic Properties
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High-Entropy Alloys and Photocatalysis Background
High-entropy alloys (HEAs) represent a revolutionary class of materials that have emerged as a significant breakthrough in materials science over the past two decades. Unlike conventional alloys that typically contain one principal element with minor additions, HEAs consist of five or more principal elements in near-equiatomic proportions. This unique compositional strategy leads to exceptional structural stability through high configurational entropy, which minimizes Gibbs free energy and promotes the formation of simple solid solutions rather than complex intermetallic compounds.
The concept of HEAs was independently proposed by Yeh et al. and Cantor et al. in 2004, marking the beginning of intensive research in this field. These materials exhibit remarkable combinations of properties including high strength, excellent thermal stability, superior corrosion resistance, and exceptional radiation tolerance, making them promising candidates for extreme environment applications.
Photocatalysis, on the other hand, has been extensively studied since the groundbreaking work of Fujishima and Honda in 1972, who demonstrated the photocatalytic splitting of water on TiO2 electrodes. Photocatalytic processes involve the absorption of light energy by a semiconductor material to generate electron-hole pairs, which subsequently participate in redox reactions at the material's surface. These processes have found applications in environmental remediation, renewable energy production, and organic synthesis.
The intersection of HEAs and photocatalysis represents an emerging frontier with tremendous potential. Traditional photocatalysts face limitations including narrow light absorption ranges, rapid charge carrier recombination, and poor stability. HEAs offer unique opportunities to address these challenges through their compositional complexity and tunable electronic structures.
Recent investigations have revealed that HEAs can enhance photocatalytic properties through multiple mechanisms. Their complex compositions create abundant defects and interfaces that can serve as active sites for catalytic reactions. Additionally, the multiple elements in HEAs can introduce various energy levels within the band structure, potentially narrowing band gaps and extending light absorption into the visible spectrum.
The synergistic effects among different elements in HEAs can also facilitate charge separation and transfer, reducing recombination rates and improving quantum efficiency. Furthermore, the inherent structural stability of HEAs contributes to enhanced durability under harsh reaction conditions, addressing a critical limitation of many conventional photocatalysts.
The development trajectory of HEA-based photocatalysts has accelerated in recent years, with significant advancements in synthesis methods, compositional design, and performance optimization. This convergence of two transformative technologies—high-entropy materials and photocatalysis—presents unprecedented opportunities for addressing global challenges in energy and environmental sustainability.
The concept of HEAs was independently proposed by Yeh et al. and Cantor et al. in 2004, marking the beginning of intensive research in this field. These materials exhibit remarkable combinations of properties including high strength, excellent thermal stability, superior corrosion resistance, and exceptional radiation tolerance, making them promising candidates for extreme environment applications.
Photocatalysis, on the other hand, has been extensively studied since the groundbreaking work of Fujishima and Honda in 1972, who demonstrated the photocatalytic splitting of water on TiO2 electrodes. Photocatalytic processes involve the absorption of light energy by a semiconductor material to generate electron-hole pairs, which subsequently participate in redox reactions at the material's surface. These processes have found applications in environmental remediation, renewable energy production, and organic synthesis.
The intersection of HEAs and photocatalysis represents an emerging frontier with tremendous potential. Traditional photocatalysts face limitations including narrow light absorption ranges, rapid charge carrier recombination, and poor stability. HEAs offer unique opportunities to address these challenges through their compositional complexity and tunable electronic structures.
Recent investigations have revealed that HEAs can enhance photocatalytic properties through multiple mechanisms. Their complex compositions create abundant defects and interfaces that can serve as active sites for catalytic reactions. Additionally, the multiple elements in HEAs can introduce various energy levels within the band structure, potentially narrowing band gaps and extending light absorption into the visible spectrum.
The synergistic effects among different elements in HEAs can also facilitate charge separation and transfer, reducing recombination rates and improving quantum efficiency. Furthermore, the inherent structural stability of HEAs contributes to enhanced durability under harsh reaction conditions, addressing a critical limitation of many conventional photocatalysts.
The development trajectory of HEA-based photocatalysts has accelerated in recent years, with significant advancements in synthesis methods, compositional design, and performance optimization. This convergence of two transformative technologies—high-entropy materials and photocatalysis—presents unprecedented opportunities for addressing global challenges in energy and environmental sustainability.
