How to Conduct Hydrofluoric Acid Reactivity Experiments Safely
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrofluoric Acid Safety Background and Objectives
Hydrofluoric acid (HF) represents one of the most hazardous chemicals encountered in laboratory and industrial settings, distinguished by its unique dual threat of corrosivity and systemic toxicity. Since its first isolation in the late 18th century, HF has evolved from a scientific curiosity to an essential industrial reagent, with applications spanning semiconductor manufacturing, glass etching, petroleum refining, and various chemical synthesis processes. The historical development of HF handling protocols has been marked by tragic incidents that have progressively informed safety standards.
The evolution of HF safety practices has followed a reactive trajectory, with major advancements often occurring in response to serious accidents. Notable incidents include the 1987 Marathon Oil refinery release in Texas and the 2012 Gumi, South Korea industrial disaster, which collectively resulted in multiple fatalities and thousands of injuries. These events catalyzed significant improvements in containment systems, personal protective equipment specifications, and emergency response protocols.
Current global usage of HF exceeds 1.5 million metric tons annually, with demand projected to increase at approximately 3-5% per year, driven primarily by growth in the electronics and petrochemical sectors. This expanding utilization underscores the critical importance of establishing standardized safety frameworks for HF reactivity experiments across research, educational, and industrial environments.
The primary objective of this technical research is to develop comprehensive, evidence-based protocols for conducting hydrofluoric acid reactivity experiments with maximum safety assurance. This entails identifying best practices for experimental design, engineering controls, personal protective measures, and emergency response procedures specifically tailored to HF's unique hazard profile.
Secondary objectives include quantifying the effectiveness of various neutralization agents and techniques, evaluating containment system designs, assessing detection technologies, and establishing exposure thresholds that incorporate the latest toxicological data. The research aims to bridge existing knowledge gaps regarding HF's reactivity with various substrates under different experimental conditions.
The technological landscape for HF safety has advanced considerably in recent years, with innovations in real-time monitoring systems, specialized containment materials, and medical countermeasures. However, standardization remains inconsistent across different sectors and geographical regions, creating opportunities for significant improvement through systematic research and protocol development.
This research initiative aligns with broader industry trends toward inherently safer design principles and the substitution of hazardous materials where feasible. When substitution is not possible, as is often the case with HF's unique properties, the focus shifts to developing robust engineering controls and administrative safeguards that minimize risk while enabling necessary scientific and industrial processes to continue.
The evolution of HF safety practices has followed a reactive trajectory, with major advancements often occurring in response to serious accidents. Notable incidents include the 1987 Marathon Oil refinery release in Texas and the 2012 Gumi, South Korea industrial disaster, which collectively resulted in multiple fatalities and thousands of injuries. These events catalyzed significant improvements in containment systems, personal protective equipment specifications, and emergency response protocols.
Current global usage of HF exceeds 1.5 million metric tons annually, with demand projected to increase at approximately 3-5% per year, driven primarily by growth in the electronics and petrochemical sectors. This expanding utilization underscores the critical importance of establishing standardized safety frameworks for HF reactivity experiments across research, educational, and industrial environments.
The primary objective of this technical research is to develop comprehensive, evidence-based protocols for conducting hydrofluoric acid reactivity experiments with maximum safety assurance. This entails identifying best practices for experimental design, engineering controls, personal protective measures, and emergency response procedures specifically tailored to HF's unique hazard profile.
Secondary objectives include quantifying the effectiveness of various neutralization agents and techniques, evaluating containment system designs, assessing detection technologies, and establishing exposure thresholds that incorporate the latest toxicological data. The research aims to bridge existing knowledge gaps regarding HF's reactivity with various substrates under different experimental conditions.
The technological landscape for HF safety has advanced considerably in recent years, with innovations in real-time monitoring systems, specialized containment materials, and medical countermeasures. However, standardization remains inconsistent across different sectors and geographical regions, creating opportunities for significant improvement through systematic research and protocol development.
This research initiative aligns with broader industry trends toward inherently safer design principles and the substitution of hazardous materials where feasible. When substitution is not possible, as is often the case with HF's unique properties, the focus shifts to developing robust engineering controls and administrative safeguards that minimize risk while enabling necessary scientific and industrial processes to continue.
