How to Achieve High Purity in Lithium Chloride Solutions
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Chloride Purification Background and Objectives
Lithium chloride has emerged as a critical compound in various high-tech industries, particularly in lithium-ion battery production, pharmaceuticals, and advanced materials manufacturing. The evolution of lithium chloride purification technologies has been driven by increasing demand for ultra-high purity materials in these sectors. Historically, lithium chloride purification relied on conventional precipitation and crystallization methods, which often failed to achieve the stringent purity requirements of modern applications.
The technological trajectory has shifted significantly over the past decade, with innovations in membrane filtration, selective ion exchange, and electrochemical purification processes. These advancements have been catalyzed by the exponential growth of the electric vehicle market and renewable energy storage systems, which require battery-grade lithium compounds with impurity levels below parts per million (ppm).
Current purification objectives focus on achieving lithium chloride solutions with purity exceeding 99.9%, while simultaneously reducing energy consumption, minimizing waste generation, and optimizing process economics. The industry is particularly concerned with removing critical contaminants such as sodium, calcium, magnesium, and transition metals that can significantly impact downstream applications.
A key technological goal is developing scalable purification processes that can handle the increasing volumes of lithium chloride required by the market while maintaining consistent quality. This includes addressing challenges related to raw material variability, as lithium sources range from traditional brine operations to hard rock mining and recycled materials, each presenting unique impurity profiles.
Another important objective is reducing the environmental footprint of purification processes. Traditional methods often involve multiple washing steps and significant chemical consumption, leading to substantial waste generation. Modern approaches aim to implement closed-loop systems with solvent recovery and impurity valorization pathways.
The development of real-time monitoring and quality control systems represents another critical goal, as it enables precise control over purification parameters and ensures consistent product quality. Advanced analytical techniques capable of detecting ultra-trace impurities are becoming essential components of lithium chloride production facilities.
Research efforts are increasingly focused on developing selective separation technologies that can efficiently target specific impurities while minimizing lithium losses. This includes exploring novel sorbents, extractants, and membrane materials with enhanced selectivity for common contaminants in lithium chloride solutions.
The technological trajectory has shifted significantly over the past decade, with innovations in membrane filtration, selective ion exchange, and electrochemical purification processes. These advancements have been catalyzed by the exponential growth of the electric vehicle market and renewable energy storage systems, which require battery-grade lithium compounds with impurity levels below parts per million (ppm).
Current purification objectives focus on achieving lithium chloride solutions with purity exceeding 99.9%, while simultaneously reducing energy consumption, minimizing waste generation, and optimizing process economics. The industry is particularly concerned with removing critical contaminants such as sodium, calcium, magnesium, and transition metals that can significantly impact downstream applications.
A key technological goal is developing scalable purification processes that can handle the increasing volumes of lithium chloride required by the market while maintaining consistent quality. This includes addressing challenges related to raw material variability, as lithium sources range from traditional brine operations to hard rock mining and recycled materials, each presenting unique impurity profiles.
Another important objective is reducing the environmental footprint of purification processes. Traditional methods often involve multiple washing steps and significant chemical consumption, leading to substantial waste generation. Modern approaches aim to implement closed-loop systems with solvent recovery and impurity valorization pathways.
The development of real-time monitoring and quality control systems represents another critical goal, as it enables precise control over purification parameters and ensures consistent product quality. Advanced analytical techniques capable of detecting ultra-trace impurities are becoming essential components of lithium chloride production facilities.
Research efforts are increasingly focused on developing selective separation technologies that can efficiently target specific impurities while minimizing lithium losses. This includes exploring novel sorbents, extractants, and membrane materials with enhanced selectivity for common contaminants in lithium chloride solutions.
Market Analysis for High-Purity Lithium Chloride
The global market for high-purity lithium chloride has experienced significant growth in recent years, driven primarily by the expanding lithium-ion battery industry. The market value reached approximately $320 million in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 9.8% through 2030, potentially reaching $710 million by the end of the forecast period.