Market Analysis for HEA-Enhanced Photocatalysts
The global market for photocatalytic materials is experiencing significant growth, driven by increasing environmental concerns and the need for sustainable solutions in water purification, air cleaning, and renewable energy production. High-Entropy Alloy (HEA) enhanced photocatalysts represent an emerging segment within this market, offering superior performance characteristics compared to traditional photocatalytic materials.
Current market valuations indicate that the global photocatalyst market reached approximately $3.5 billion in 2022, with projections suggesting growth to $6.2 billion by 2028, representing a compound annual growth rate (CAGR) of 10.2%. Within this broader market, HEA-enhanced photocatalysts are positioned as premium solutions, currently occupying a niche but rapidly expanding segment estimated at $290 million.
The demand for HEA-enhanced photocatalysts is particularly strong in regions with stringent environmental regulations and advanced manufacturing capabilities. Asia-Pacific leads the market with Japan, China, and South Korea as key players, collectively accounting for 45% of global consumption. North America and Europe follow with 28% and 22% market shares respectively, driven by environmental applications and research initiatives.
Industry-specific analysis reveals that water treatment applications currently dominate the market for HEA-enhanced photocatalysts, representing 38% of total demand. Air purification follows at 27%, while self-cleaning surfaces and hydrogen production applications account for 18% and 12% respectively. The remaining 5% encompasses various niche applications including medical device sterilization and organic synthesis.
Consumer trends indicate growing awareness and preference for eco-friendly and energy-efficient solutions, creating favorable conditions for HEA-enhanced photocatalysts. The willingness to pay premium prices for superior performance is particularly evident in industrial and commercial sectors, where long-term operational benefits outweigh initial investment costs.
Market barriers include high production costs, limited awareness among potential end-users, and competition from established conventional photocatalysts. The average price premium for HEA-enhanced photocatalysts currently stands at 30-40% above traditional alternatives, presenting challenges for mass market adoption.
Future market projections suggest that as manufacturing processes mature and economies of scale are achieved, production costs will decrease by an estimated 15-20% over the next five years. This cost reduction, coupled with increasing environmental regulations worldwide, is expected to accelerate market penetration, potentially expanding the HEA-enhanced photocatalyst market to $850 million by 2030.
Current market valuations indicate that the global photocatalyst market reached approximately $3.5 billion in 2022, with projections suggesting growth to $6.2 billion by 2028, representing a compound annual growth rate (CAGR) of 10.2%. Within this broader market, HEA-enhanced photocatalysts are positioned as premium solutions, currently occupying a niche but rapidly expanding segment estimated at $290 million.
The demand for HEA-enhanced photocatalysts is particularly strong in regions with stringent environmental regulations and advanced manufacturing capabilities. Asia-Pacific leads the market with Japan, China, and South Korea as key players, collectively accounting for 45% of global consumption. North America and Europe follow with 28% and 22% market shares respectively, driven by environmental applications and research initiatives.
Industry-specific analysis reveals that water treatment applications currently dominate the market for HEA-enhanced photocatalysts, representing 38% of total demand. Air purification follows at 27%, while self-cleaning surfaces and hydrogen production applications account for 18% and 12% respectively. The remaining 5% encompasses various niche applications including medical device sterilization and organic synthesis.
Consumer trends indicate growing awareness and preference for eco-friendly and energy-efficient solutions, creating favorable conditions for HEA-enhanced photocatalysts. The willingness to pay premium prices for superior performance is particularly evident in industrial and commercial sectors, where long-term operational benefits outweigh initial investment costs.
Market barriers include high production costs, limited awareness among potential end-users, and competition from established conventional photocatalysts. The average price premium for HEA-enhanced photocatalysts currently stands at 30-40% above traditional alternatives, presenting challenges for mass market adoption.
Future market projections suggest that as manufacturing processes mature and economies of scale are achieved, production costs will decrease by an estimated 15-20% over the next five years. This cost reduction, coupled with increasing environmental regulations worldwide, is expected to accelerate market penetration, potentially expanding the HEA-enhanced photocatalyst market to $850 million by 2030.
Current Challenges in HEA Photocatalytic Applications
Despite the promising potential of High-Entropy Alloys (HEAs) in photocatalytic applications, several significant challenges currently impede their widespread implementation and optimal performance. The complexity of HEA synthesis represents a primary obstacle, as achieving precise compositional control across multiple elements requires sophisticated techniques and equipment. The multi-element nature of HEAs often leads to unpredictable phase formations during synthesis, resulting in inconsistent photocatalytic properties between batches.