Industrial Applications and Demand Analysis
Hydrofluoric acid (HF) plays a critical role across multiple industries due to its unique chemical properties, driving significant market demand for safe experimental protocols. The semiconductor industry represents one of the largest consumers of HF, utilizing it extensively in wafer cleaning, etching, and surface preparation processes. As semiconductor devices continue to shrink in size while increasing in complexity, the demand for precise HF reactivity experiments has grown substantially, with the global semiconductor manufacturing equipment market projected to reach $103.5 billion by 2025.
The petroleum refining sector constitutes another major application area, where HF serves as a catalyst in alkylation processes to produce high-octane gasoline components. With increasing global energy demands and stricter fuel quality regulations, refineries require ongoing reactivity experiments to optimize processes and enhance safety protocols. This demand is particularly evident in regions with expanding refining capacity such as Asia-Pacific and the Middle East.
Glass etching and manufacturing represents a traditional yet still significant market for HF applications. The growing demand for specialty glass products in electronics, automotive, and construction industries necessitates continuous improvement in HF handling techniques. The global specialty glass market, valued at $53.8 billion in 2022, continues to drive demand for safe HF reactivity testing.
The pharmaceutical and chemical synthesis sectors utilize HF as a reagent in various organic transformations and as a catalyst in numerous chemical processes. As these industries pursue more complex molecules and greener chemistry approaches, understanding HF reactivity under controlled conditions becomes increasingly important for process development and scale-up operations.
Environmental considerations are significantly influencing market dynamics, with regulatory bodies worldwide implementing stricter guidelines for HF handling and disposal. This regulatory landscape has created a substantial demand for improved safety protocols and containment technologies, particularly in densely populated regions and environmentally sensitive areas.
The medical research field represents an emerging application area, where controlled HF reactivity experiments support the development of biocompatible materials, dental products, and specialized medical devices. This sector's growth is driven by aging populations in developed economies and expanding healthcare infrastructure in developing regions.
Market analysis indicates that companies investing in advanced safety technologies for HF handling gain significant competitive advantages through reduced insurance costs, fewer workplace incidents, and enhanced regulatory compliance. This economic incentive, coupled with corporate sustainability commitments, continues to drive investment in safer experimental methodologies across all industrial sectors utilizing hydrofluoric acid.
The petroleum refining sector constitutes another major application area, where HF serves as a catalyst in alkylation processes to produce high-octane gasoline components. With increasing global energy demands and stricter fuel quality regulations, refineries require ongoing reactivity experiments to optimize processes and enhance safety protocols. This demand is particularly evident in regions with expanding refining capacity such as Asia-Pacific and the Middle East.
Glass etching and manufacturing represents a traditional yet still significant market for HF applications. The growing demand for specialty glass products in electronics, automotive, and construction industries necessitates continuous improvement in HF handling techniques. The global specialty glass market, valued at $53.8 billion in 2022, continues to drive demand for safe HF reactivity testing.
The pharmaceutical and chemical synthesis sectors utilize HF as a reagent in various organic transformations and as a catalyst in numerous chemical processes. As these industries pursue more complex molecules and greener chemistry approaches, understanding HF reactivity under controlled conditions becomes increasingly important for process development and scale-up operations.
Environmental considerations are significantly influencing market dynamics, with regulatory bodies worldwide implementing stricter guidelines for HF handling and disposal. This regulatory landscape has created a substantial demand for improved safety protocols and containment technologies, particularly in densely populated regions and environmentally sensitive areas.
The medical research field represents an emerging application area, where controlled HF reactivity experiments support the development of biocompatible materials, dental products, and specialized medical devices. This sector's growth is driven by aging populations in developed economies and expanding healthcare infrastructure in developing regions.
Market analysis indicates that companies investing in advanced safety technologies for HF handling gain significant competitive advantages through reduced insurance costs, fewer workplace incidents, and enhanced regulatory compliance. This economic incentive, coupled with corporate sustainability commitments, continues to drive investment in safer experimental methodologies across all industrial sectors utilizing hydrofluoric acid.