Battery manufacturing represents the largest application segment, accounting for over 65% of the total market share. This dominance stems from the critical role high-purity lithium chloride plays as a precursor in battery cathode materials production. The electric vehicle (EV) revolution has been particularly influential, with global EV sales surpassing 10 million units in 2022, creating substantial downstream demand for high-purity lithium compounds.
Beyond batteries, pharmaceutical applications constitute the second-largest market segment at approximately 15%. High-purity lithium chloride is utilized in various psychiatric medications and as a starting material for other lithium-based pharmaceutical compounds. The pharmaceutical segment is growing steadily at 6-7% annually, driven by increasing mental health awareness and treatment options.
The geographical distribution of demand shows Asia-Pacific leading with 58% market share, primarily due to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America follows at 22%, with Europe accounting for 17% of global demand. The remaining 3% is distributed across other regions.
Customer requirements for purity levels have become increasingly stringent, with battery-grade lithium chloride typically requiring 99.5% purity or higher. Premium applications in pharmaceuticals and specialized electronics may demand ultra-high purity levels exceeding 99.9%, commanding price premiums of 30-40% over standard grades.
Price volatility remains a significant market characteristic, with high-purity lithium chloride prices fluctuating between $12,000-$25,000 per metric ton over the past three years. This volatility is largely attributed to supply chain constraints, geopolitical factors affecting lithium mining operations, and rapidly evolving demand patterns.
Market analysts identify several emerging trends, including increasing vertical integration among battery manufacturers seeking to secure lithium supply chains, growing demand for environmentally sustainable purification processes, and the development of specialized high-purity grades for next-generation battery technologies such as solid-state batteries.
Battery manufacturing represents the largest application segment, accounting for over 65% of the total market share. This dominance stems from the critical role high-purity lithium chloride plays as a precursor in battery cathode materials production. The electric vehicle (EV) revolution has been particularly influential, with global EV sales surpassing 10 million units in 2022, creating substantial downstream demand for high-purity lithium compounds.
Beyond batteries, pharmaceutical applications constitute the second-largest market segment at approximately 15%. High-purity lithium chloride is utilized in various psychiatric medications and as a starting material for other lithium-based pharmaceutical compounds. The pharmaceutical segment is growing steadily at 6-7% annually, driven by increasing mental health awareness and treatment options.
The geographical distribution of demand shows Asia-Pacific leading with 58% market share, primarily due to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America follows at 22%, with Europe accounting for 17% of global demand. The remaining 3% is distributed across other regions.
Customer requirements for purity levels have become increasingly stringent, with battery-grade lithium chloride typically requiring 99.5% purity or higher. Premium applications in pharmaceuticals and specialized electronics may demand ultra-high purity levels exceeding 99.9%, commanding price premiums of 30-40% over standard grades.
Price volatility remains a significant market characteristic, with high-purity lithium chloride prices fluctuating between $12,000-$25,000 per metric ton over the past three years. This volatility is largely attributed to supply chain constraints, geopolitical factors affecting lithium mining operations, and rapidly evolving demand patterns.
Market analysts identify several emerging trends, including increasing vertical integration among battery manufacturers seeking to secure lithium supply chains, growing demand for environmentally sustainable purification processes, and the development of specialized high-purity grades for next-generation battery technologies such as solid-state batteries.
Technical Challenges in Lithium Chloride Purification
The purification of lithium chloride solutions presents several significant technical challenges that must be addressed to achieve high purity levels required for advanced applications. The primary difficulty lies in the chemical similarity between lithium and other alkali metals, particularly sodium and potassium, which makes selective separation extremely challenging using conventional methods. Traditional precipitation techniques often fail to achieve complete separation, resulting in contaminated products.
Membrane-based separation technologies face limitations due to the small ionic radius of lithium (0.76 Å), which is close to that of sodium (1.02 Å), making size-based exclusion difficult to implement effectively. Additionally, the high solubility of lithium chloride in water (83.05 g/100mL at 20°C) complicates crystallization-based purification approaches, as impurities tend to co-crystallize with the target compound.