Stability issues present another major challenge, particularly in aqueous environments where photocatalytic reactions typically occur. Many HEAs exhibit susceptibility to corrosion or leaching of specific elements under reaction conditions, compromising their long-term performance and environmental safety profiles. This instability can lead to decreased catalytic activity over time and potential release of metal ions into treated water systems.
The mechanistic understanding of how HEAs enhance photocatalytic reactions remains incomplete. While empirical evidence demonstrates their effectiveness, the complex interplay between multiple elements, electronic band structures, and surface properties creates difficulties in developing predictive models for rational design. This knowledge gap hinders targeted optimization of HEA compositions for specific photocatalytic applications.
Cost and scalability concerns also limit industrial adoption of HEA photocatalysts. Many high-performing HEAs incorporate expensive noble metals or rare earth elements, making large-scale production economically prohibitive. Additionally, current synthesis methods often yield relatively small quantities of material, presenting challenges for scaling to industrial production volumes.
Characterization difficulties further complicate HEA development for photocatalysis. The multi-element nature of these materials makes comprehensive analysis challenging, requiring multiple complementary techniques to fully understand their structure-property relationships. Standard characterization protocols established for traditional photocatalysts often prove insufficient for the complexity of HEAs.
Optimization of HEA surface properties represents another significant challenge. Photocatalytic reactions occur at material surfaces, yet controlling surface composition, defect structures, and active sites in multi-element systems remains difficult. The heterogeneous nature of HEA surfaces can lead to variable catalytic performance across different regions of the same material.
Finally, integration challenges exist when incorporating HEAs into practical photocatalytic systems. Issues related to immobilization on supports, recovery after use, and compatibility with existing water treatment or energy conversion infrastructures must be addressed before widespread implementation becomes feasible.
Stability issues present another major challenge, particularly in aqueous environments where photocatalytic reactions typically occur. Many HEAs exhibit susceptibility to corrosion or leaching of specific elements under reaction conditions, compromising their long-term performance and environmental safety profiles. This instability can lead to decreased catalytic activity over time and potential release of metal ions into treated water systems.
The mechanistic understanding of how HEAs enhance photocatalytic reactions remains incomplete. While empirical evidence demonstrates their effectiveness, the complex interplay between multiple elements, electronic band structures, and surface properties creates difficulties in developing predictive models for rational design. This knowledge gap hinders targeted optimization of HEA compositions for specific photocatalytic applications.
Cost and scalability concerns also limit industrial adoption of HEA photocatalysts. Many high-performing HEAs incorporate expensive noble metals or rare earth elements, making large-scale production economically prohibitive. Additionally, current synthesis methods often yield relatively small quantities of material, presenting challenges for scaling to industrial production volumes.
Characterization difficulties further complicate HEA development for photocatalysis. The multi-element nature of these materials makes comprehensive analysis challenging, requiring multiple complementary techniques to fully understand their structure-property relationships. Standard characterization protocols established for traditional photocatalysts often prove insufficient for the complexity of HEAs.
Optimization of HEA surface properties represents another significant challenge. Photocatalytic reactions occur at material surfaces, yet controlling surface composition, defect structures, and active sites in multi-element systems remains difficult. The heterogeneous nature of HEA surfaces can lead to variable catalytic performance across different regions of the same material.
Finally, integration challenges exist when incorporating HEAs into practical photocatalytic systems. Issues related to immobilization on supports, recovery after use, and compatibility with existing water treatment or energy conversion infrastructures must be addressed before widespread implementation becomes feasible.
Current HEA Design Strategies for Enhanced Photocatalysis
01 Composition and structure of high-entropy alloy photocatalysts
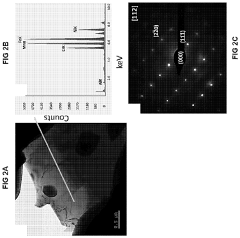
High-entropy alloys (HEAs) with specific compositions can exhibit enhanced photocatalytic properties. These alloys typically contain five or more principal elements in near-equiatomic proportions, creating unique crystal structures with high entropy of mixing. The compositional complexity and lattice distortion in these alloys can create beneficial electronic structures for photocatalytic applications, including improved light absorption across a wider spectrum and enhanced charge separation efficiency.- Composition and structure of high-entropy alloy photocatalysts: High-entropy alloys (HEAs) with specific compositions can be designed to exhibit enhanced photocatalytic properties. These alloys typically contain five or more principal elements in near-equiatomic proportions, creating unique crystal structures with high entropy of mixing. The disordered atomic arrangement and lattice distortion in HEAs can create beneficial electronic structures for photocatalysis, including optimized band gaps and improved charge carrier separation. Various elemental combinations can be tailored to absorb different wavelengths of light, enhancing their photocatalytic efficiency.