Current Safety Protocols and Technical Challenges
Current safety protocols for hydrofluoric acid (HF) reactivity experiments vary significantly across industries and research institutions, creating inconsistencies in implementation and effectiveness. Standard protocols typically include comprehensive personal protective equipment (PPE) requirements such as chemical-resistant suits, face shields, specialized gloves (often nitrile under neoprene), and respiratory protection depending on concentration levels and experiment scale. Most facilities mandate the presence of calcium gluconate gel within immediate reach as a first-aid countermeasure for HF exposure.
Engineering controls represent another critical safety layer, with properly functioning fume hoods being non-negotiable for any HF work. These systems must maintain face velocities of 80-120 feet per minute and undergo regular certification. Closed systems and remote handling apparatus are increasingly becoming standard for higher-concentration HF applications, though implementation remains inconsistent across smaller laboratories.
Administrative controls present significant challenges, with training programs varying dramatically in quality and comprehensiveness. While larger organizations typically maintain robust HF-specific training modules, smaller research facilities often rely on general chemical safety training that inadequately addresses HF's unique hazards. Standard operating procedures (SOPs) for HF experiments frequently lack sufficient detail regarding emergency response scenarios.
Technical challenges in HF safety primarily stem from its unique chemical properties. Unlike other acids, HF's delayed symptom onset complicates immediate hazard recognition, potentially delaying critical treatment. The acid's exceptional ability to penetrate standard laboratory materials creates containment difficulties, with many conventional containers experiencing degradation over time, leading to potential leaks or failures during experiments.
Detection represents another significant technical hurdle. While electronic HF monitors exist, their reliability in diverse laboratory environments remains questionable, with false positives and calibration drift being common issues. The technology for real-time personal HF exposure monitoring remains underdeveloped compared to other hazardous substances.
Waste management protocols for HF experiments face considerable challenges. Neutralization procedures must be carefully controlled to prevent dangerous heat generation and potential splashing. Many facilities lack specialized waste streams for HF-containing materials, resulting in improper disposal practices that create environmental and safety risks.
Emergency response capabilities vary dramatically across institutions. While some maintain dedicated HF response teams with specialized training and equipment, many rely on general emergency services that may lack HF-specific expertise. The availability of calcium gluconate in sufficient quantities for serious exposure incidents remains inconsistent, with some facilities maintaining inadequate supplies for worst-case scenarios.
Engineering controls represent another critical safety layer, with properly functioning fume hoods being non-negotiable for any HF work. These systems must maintain face velocities of 80-120 feet per minute and undergo regular certification. Closed systems and remote handling apparatus are increasingly becoming standard for higher-concentration HF applications, though implementation remains inconsistent across smaller laboratories.
Administrative controls present significant challenges, with training programs varying dramatically in quality and comprehensiveness. While larger organizations typically maintain robust HF-specific training modules, smaller research facilities often rely on general chemical safety training that inadequately addresses HF's unique hazards. Standard operating procedures (SOPs) for HF experiments frequently lack sufficient detail regarding emergency response scenarios.
Technical challenges in HF safety primarily stem from its unique chemical properties. Unlike other acids, HF's delayed symptom onset complicates immediate hazard recognition, potentially delaying critical treatment. The acid's exceptional ability to penetrate standard laboratory materials creates containment difficulties, with many conventional containers experiencing degradation over time, leading to potential leaks or failures during experiments.
Detection represents another significant technical hurdle. While electronic HF monitors exist, their reliability in diverse laboratory environments remains questionable, with false positives and calibration drift being common issues. The technology for real-time personal HF exposure monitoring remains underdeveloped compared to other hazardous substances.
Waste management protocols for HF experiments face considerable challenges. Neutralization procedures must be carefully controlled to prevent dangerous heat generation and potential splashing. Many facilities lack specialized waste streams for HF-containing materials, resulting in improper disposal practices that create environmental and safety risks.
Emergency response capabilities vary dramatically across institutions. While some maintain dedicated HF response teams with specialized training and equipment, many rely on general emergency services that may lack HF-specific expertise. The availability of calcium gluconate in sufficient quantities for serious exposure incidents remains inconsistent, with some facilities maintaining inadequate supplies for worst-case scenarios.