Ion exchange resins, while promising, suffer from limited selectivity for lithium over other monovalent cations, leading to reduced efficiency in multi-cycle purification processes. The resins also experience degradation under repeated regeneration cycles, particularly in highly concentrated solutions, necessitating frequent replacement and increasing operational costs.
Electrochemical purification methods encounter challenges related to electrode fouling and degradation when processing solutions with complex impurity profiles. The energy consumption of these processes remains prohibitively high for large-scale applications, with current densities often exceeding 100 mA/cm² to achieve reasonable production rates.
Solvent extraction techniques face issues with phase separation, emulsion formation, and solvent loss during operation. The organic extractants commonly used also demonstrate insufficient selectivity between lithium and other alkali metals, particularly in concentrated brine solutions where ionic strength effects become significant.
Environmental considerations add another layer of complexity, as many purification processes generate substantial waste streams containing heavy metals, organic compounds, and concentrated salt solutions that require additional treatment before disposal. Regulatory compliance for these waste streams varies globally, creating challenges for standardized process implementation.
Scale-up from laboratory to industrial production introduces further complications, including heat and mass transfer limitations, mixing inefficiencies, and materials compatibility issues. Equipment corrosion becomes particularly problematic when processing acidic lithium-containing solutions, necessitating expensive corrosion-resistant materials that significantly increase capital expenditure.
Membrane-based separation technologies face limitations due to the small ionic radius of lithium (0.76 Å), which is close to that of sodium (1.02 Å), making size-based exclusion difficult to implement effectively. Additionally, the high solubility of lithium chloride in water (83.05 g/100mL at 20°C) complicates crystallization-based purification approaches, as impurities tend to co-crystallize with the target compound.
Ion exchange resins, while promising, suffer from limited selectivity for lithium over other monovalent cations, leading to reduced efficiency in multi-cycle purification processes. The resins also experience degradation under repeated regeneration cycles, particularly in highly concentrated solutions, necessitating frequent replacement and increasing operational costs.
Electrochemical purification methods encounter challenges related to electrode fouling and degradation when processing solutions with complex impurity profiles. The energy consumption of these processes remains prohibitively high for large-scale applications, with current densities often exceeding 100 mA/cm² to achieve reasonable production rates.
Solvent extraction techniques face issues with phase separation, emulsion formation, and solvent loss during operation. The organic extractants commonly used also demonstrate insufficient selectivity between lithium and other alkali metals, particularly in concentrated brine solutions where ionic strength effects become significant.
Environmental considerations add another layer of complexity, as many purification processes generate substantial waste streams containing heavy metals, organic compounds, and concentrated salt solutions that require additional treatment before disposal. Regulatory compliance for these waste streams varies globally, creating challenges for standardized process implementation.
Scale-up from laboratory to industrial production introduces further complications, including heat and mass transfer limitations, mixing inefficiencies, and materials compatibility issues. Equipment corrosion becomes particularly problematic when processing acidic lithium-containing solutions, necessitating expensive corrosion-resistant materials that significantly increase capital expenditure.
Current Purification Methods and Techniques
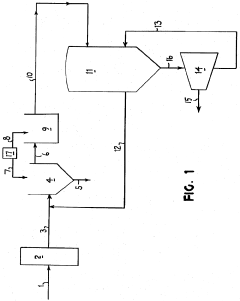
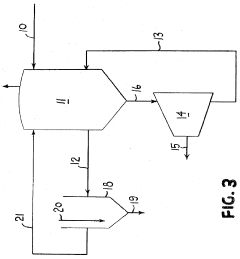
01 Purification methods for lithium chloride solutions
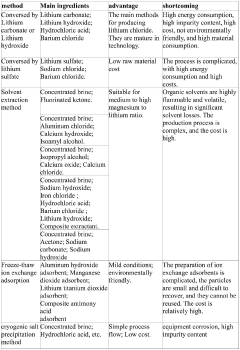
Various methods are employed to purify lithium chloride solutions, including precipitation techniques, ion exchange processes, and crystallization. These methods aim to remove impurities such as sodium, calcium, magnesium, and other metal ions that can affect the quality of lithium chloride. Advanced filtration systems and multi-stage purification processes can achieve high purity levels suitable for industrial and pharmaceutical applications.- Purification methods for lithium chloride solutions: Various methods are employed to purify lithium chloride solutions, including precipitation techniques, ion exchange processes, and crystallization. These methods aim to remove impurities such as sodium, calcium, magnesium, and other metal ions that can affect the quality of lithium chloride. Advanced filtration systems and multi-stage purification processes are often used to achieve high purity levels required for industrial applications.