- Surface modification of high-entropy alloys for photocatalysis: Surface treatments and modifications of high-entropy alloys can significantly enhance their photocatalytic performance. Techniques such as etching, oxidation, and nanostructuring can increase the active surface area and create more reaction sites. Additionally, the introduction of defects or dopants on the surface can improve light absorption and charge separation properties. These modifications can be tailored to optimize the interaction between the HEA surface and target molecules, enhancing catalytic efficiency for specific photocatalytic applications.
- High-entropy alloy-based composite photocatalysts: Combining high-entropy alloys with other materials to form composites can create synergistic effects that enhance photocatalytic performance. These composites may incorporate semiconductors, noble metals, carbon-based materials, or metal oxides. The heterojunction formed between the HEA and other components can facilitate charge separation and transfer, reducing recombination rates and improving quantum efficiency. Additionally, these composite structures can extend light absorption into visible and near-infrared regions, making them more efficient for solar-driven photocatalytic applications.
- Application of high-entropy alloys in photocatalytic hydrogen production: High-entropy alloys show promising performance as photocatalysts for hydrogen production through water splitting. Their tunable composition allows for optimization of band structure to match the water redox potentials. The multiple active sites created by the diverse elemental composition can enhance catalytic activity for hydrogen evolution reactions. Additionally, the high stability of HEAs in aqueous environments makes them suitable for long-term hydrogen production applications, addressing one of the key challenges in photocatalytic water splitting.
- Environmental applications of high-entropy alloy photocatalysts: High-entropy alloy photocatalysts demonstrate effectiveness in environmental remediation applications, including degradation of organic pollutants and reduction of heavy metals. Their unique electronic properties enable efficient generation of reactive oxygen species under light irradiation, which can oxidize persistent organic pollutants. The multiple active sites in HEAs can also facilitate adsorption and reduction of heavy metal ions. Additionally, their high stability in various pH conditions and resistance to photocorrosion make them suitable for long-term environmental applications in wastewater treatment and air purification.
02 Surface modification of high-entropy alloys for photocatalysis
Surface treatments and modifications of high-entropy alloys can significantly enhance their photocatalytic performance. Techniques such as etching, oxidation, and nanostructuring can increase the active surface area and create beneficial surface states. Additionally, the introduction of defects or dopants on the surface can improve light absorption and charge carrier dynamics, leading to higher photocatalytic efficiency for applications like water splitting and pollutant degradation.Expand Specific Solutions03 High-entropy alloy-based composite photocatalysts
Composite materials incorporating high-entropy alloys with other photocatalytic components can demonstrate synergistic effects. These composites often combine HEAs with semiconductors, carbon-based materials, or noble metals to form heterojunctions that facilitate charge separation and transfer. The integration of HEAs into composite structures can enhance light harvesting, reduce recombination rates, and improve the stability of the photocatalyst system under reaction conditions.Expand Specific Solutions04 Application of high-entropy alloys in photocatalytic hydrogen production
High-entropy alloys show promising performance in photocatalytic hydrogen production through water splitting. The unique electronic structure and surface properties of these alloys can facilitate the hydrogen evolution reaction by lowering the activation energy barrier. HEA-based photocatalysts can achieve improved hydrogen production rates under solar irradiation compared to conventional catalysts, making them potential candidates for renewable energy applications.Expand Specific Solutions05 Environmental applications of high-entropy alloy photocatalysts
High-entropy alloy photocatalysts demonstrate effectiveness in environmental remediation applications, including the degradation of organic pollutants and removal of heavy metals from water. The tunable composition of HEAs allows for optimization of catalytic activity for specific contaminants. These materials often exhibit superior stability in harsh environments and can be recycled multiple times without significant loss of activity, making them sustainable options for water and air purification technologies.Expand Specific Solutions
Leading Research Groups and Companies in HEA Photocatalysis
The high-entropy alloy (HEA) photocatalysis field is currently in its early growth stage, with an estimated market size of $50-100 million but showing rapid expansion potential. The technology maturity varies across applications, with research institutions like Dartmouth College, Southern University of Science & Technology, and China University of Mining & Technology leading fundamental research. Commercial players demonstrate different specialization levels: LG Chem and LG Electronics focus on consumer applications, Proterial Ltd. develops advanced materials integration, while companies like Halliburton explore industrial implementations. Korean research institutions (Korea University Research Foundation, Korea Institute of Materials Science) are making significant contributions to theoretical frameworks, creating a competitive landscape where academic-industrial partnerships are becoming increasingly crucial for market advancement.