Standard Operating Procedures for HF Reactivity Testing
01 Safety protocols for handling hydrofluoric acid
Hydrofluoric acid requires strict safety protocols due to its highly corrosive and toxic nature. These protocols include the use of appropriate personal protective equipment (PPE) such as chemical-resistant gloves, face shields, and acid-resistant clothing. Proper ventilation systems must be in place to prevent inhalation of toxic fumes. Emergency response procedures, including access to calcium gluconate for treating HF burns, should be established before conducting any experiments with hydrofluoric acid.- Protective equipment and handling procedures: When conducting experiments with hydrofluoric acid, proper protective equipment and handling procedures are essential for safety. This includes using specialized gloves, face shields, and acid-resistant clothing. Proper ventilation systems must be in place to prevent inhalation of toxic fumes. Standard operating procedures should include guidelines for safe handling, storage, and disposal of hydrofluoric acid to minimize exposure risks during reactivity experiments.
- Neutralization and emergency response protocols: Effective neutralization methods and emergency response protocols are critical when working with hydrofluoric acid. Calcium gluconate gel should be readily available as a first-aid treatment for skin exposure. Specialized neutralizing agents and spill kits designed specifically for hydrofluoric acid should be accessible in the laboratory. Emergency shower and eyewash stations must be functional and located within quick reach of experiment areas.
- Containment systems and reaction vessel design: Specialized containment systems and reaction vessel designs are necessary for safely conducting hydrofluoric acid experiments. These include corrosion-resistant materials such as specific grades of polyethylene, PTFE, or certain alloys that can withstand hydrofluoric acid's aggressive nature. Double containment systems should be employed to prevent leaks, and reaction vessels should incorporate pressure relief mechanisms to prevent dangerous buildup during exothermic reactions.
- Monitoring and detection systems: Advanced monitoring and detection systems are essential for maintaining safety during hydrofluoric acid experiments. These include HF-specific gas detectors, pH monitoring systems, and temperature controls to prevent runaway reactions. Real-time monitoring equipment should be calibrated regularly and connected to alarm systems that alert researchers to dangerous conditions before they become critical. Automated shutdown systems can be integrated to halt reactions if safety parameters are exceeded.
- Alternative reagents and reaction pathways: Research into alternative reagents and reaction pathways can reduce the hazards associated with hydrofluoric acid experiments. This includes developing less hazardous fluorinating agents, using ionic liquids as safer reaction media, or employing flow chemistry techniques to minimize the quantity of hydrofluoric acid present at any given time. Modified synthetic routes that achieve similar chemical transformations without requiring hydrofluoric acid can significantly improve laboratory safety.
02 Neutralization and containment methods
Effective neutralization and containment methods are essential when working with hydrofluoric acid. These include using appropriate neutralizing agents such as calcium carbonate or sodium bicarbonate to safely neutralize spills. Specialized containment systems with acid-resistant materials prevent leakage and environmental contamination. Double containment strategies are recommended for storage and transport of hydrofluoric acid to minimize risks in case of primary containment failure.Expand Specific Solutions03 Specialized equipment for hydrofluoric acid experiments
Conducting experiments with hydrofluoric acid requires specialized equipment designed to withstand its corrosive properties. This includes PTFE (polytetrafluoroethylene) or other fluoropolymer-lined vessels and tools, specialized glass etching equipment with proper barriers, and automated handling systems to minimize direct human contact. Temperature-controlled reaction vessels are important for maintaining safe reaction conditions, as many reactions involving hydrofluoric acid are exothermic.Expand Specific Solutions04 Waste management and disposal procedures
Proper waste management and disposal procedures are critical when working with hydrofluoric acid. This includes neutralization before disposal, specialized containment for waste products, and compliance with local regulations for hazardous waste disposal. Treatment methods may involve precipitation of fluoride ions as calcium fluoride or other insoluble fluoride salts. Documentation and tracking of all waste disposal activities should be maintained for regulatory compliance and safety auditing.Expand Specific Solutions05 Reaction monitoring and control systems