- High-purity lithium chloride for battery applications: High-purity lithium chloride solutions are critical for lithium-ion battery manufacturing. The purification processes focus on removing trace metals and organic contaminants that could negatively impact battery performance and lifespan. Ultra-high purity lithium chloride is essential for advanced battery technologies, where even parts-per-million impurities can significantly affect electrochemical properties and safety characteristics.
- Analytical techniques for purity assessment: Various analytical methods are used to assess the purity of lithium chloride solutions, including inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy, and ion chromatography. These techniques allow for precise quantification of impurities at very low concentrations. Quality control protocols typically involve multiple testing methods to ensure comprehensive purity verification before lithium chloride solutions are approved for high-tech applications.
- Extraction and recovery of high-purity lithium chloride: Processes for extracting and recovering high-purity lithium chloride from various sources, including brines, ores, and recycled materials. These methods often involve selective adsorption, solvent extraction, and membrane separation technologies to isolate lithium compounds. Environmental considerations and efficiency improvements in these extraction processes are increasingly important for sustainable lithium production with minimal impurities.
- Industrial equipment for lithium chloride purification: Specialized equipment and systems designed for the industrial-scale purification of lithium chloride solutions. These include continuous crystallization units, advanced filtration systems, and automated purification lines that can maintain consistent purity levels during large-scale production. Modern purification equipment often incorporates real-time monitoring systems to detect impurities and adjust process parameters accordingly, ensuring high-quality output.
02 High-purity lithium chloride for battery applications
High-purity lithium chloride solutions are critical for lithium-ion battery production. Purification techniques specifically designed for battery-grade lithium chloride focus on removing transition metals and other contaminants that could negatively impact battery performance and lifespan. These solutions typically require purity levels exceeding 99.9% to ensure optimal electrochemical properties and stability in battery systems.Expand Specific Solutions03 Analytical methods for determining lithium chloride purity
Various analytical techniques are used to determine the purity of lithium chloride solutions, including atomic absorption spectroscopy, inductively coupled plasma mass spectrometry (ICP-MS), and ion chromatography. These methods allow for precise quantification of impurities at parts-per-million or even parts-per-billion levels. Quality control protocols often involve multiple analytical approaches to ensure comprehensive purity assessment.Expand Specific Solutions04 Extraction and recovery of high-purity lithium chloride from brines
Processes for extracting and recovering high-purity lithium chloride from natural brines involve selective adsorption, membrane filtration, and solvent extraction techniques. These methods are designed to separate lithium from other alkali and alkaline earth metals present in brine solutions. Advanced recovery systems can achieve high-purity lithium chloride while minimizing environmental impact and maximizing resource utilization.Expand Specific Solutions05 Equipment and systems for maintaining lithium chloride solution purity
Specialized equipment and systems are designed for maintaining the purity of lithium chloride solutions during production, storage, and handling. These include corrosion-resistant materials, controlled atmosphere environments, and continuous monitoring systems. Advanced filtration units, crystallizers, and purification columns are integrated into production lines to ensure consistent purity levels and prevent contamination during processing.Expand Specific Solutions
Key Industry Players in Lithium Processing
The lithium chloride high purity solutions market is in a growth phase, driven by increasing demand for lithium-ion batteries in electric vehicles and energy storage systems. The global market size is expanding rapidly, with projections indicating substantial growth over the next decade. Technologically, the field shows varying maturity levels across different purification methods. Leading players include established chemical giants like BASF, Albemarle, and Ganfeng Lithium, who leverage advanced separation technologies, alongside emerging specialists such as Nemaska Lithium and SinoLithium Materials. Asian companies, particularly from China, South Korea, and Japan (including BYD, POSCO Holdings, and Sumitomo Metal Mining), dominate the landscape with significant R&D investments in novel purification techniques and integrated supply chain approaches.