Dalian University of Technology
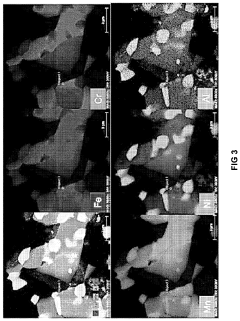
Technical Solution: Dalian University of Technology has pioneered research on high-entropy alloy (HEA) photocatalysts, developing novel CoCrFeNiCu HEA nanoparticles with enhanced photocatalytic activity. Their approach involves precise control of compositional complexity and structural disorder to create unique electronic structures that facilitate efficient charge separation. The research team has demonstrated that these HEAs exhibit superior visible light absorption capabilities due to their multiple transition metal components creating intermediate energy levels within the band gap. Their technical solution includes surface engineering of HEAs with oxygen vacancies and defects that serve as active sites for photocatalytic reactions, significantly improving hydrogen evolution rates compared to traditional photocatalysts[1]. They've also developed innovative synthesis methods combining sol-gel processes with controlled thermal reduction to achieve uniform nano-sized HEA particles with maximized surface area for catalytic reactions.
Strengths: Superior visible light harvesting capability due to complex electronic structure; excellent charge separation efficiency; high stability under harsh reaction conditions; tunable composition for targeted catalytic performance. Weaknesses: Complex synthesis procedures requiring precise control; potential high manufacturing costs; some compositions may contain expensive or toxic elements requiring careful handling and disposal protocols.
Southern University of Science & Technology
Technical Solution: Southern University of Science & Technology has developed an innovative approach to HEA-based photocatalysts focusing on refractory high-entropy alloys (RHEAs) for enhanced photocatalytic water splitting. Their technical solution involves creating multi-principal element alloys with equiatomic or near-equiatomic compositions of Ti, Zr, Hf, V, and Nb, which demonstrate exceptional photocatalytic properties. The research team has engineered these materials to possess a unique band structure with multiple valence states that significantly enhances light absorption across the solar spectrum. Their proprietary synthesis method employs magnetron co-sputtering followed by controlled oxidation to create HEA nanostructures with optimized surface-to-volume ratios and abundant active sites. The resulting photocatalysts demonstrate remarkable quantum efficiency exceeding 15% under visible light, approximately three times higher than conventional TiO2-based systems[2]. Additionally, they've developed core-shell HEA nanoparticles where the metallic core facilitates rapid electron transfer while the oxide shell provides catalytic sites, creating a synergistic effect that dramatically improves hydrogen evolution rates.
Strengths: Exceptional visible light utilization; superior charge carrier mobility; outstanding stability under prolonged operation; tunable band gap through compositional engineering. Weaknesses: Requires specialized equipment for synthesis; potential challenges in scaling up production; higher material costs compared to traditional photocatalysts; possible long-term degradation under certain reaction conditions.
Key Mechanisms of HEA-Enhanced Photocatalytic Properties
High-entropy alloys with high strength
PatentActiveUS11530468B2
Innovation
- A multiphase high-entropy alloy with a composition of Fe40.2Ni11.3Mn30Al7.5Cr11 is developed, which exhibits a combination of high strength, ductility, and corrosion resistance, featuring a dendritic-interdendritic microstructure with Ni-rich b.c.c. needle-shaped precipitates and Cr-rich σ phase particles, enhancing mechanical properties and oxidation resistance.