Advanced monitoring and control systems are essential for safely conducting hydrofluoric acid reactivity experiments. These include real-time pH and temperature monitoring equipment, automated emergency shutdown systems that activate when parameters exceed safe limits, and remote operation capabilities to minimize exposure risks. Continuous monitoring of hydrogen fluoride gas levels in the laboratory atmosphere is also crucial, with alarm systems that alert personnel when concentrations approach dangerous levels.Expand Specific Solutions
Leading Organizations in HF Safety Research
The hydrofluoric acid reactivity experiments safety landscape is currently in a mature development stage, with established protocols being continuously refined as technology advances. The market for safety equipment and procedures is estimated at $2.5 billion globally, driven by stringent regulations and increasing industrial applications. Leading companies demonstrate varying levels of technical maturity in this field: Honeywell International and DuPont de Nemours have established comprehensive safety systems with advanced containment technologies, while Arkema France and AGC Inc. focus on specialized handling equipment. Corning and Abbott Laboratories contribute through innovative materials science approaches for safer containment vessels. Schneider Electric Systems provides automation solutions that minimize human exposure during experiments, representing the industry's shift toward integrated safety systems combining material science, automation, and monitoring technologies.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed an integrated safety system for hydrofluoric acid handling that combines advanced engineering controls with digital monitoring technologies. Their approach centers on a three-tier containment strategy with primary reaction vessels constructed of specialized HF-resistant materials, secondary containment systems with automated neutralization capabilities, and tertiary environmental controls that manage workspace air quality. The system incorporates Honeywell's proprietary Safety Manager technology that provides real-time monitoring of multiple parameters including temperature, pressure, pH, and HF vapor concentrations. Their digital platform integrates with automated emergency response systems that can initiate containment protocols without human intervention when unsafe conditions are detected. Honeywell's system also includes specialized personal protective equipment with integrated sensors that alert wearers to exposure risks.
Strengths: Comprehensive integration of digital monitoring with physical safety systems; extensive automation reduces human error potential; scalable solutions for different facility sizes. Weaknesses: Complex digital systems require specialized IT support; high dependence on sensor reliability; significant initial implementation costs.
Corning, Inc.
Technical Solution: Corning has developed specialized glass-lined reaction vessels specifically designed for hydrofluoric acid experiments. Their technology incorporates fluoropolymer-coated glass that resists HF etching while maintaining visibility for reaction monitoring. The system features integrated temperature control mechanisms that prevent overheating and potential runaway reactions, along with automated pressure relief systems calibrated specifically for HF-related processes. Corning's approach includes modular containment units with redundant sealing mechanisms and specialized ventilation systems that direct any potential emissions through multiple scrubber stages. Their vessels incorporate real-time monitoring capabilities with specialized sensors that can detect microscopic breaches before full failures occur, triggering automated shutdown procedures.
Strengths: Unparalleled materials expertise specifically relevant to HF containment; transparent reaction vessels allow visual monitoring; highly specialized equipment designed specifically for HF applications. Weaknesses: Higher initial equipment costs; requires specialized maintenance protocols; system components may have shorter lifespans due to eventual HF degradation.
Critical Safety Technologies and Protective Measures
Methods for performing flow reactions utilizing high temperature hydrofluoric acid
PatentActiveUS20180104665A1
Innovation
- The use of fluoroelastomer O-rings or gaskets with specific pre-use tensile strength and compressive set properties, ranging from 0.1 to 14 MPa and 0 to 12% respectively, in combination with HF-resistant materials, to maintain reliable operation at temperatures from 50° C. to 220° C.
System and Method for Alkylation Process Analysis
PatentActiveUS20110111509A1
Innovation
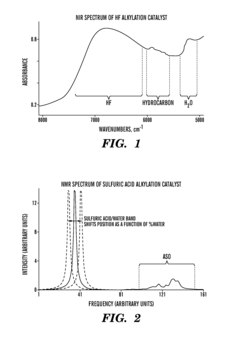
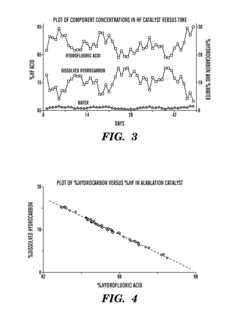
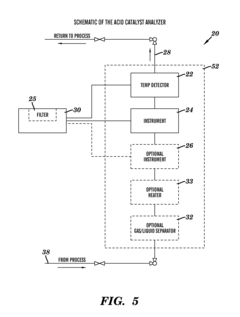
- A closed-loop system utilizing a combination of low-cost sensors such as conductivity detectors and Raman spectrometers, along with a process control module, to continuously analyze the acid catalyst composition, providing temperature-compensated concentration data for real-time process control, thereby overcoming the limitations of manual methods and improving safety.