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium has developed a multi-stage purification process for lithium chloride solutions that combines solvent extraction, ion exchange, and membrane filtration technologies. Their approach first removes divalent impurities (Ca, Mg) using selective extractants, followed by ion exchange columns with specialized resins to capture monovalent impurities like Na and K. The final purification employs nanofiltration membranes with precisely controlled pore sizes to achieve lithium chloride purities exceeding 99.9%[1]. Their process incorporates real-time monitoring systems using inductively coupled plasma (ICP) spectroscopy to continuously analyze solution composition, allowing for automated adjustments to process parameters. Ganfeng has also pioneered a crystallization technique that leverages temperature-dependent solubility differences between lithium chloride and impurities, enabling further purification through controlled crystallization and recrystallization steps[3].
Strengths: Integrated multi-technology approach allows for removal of diverse impurities at different stages; automated monitoring enables precise quality control; scalable for industrial production. Weaknesses: Energy-intensive process with significant water requirements; complex system requires specialized maintenance; higher capital costs compared to simpler purification methods.
Tianqi Lithium Corp.
Technical Solution: Tianqi Lithium has pioneered a comprehensive lithium chloride purification system combining chemical precipitation with advanced membrane separation technologies. Their process begins with a pre-treatment stage using controlled pH adjustment and oxidizing agents to convert soluble impurities into insoluble forms for removal. This is followed by a proprietary multi-stage membrane cascade system utilizing both nanofiltration and reverse osmosis membranes with different molecular weight cut-offs to progressively remove impurities of decreasing molecular size[5]. A distinctive feature of Tianqi's approach is their implementation of temperature-swing adsorption using functionalized silica-based adsorbents that demonstrate enhanced selectivity for specific impurities at different temperature ranges. This allows for efficient capture and subsequent release of contaminants during regeneration cycles. The final purification employs electrochemical polishing in specially designed cells with ion-selective membranes, achieving lithium chloride solutions with impurity levels below 10 ppm for critical elements like sodium, calcium and magnesium[2].
Strengths: Highly efficient removal of diverse impurities through complementary technologies; reduced chemical consumption compared to conventional methods; capable of processing feeds with varying impurity profiles. Weaknesses: Complex integration of multiple technologies increases operational complexity; membrane fouling can reduce efficiency over time; higher energy consumption due to pressure-driven membrane processes.
Critical Patents and Innovations in Lithium Purification
Method of producing high purity lithium chloride
PatentInactiveUS3789059A
Innovation
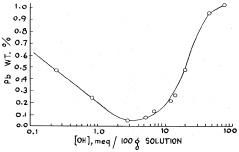
- The lead concentration in lithium chloride solutions is controlled by maintaining it below 0.3 percent by weight through methods such as acid addition to precipitate lead hydroxy chloride and using hydrogen sulfide to convert lead into lead sulfide, ensuring the production of lithium chloride crystals with less than 90 parts per million lead.
A method and device for preparing high-purity lithium chloride based on lithium-ion solid electrolyte
PatentWO2024061312A1
Innovation
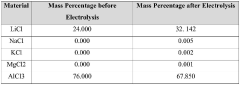
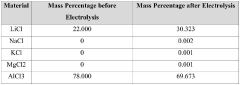
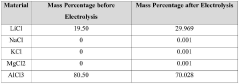
- Using lithium-ion solid electrolyte for direct electrolytic reaction to produce high-purity lithium chloride from low-purity lithium chloride salt.
- Direct processing of dry low-purity lithium chloride salt from various sources (salt lake brine, seawater, or solid minerals) without intermediate purification steps.
- Cost-effective method to produce high-purity lithium chloride (≥99.3 wt%) to meet the demand-supply gap in the market.