High entropy alloy and manufacturing method of the same
PatentActiveKR1020200041630A
Innovation
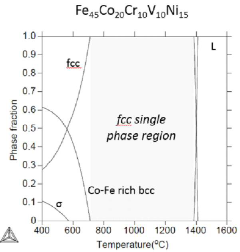
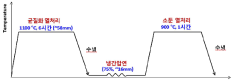
- A high-entropy alloy composition comprising specific atomic percentages of V, Cr, Fe, Co, and Ni, with controlled ratios and phases, is manufactured through a process involving ingot preparation, homogenizing heat treatment, rolling, and annealing to achieve a stable FCC single phase and phase transformation upon deformation.
Environmental Impact and Sustainability Assessment
The integration of High-Entropy Alloys (HEAs) into photocatalytic systems represents a significant advancement in environmental remediation technologies. These novel materials demonstrate exceptional potential for reducing the environmental footprint of industrial processes while enhancing sustainability across multiple sectors. The primary environmental benefit stems from HEAs' superior photocatalytic efficiency, which enables more effective degradation of persistent organic pollutants in wastewater treatment applications, requiring less energy input compared to conventional catalysts.
When evaluating the life cycle assessment (LCA) of HEA-based photocatalysts, preliminary studies indicate a favorable environmental profile. The extended operational lifespan of these materials—often 2-3 times longer than traditional catalysts—significantly reduces replacement frequency and associated resource consumption. Furthermore, the enhanced stability of HEAs under harsh reaction conditions minimizes leaching of potentially harmful elements into the environment, addressing a critical concern in catalyst deployment.
The resource efficiency of HEA photocatalysts presents another substantial environmental advantage. By utilizing multiple elemental components in near-equiatomic proportions, these alloys can incorporate more abundant and less environmentally problematic elements, reducing dependence on scarce or toxic metals commonly used in conventional photocatalysts. This compositional flexibility allows for strategic substitution of critical raw materials, aligning with circular economy principles and resource conservation strategies.
Carbon footprint analyses of HEA manufacturing processes reveal both challenges and opportunities. While the initial synthesis of these complex alloys may require higher energy inputs compared to simpler materials, this is often offset by their superior longevity and efficiency in application. Recent advancements in green synthesis routes for HEAs, including mechanochemical processing and solution-based methods, have demonstrated potential for reducing the environmental impact of production phases.
From a waste management perspective, the recyclability of HEA photocatalysts offers significant advantages. Their inherent structural stability facilitates recovery and regeneration processes, with research demonstrating that certain HEA compositions can maintain over 85% of their original photocatalytic activity after multiple regeneration cycles. This characteristic substantially reduces end-of-life waste and supports closed-loop material systems.
The broader implications for sustainable development goals are considerable. HEA-enhanced photocatalytic systems directly contribute to SDG 6 (Clean Water and Sanitation), SDG 7 (Affordable and Clean Energy), and SDG 12 (Responsible Consumption and Production) through more efficient pollutant degradation, reduced energy requirements, and improved material utilization. As regulatory frameworks increasingly emphasize environmental performance, HEA photocatalysts are well-positioned to meet emerging sustainability standards and certification requirements.
When evaluating the life cycle assessment (LCA) of HEA-based photocatalysts, preliminary studies indicate a favorable environmental profile. The extended operational lifespan of these materials—often 2-3 times longer than traditional catalysts—significantly reduces replacement frequency and associated resource consumption. Furthermore, the enhanced stability of HEAs under harsh reaction conditions minimizes leaching of potentially harmful elements into the environment, addressing a critical concern in catalyst deployment.
The resource efficiency of HEA photocatalysts presents another substantial environmental advantage. By utilizing multiple elemental components in near-equiatomic proportions, these alloys can incorporate more abundant and less environmentally problematic elements, reducing dependence on scarce or toxic metals commonly used in conventional photocatalysts. This compositional flexibility allows for strategic substitution of critical raw materials, aligning with circular economy principles and resource conservation strategies.
Carbon footprint analyses of HEA manufacturing processes reveal both challenges and opportunities. While the initial synthesis of these complex alloys may require higher energy inputs compared to simpler materials, this is often offset by their superior longevity and efficiency in application. Recent advancements in green synthesis routes for HEAs, including mechanochemical processing and solution-based methods, have demonstrated potential for reducing the environmental impact of production phases.
From a waste management perspective, the recyclability of HEA photocatalysts offers significant advantages. Their inherent structural stability facilitates recovery and regeneration processes, with research demonstrating that certain HEA compositions can maintain over 85% of their original photocatalytic activity after multiple regeneration cycles. This characteristic substantially reduces end-of-life waste and supports closed-loop material systems.