Emergency Response and First Aid Protocols
Establishing comprehensive Emergency Response and First Aid Protocols is critical when conducting hydrofluoric acid (HF) reactivity experiments. Due to HF's unique properties—particularly its ability to penetrate tissue rapidly and cause delayed symptoms—standard acid protocols are insufficient. Organizations must implement a multi-tiered emergency response system specifically designed for HF exposure incidents.
The primary response protocol must include immediate access to calcium gluconate gel (2.5%), which counteracts fluoride ions by forming insoluble calcium fluoride. This antidote should be strategically placed throughout laboratory facilities, with clear signage and regular inventory checks to ensure availability. Laboratory personnel must be trained to apply the gel within 2-3 minutes of skin exposure, even when symptoms are not immediately apparent.
Emergency eyewash stations and safety showers must be modified specifically for HF incidents, featuring extended operation times (minimum 15 minutes) and appropriate water temperature control. These stations require weekly testing and documentation to ensure proper functionality. Additionally, specialized HF spill kits containing neutralizing agents such as calcium carbonate or magnesium oxide should supplement standard acid spill materials.
Communication protocols represent another critical component, requiring clear escalation procedures and emergency contact information posted visibly throughout the facility. Laboratories should establish direct lines to medical facilities with experience treating HF exposures, as general emergency departments may lack specialized knowledge. Documentation must include detailed exposure information to accompany victims to medical facilities, including concentration, duration, and body areas affected.
Medical response coordination demands particular attention, with arrangements made with local medical facilities before experiments begin. This preparation should include providing calcium gluconate supplies to medical partners and ensuring they understand HF-specific treatment protocols. For facilities in remote locations, on-site medical capabilities may need enhancement, potentially including injectable calcium gluconate and trained personnel to administer it under medical guidance.
Regular emergency drills specific to HF incidents must be conducted quarterly, simulating various exposure scenarios and evaluating response times and protocol adherence. These drills should involve all laboratory personnel and include coordination with external emergency services when possible. Post-drill evaluations should identify improvement opportunities and update protocols accordingly.
Psychological support mechanisms must also be established for both victims and witnesses of HF incidents, recognizing the traumatic nature of chemical emergencies and their potential long-term psychological impacts. This support framework should include immediate crisis intervention resources and longer-term counseling options.
The primary response protocol must include immediate access to calcium gluconate gel (2.5%), which counteracts fluoride ions by forming insoluble calcium fluoride. This antidote should be strategically placed throughout laboratory facilities, with clear signage and regular inventory checks to ensure availability. Laboratory personnel must be trained to apply the gel within 2-3 minutes of skin exposure, even when symptoms are not immediately apparent.
Emergency eyewash stations and safety showers must be modified specifically for HF incidents, featuring extended operation times (minimum 15 minutes) and appropriate water temperature control. These stations require weekly testing and documentation to ensure proper functionality. Additionally, specialized HF spill kits containing neutralizing agents such as calcium carbonate or magnesium oxide should supplement standard acid spill materials.
Communication protocols represent another critical component, requiring clear escalation procedures and emergency contact information posted visibly throughout the facility. Laboratories should establish direct lines to medical facilities with experience treating HF exposures, as general emergency departments may lack specialized knowledge. Documentation must include detailed exposure information to accompany victims to medical facilities, including concentration, duration, and body areas affected.
Medical response coordination demands particular attention, with arrangements made with local medical facilities before experiments begin. This preparation should include providing calcium gluconate supplies to medical partners and ensuring they understand HF-specific treatment protocols. For facilities in remote locations, on-site medical capabilities may need enhancement, potentially including injectable calcium gluconate and trained personnel to administer it under medical guidance.