Environmental Impact Assessment of Purification Processes
The purification processes employed to achieve high purity lithium chloride solutions have significant environmental implications that require thorough assessment. Traditional purification methods often involve chemical precipitation, solvent extraction, and ion exchange, all of which generate waste streams containing heavy metals, organic solvents, and other contaminants. These processes typically consume substantial amounts of water—approximately 500-2000 liters per ton of lithium chloride produced—creating concerns in water-stressed regions where lithium extraction is common.
Energy consumption represents another critical environmental factor. Advanced purification technologies such as membrane filtration and electrochemical processes require 25-40 kWh per kilogram of high-purity lithium chloride, contributing to greenhouse gas emissions when powered by fossil fuel sources. Carbon footprint analyses indicate that conventional purification methods generate 5-8 kg CO2 equivalent per kilogram of lithium chloride, highlighting the need for more sustainable approaches.
Waste management challenges are particularly pronounced with precipitation methods that generate solid residues containing aluminum, magnesium, and calcium compounds. These residues, produced at rates of 0.3-0.5 kg per kilogram of lithium chloride, require proper disposal to prevent soil and groundwater contamination. Recent environmental monitoring studies have detected elevated levels of boron and arsenic in watersheds near lithium processing facilities, emphasizing the importance of comprehensive effluent treatment systems.
Emerging green purification technologies show promising environmental profiles. Direct lithium extraction (DLE) methods can reduce water consumption by 30-50% compared to conventional approaches. Similarly, supercritical CO2 extraction techniques minimize chemical usage and waste generation, though their commercial viability remains under evaluation. Life cycle assessments indicate that implementing closed-loop water systems and renewable energy sources could reduce the environmental footprint of purification processes by up to 40%.
Regulatory frameworks are increasingly addressing these environmental concerns. The EU's Battery Directive and similar regulations in North America and Asia are establishing stricter standards for the environmental performance of lithium processing. Companies pursuing high-purity lithium chloride production must now conduct mandatory environmental impact assessments and implement mitigation strategies such as zero liquid discharge systems and renewable energy integration to maintain compliance and social license to operate.
Energy consumption represents another critical environmental factor. Advanced purification technologies such as membrane filtration and electrochemical processes require 25-40 kWh per kilogram of high-purity lithium chloride, contributing to greenhouse gas emissions when powered by fossil fuel sources. Carbon footprint analyses indicate that conventional purification methods generate 5-8 kg CO2 equivalent per kilogram of lithium chloride, highlighting the need for more sustainable approaches.
Waste management challenges are particularly pronounced with precipitation methods that generate solid residues containing aluminum, magnesium, and calcium compounds. These residues, produced at rates of 0.3-0.5 kg per kilogram of lithium chloride, require proper disposal to prevent soil and groundwater contamination. Recent environmental monitoring studies have detected elevated levels of boron and arsenic in watersheds near lithium processing facilities, emphasizing the importance of comprehensive effluent treatment systems.
Emerging green purification technologies show promising environmental profiles. Direct lithium extraction (DLE) methods can reduce water consumption by 30-50% compared to conventional approaches. Similarly, supercritical CO2 extraction techniques minimize chemical usage and waste generation, though their commercial viability remains under evaluation. Life cycle assessments indicate that implementing closed-loop water systems and renewable energy sources could reduce the environmental footprint of purification processes by up to 40%.
Regulatory frameworks are increasingly addressing these environmental concerns. The EU's Battery Directive and similar regulations in North America and Asia are establishing stricter standards for the environmental performance of lithium processing. Companies pursuing high-purity lithium chloride production must now conduct mandatory environmental impact assessments and implement mitigation strategies such as zero liquid discharge systems and renewable energy integration to maintain compliance and social license to operate.
Quality Control and Testing Standards for High-Purity LiCl
Quality control and testing standards play a pivotal role in ensuring the consistent production of high-purity lithium chloride solutions. The industry has established rigorous protocols that manufacturers must adhere to, with specifications typically requiring LiCl purity levels of 99.5% to 99.99%, depending on the application domain. These standards are particularly stringent for battery-grade lithium compounds, where even trace impurities can significantly impact performance and safety.