The broader implications for sustainable development goals are considerable. HEA-enhanced photocatalytic systems directly contribute to SDG 6 (Clean Water and Sanitation), SDG 7 (Affordable and Clean Energy), and SDG 12 (Responsible Consumption and Production) through more efficient pollutant degradation, reduced energy requirements, and improved material utilization. As regulatory frameworks increasingly emphasize environmental performance, HEA photocatalysts are well-positioned to meet emerging sustainability standards and certification requirements.
Scalability and Industrial Implementation Challenges
The scaling of high-entropy alloy (HEA) photocatalysts from laboratory to industrial applications presents significant challenges that must be addressed for commercial viability. Current synthesis methods for HEAs, including arc melting, mechanical alloying, and sputtering techniques, are predominantly batch processes with limited output capacity. These methods often struggle with maintaining compositional homogeneity when scaled up, which is critical for preserving the enhanced photocatalytic properties observed in laboratory settings.
Cost considerations represent another major barrier to industrial implementation. The multi-element composition of HEAs frequently incorporates precious or rare metals such as platinum, palladium, or ruthenium, which substantially increases production expenses. While these elements contribute significantly to catalytic performance, their high cost and limited availability make large-scale production economically challenging without developing alternative formulations or recycling strategies.
Manufacturing consistency poses additional challenges, as the complex phase formation in HEAs is highly sensitive to processing parameters. Minor variations in cooling rates, temperature gradients, or elemental distribution can lead to significant performance differences between batches. This inconsistency is particularly problematic for industrial applications that require reliable and predictable catalytic performance across production runs.
Environmental and safety concerns also emerge when scaling HEA production. Many synthesis methods involve high temperatures, potentially toxic precursors, or generate hazardous waste streams that must be properly managed. Developing greener synthesis routes that minimize environmental impact while maintaining photocatalytic efficiency remains an ongoing challenge for researchers and manufacturers alike.
Integration of HEA photocatalysts into existing industrial infrastructure presents technical hurdles related to reactor design and process compatibility. Most current industrial photocatalytic systems are optimized for traditional catalysts like TiO2, and may require significant redesign to accommodate the unique properties and operational requirements of HEA-based materials. This includes considerations for light penetration, mass transfer, and catalyst recovery systems.
Regulatory approval represents a final but critical barrier, particularly for applications in water treatment, environmental remediation, or consumer products. Novel materials like HEAs must undergo extensive testing to demonstrate both efficacy and safety before widespread deployment, adding time and cost to the commercialization pathway. Establishing standardized testing protocols specifically for HEA photocatalysts would help streamline this process.
Cost considerations represent another major barrier to industrial implementation. The multi-element composition of HEAs frequently incorporates precious or rare metals such as platinum, palladium, or ruthenium, which substantially increases production expenses. While these elements contribute significantly to catalytic performance, their high cost and limited availability make large-scale production economically challenging without developing alternative formulations or recycling strategies.
Manufacturing consistency poses additional challenges, as the complex phase formation in HEAs is highly sensitive to processing parameters. Minor variations in cooling rates, temperature gradients, or elemental distribution can lead to significant performance differences between batches. This inconsistency is particularly problematic for industrial applications that require reliable and predictable catalytic performance across production runs.
Environmental and safety concerns also emerge when scaling HEA production. Many synthesis methods involve high temperatures, potentially toxic precursors, or generate hazardous waste streams that must be properly managed. Developing greener synthesis routes that minimize environmental impact while maintaining photocatalytic efficiency remains an ongoing challenge for researchers and manufacturers alike.
Integration of HEA photocatalysts into existing industrial infrastructure presents technical hurdles related to reactor design and process compatibility. Most current industrial photocatalytic systems are optimized for traditional catalysts like TiO2, and may require significant redesign to accommodate the unique properties and operational requirements of HEA-based materials. This includes considerations for light penetration, mass transfer, and catalyst recovery systems.
Regulatory approval represents a final but critical barrier, particularly for applications in water treatment, environmental remediation, or consumer products. Novel materials like HEAs must undergo extensive testing to demonstrate both efficacy and safety before widespread deployment, adding time and cost to the commercialization pathway. Establishing standardized testing protocols specifically for HEA photocatalysts would help streamline this process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!