Regular emergency drills specific to HF incidents must be conducted quarterly, simulating various exposure scenarios and evaluating response times and protocol adherence. These drills should involve all laboratory personnel and include coordination with external emergency services when possible. Post-drill evaluations should identify improvement opportunities and update protocols accordingly.
Psychological support mechanisms must also be established for both victims and witnesses of HF incidents, recognizing the traumatic nature of chemical emergencies and their potential long-term psychological impacts. This support framework should include immediate crisis intervention resources and longer-term counseling options.
Environmental Impact and Waste Management
Hydrofluoric acid (HF) experiments generate significant environmental concerns that require comprehensive management strategies. The disposal of HF waste presents serious environmental hazards due to its high toxicity and corrosive properties. When released into aquatic environments, even at low concentrations, HF can drastically alter pH levels, causing severe damage to aquatic ecosystems and endangering various species. Furthermore, fluoride compounds resulting from HF reactions can persist in soil and water systems, potentially entering the food chain.
Proper waste management protocols for HF experiments must adhere to strict regulatory frameworks established by environmental protection agencies. These typically include neutralization procedures using calcium compounds that convert HF into less hazardous calcium fluoride precipitates. All neutralized waste must undergo verification testing to ensure pH levels fall within acceptable ranges (typically 6.0-9.0) before disposal through approved channels.
Air emissions during HF experiments also require careful management through properly designed ventilation systems equipped with scrubbers or absorption units. These systems should capture and neutralize HF vapors before release, with regular monitoring of emission levels to ensure compliance with local air quality regulations. Laboratory facilities conducting regular HF experiments should implement continuous air monitoring systems with appropriate alarm thresholds.
Spill management constitutes another critical aspect of environmental protection. Dedicated HF spill kits containing specialized neutralizing agents (calcium gluconate or calcium carbonate) must be readily accessible in all experimental areas. Staff should receive specific training on spill containment procedures to prevent environmental contamination through drainage systems.
Documentation and tracking of all HF waste streams represent essential components of responsible environmental stewardship. Detailed records should be maintained regarding quantities generated, treatment methods applied, and final disposal pathways. Many jurisdictions require formal waste manifests and regular reporting of hazardous waste management activities.
Emerging technologies are improving HF waste management practices, including advanced ion exchange resins specifically designed for fluoride removal, membrane filtration systems capable of separating fluoride compounds from aqueous solutions, and regenerative scrubbing systems that minimize secondary waste generation. Research facilities should regularly evaluate these developing technologies to optimize their environmental protection measures while maintaining experimental capabilities.
Proper waste management protocols for HF experiments must adhere to strict regulatory frameworks established by environmental protection agencies. These typically include neutralization procedures using calcium compounds that convert HF into less hazardous calcium fluoride precipitates. All neutralized waste must undergo verification testing to ensure pH levels fall within acceptable ranges (typically 6.0-9.0) before disposal through approved channels.
Air emissions during HF experiments also require careful management through properly designed ventilation systems equipped with scrubbers or absorption units. These systems should capture and neutralize HF vapors before release, with regular monitoring of emission levels to ensure compliance with local air quality regulations. Laboratory facilities conducting regular HF experiments should implement continuous air monitoring systems with appropriate alarm thresholds.
Spill management constitutes another critical aspect of environmental protection. Dedicated HF spill kits containing specialized neutralizing agents (calcium gluconate or calcium carbonate) must be readily accessible in all experimental areas. Staff should receive specific training on spill containment procedures to prevent environmental contamination through drainage systems.
Documentation and tracking of all HF waste streams represent essential components of responsible environmental stewardship. Detailed records should be maintained regarding quantities generated, treatment methods applied, and final disposal pathways. Many jurisdictions require formal waste manifests and regular reporting of hazardous waste management activities.
Emerging technologies are improving HF waste management practices, including advanced ion exchange resins specifically designed for fluoride removal, membrane filtration systems capable of separating fluoride compounds from aqueous solutions, and regenerative scrubbing systems that minimize secondary waste generation. Research facilities should regularly evaluate these developing technologies to optimize their environmental protection measures while maintaining experimental capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!