The analytical methods employed for purity verification encompass a comprehensive suite of techniques. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) serve as primary tools for detecting metallic impurities at parts-per-billion levels. These techniques can identify critical contaminants such as sodium, calcium, magnesium, and transition metals that may compromise lithium chloride quality.
X-ray Fluorescence (XRF) and X-ray Diffraction (XRD) provide complementary structural analysis, enabling the identification of crystalline impurities and verification of the lithium chloride crystal structure. For organic contaminants, Gas Chromatography-Mass Spectrometry (GC-MS) and High-Performance Liquid Chromatography (HPLC) are deployed to detect residual solvents or organic compounds that might have been introduced during processing steps.
International standards organizations, including ISO, ASTM, and USP, have developed specific protocols for lithium compound testing. For instance, ASTM E1613 outlines standard test methods for the analysis of lithium compounds, while USP <232> and <233> provide guidelines for elemental impurities in pharmaceutical-grade materials. These standards establish not only the acceptable limits for various impurities but also the validated methodologies for their detection and quantification.
Statistical process control (SPC) methodologies have become increasingly important in maintaining consistent quality. Manufacturers implement control charts, capability analyses, and design of experiments (DOE) to monitor process stability and identify potential sources of contamination before they impact product quality. Real-time monitoring systems equipped with spectroscopic techniques allow for continuous quality verification during production, enabling immediate corrective actions when deviations occur.
Certification processes typically involve third-party testing laboratories that provide independent verification of compliance with industry standards. Many end-users, particularly in the battery and pharmaceutical sectors, require certificates of analysis (CoA) that document the exact composition and impurity profile of each lithium chloride batch. These certificates must include test results for all specified impurities, analytical methods employed, and confirmation that the material meets the agreed-upon specifications.
The evolution of quality standards continues to advance with technological developments. Emerging techniques such as Nuclear Magnetic Resonance (NMR) spectroscopy and advanced mass spectrometry methods are being incorporated into testing protocols to provide even more detailed characterization of lithium chloride purity and impurity profiles, further enhancing quality assurance capabilities in this critical material.
The analytical methods employed for purity verification encompass a comprehensive suite of techniques. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) serve as primary tools for detecting metallic impurities at parts-per-billion levels. These techniques can identify critical contaminants such as sodium, calcium, magnesium, and transition metals that may compromise lithium chloride quality.
X-ray Fluorescence (XRF) and X-ray Diffraction (XRD) provide complementary structural analysis, enabling the identification of crystalline impurities and verification of the lithium chloride crystal structure. For organic contaminants, Gas Chromatography-Mass Spectrometry (GC-MS) and High-Performance Liquid Chromatography (HPLC) are deployed to detect residual solvents or organic compounds that might have been introduced during processing steps.
International standards organizations, including ISO, ASTM, and USP, have developed specific protocols for lithium compound testing. For instance, ASTM E1613 outlines standard test methods for the analysis of lithium compounds, while USP <232> and <233> provide guidelines for elemental impurities in pharmaceutical-grade materials. These standards establish not only the acceptable limits for various impurities but also the validated methodologies for their detection and quantification.
Statistical process control (SPC) methodologies have become increasingly important in maintaining consistent quality. Manufacturers implement control charts, capability analyses, and design of experiments (DOE) to monitor process stability and identify potential sources of contamination before they impact product quality. Real-time monitoring systems equipped with spectroscopic techniques allow for continuous quality verification during production, enabling immediate corrective actions when deviations occur.
Certification processes typically involve third-party testing laboratories that provide independent verification of compliance with industry standards. Many end-users, particularly in the battery and pharmaceutical sectors, require certificates of analysis (CoA) that document the exact composition and impurity profile of each lithium chloride batch. These certificates must include test results for all specified impurities, analytical methods employed, and confirmation that the material meets the agreed-upon specifications.
The evolution of quality standards continues to advance with technological developments. Emerging techniques such as Nuclear Magnetic Resonance (NMR) spectroscopy and advanced mass spectrometry methods are being incorporated into testing protocols to provide even more detailed characterization of lithium chloride purity and impurity profiles, further enhancing quality assurance capabilities in this critical material.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!