How to Develop and Implement an Effective ICP-MS Quality Control Protocol
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines a high-temperature ICP source with a mass spectrometer to detect and quantify metals and several non-metals at concentrations as low as one part per trillion. The technology emerged from the limitations of earlier techniques such as atomic absorption spectroscopy and ICP optical emission spectroscopy, offering superior detection limits and analytical capabilities.
The evolution of ICP-MS technology has been marked by several key advancements. Early systems faced challenges with matrix interferences and limited dynamic range. Modern instruments incorporate collision/reaction cell technology, which effectively reduces polyatomic interferences, and high-resolution mass analyzers that provide improved selectivity. Additionally, the integration of laser ablation systems has expanded ICP-MS applications to solid sample analysis without extensive preparation.
Current technological trends in ICP-MS include the development of more compact and user-friendly systems, improved sample introduction techniques, and enhanced software for data processing and interpretation. The miniaturization of components and the implementation of advanced electronics have contributed to increased sensitivity and stability, making the technology more accessible to a broader range of laboratories.
The primary objective of developing an effective ICP-MS quality control protocol is to ensure consistent, accurate, and reliable analytical results across various applications. This involves establishing standardized procedures for instrument calibration, sample preparation, analysis execution, and data validation. A robust protocol must address the specific challenges associated with ICP-MS, including matrix effects, spectral interferences, and instrument drift.
Another critical goal is to align ICP-MS quality control practices with regulatory requirements and industry standards. This includes compliance with guidelines from organizations such as the FDA, EPA, ISO, and USP, which specify performance criteria for analytical methods used in pharmaceutical, environmental, and clinical applications.
Furthermore, an effective protocol aims to optimize laboratory efficiency by minimizing unnecessary repetition of analyses while maintaining data integrity. This balance requires careful consideration of quality control sample frequency, acceptance criteria, and corrective action procedures when deviations occur.
The long-term technological objective extends beyond immediate analytical needs to establishing a framework that can adapt to evolving instrumentation capabilities and regulatory expectations. As ICP-MS technology continues to advance, quality control protocols must remain flexible enough to incorporate new features while preserving the fundamental principles of analytical quality assurance.
The evolution of ICP-MS technology has been marked by several key advancements. Early systems faced challenges with matrix interferences and limited dynamic range. Modern instruments incorporate collision/reaction cell technology, which effectively reduces polyatomic interferences, and high-resolution mass analyzers that provide improved selectivity. Additionally, the integration of laser ablation systems has expanded ICP-MS applications to solid sample analysis without extensive preparation.
Current technological trends in ICP-MS include the development of more compact and user-friendly systems, improved sample introduction techniques, and enhanced software for data processing and interpretation. The miniaturization of components and the implementation of advanced electronics have contributed to increased sensitivity and stability, making the technology more accessible to a broader range of laboratories.
The primary objective of developing an effective ICP-MS quality control protocol is to ensure consistent, accurate, and reliable analytical results across various applications. This involves establishing standardized procedures for instrument calibration, sample preparation, analysis execution, and data validation. A robust protocol must address the specific challenges associated with ICP-MS, including matrix effects, spectral interferences, and instrument drift.
Another critical goal is to align ICP-MS quality control practices with regulatory requirements and industry standards. This includes compliance with guidelines from organizations such as the FDA, EPA, ISO, and USP, which specify performance criteria for analytical methods used in pharmaceutical, environmental, and clinical applications.
Furthermore, an effective protocol aims to optimize laboratory efficiency by minimizing unnecessary repetition of analyses while maintaining data integrity. This balance requires careful consideration of quality control sample frequency, acceptance criteria, and corrective action procedures when deviations occur.
The long-term technological objective extends beyond immediate analytical needs to establishing a framework that can adapt to evolving instrumentation capabilities and regulatory expectations. As ICP-MS technology continues to advance, quality control protocols must remain flexible enough to incorporate new features while preserving the fundamental principles of analytical quality assurance.
Market Demand for Analytical Quality Control
The global market for analytical quality control in ICP-MS (Inductively Coupled Plasma Mass Spectrometry) has experienced significant growth over the past decade, driven primarily by increasing regulatory requirements across pharmaceutical, environmental, and food safety sectors. The demand for robust quality control protocols has become paramount as industries face stricter compliance standards and higher expectations for data reliability.
In the pharmaceutical industry, regulatory bodies including the FDA, EMA, and NMPA have strengthened their requirements for elemental impurity testing following the implementation of ICH Q3D guidelines. This has created a substantial market need for standardized QC protocols that can demonstrate consistent performance and reliability in ICP-MS analysis.
Environmental monitoring represents another major market driver, with government agencies worldwide mandating more frequent and precise testing of heavy metals and other elemental contaminants in water, soil, and air samples. The EPA in the United States and similar organizations globally have established increasingly stringent detection limits that necessitate highly controlled analytical procedures.
The food and beverage industry has similarly witnessed growing demand for analytical quality control, particularly following high-profile contamination incidents that have heightened consumer awareness and regulatory scrutiny. Major food producers and processors are investing in comprehensive quality control systems to ensure product safety and maintain consumer trust.
Clinical laboratories constitute a rapidly expanding market segment, with ICP-MS increasingly utilized for biomonitoring and diagnostic applications. The need for reliable results in patient testing has created demand for quality control protocols that can ensure consistent performance across different operators, instruments, and laboratory conditions.
Contract research organizations (CROs) and testing laboratories have emerged as significant consumers of quality control solutions, as they seek to differentiate their services through demonstrated analytical excellence and reliability. These organizations often serve multiple industries and must maintain diverse quality control protocols to meet varying client requirements.
The market is further characterized by regional variations in regulatory frameworks and implementation timelines, creating opportunities for specialized quality control solutions tailored to specific geographic markets. Developing economies in Asia and Latin America are showing accelerated growth rates as their regulatory systems mature and industrial quality standards rise.
In the pharmaceutical industry, regulatory bodies including the FDA, EMA, and NMPA have strengthened their requirements for elemental impurity testing following the implementation of ICH Q3D guidelines. This has created a substantial market need for standardized QC protocols that can demonstrate consistent performance and reliability in ICP-MS analysis.
Environmental monitoring represents another major market driver, with government agencies worldwide mandating more frequent and precise testing of heavy metals and other elemental contaminants in water, soil, and air samples. The EPA in the United States and similar organizations globally have established increasingly stringent detection limits that necessitate highly controlled analytical procedures.
The food and beverage industry has similarly witnessed growing demand for analytical quality control, particularly following high-profile contamination incidents that have heightened consumer awareness and regulatory scrutiny. Major food producers and processors are investing in comprehensive quality control systems to ensure product safety and maintain consumer trust.
Clinical laboratories constitute a rapidly expanding market segment, with ICP-MS increasingly utilized for biomonitoring and diagnostic applications. The need for reliable results in patient testing has created demand for quality control protocols that can ensure consistent performance across different operators, instruments, and laboratory conditions.
Contract research organizations (CROs) and testing laboratories have emerged as significant consumers of quality control solutions, as they seek to differentiate their services through demonstrated analytical excellence and reliability. These organizations often serve multiple industries and must maintain diverse quality control protocols to meet varying client requirements.
The market is further characterized by regional variations in regulatory frameworks and implementation timelines, creating opportunities for specialized quality control solutions tailored to specific geographic markets. Developing economies in Asia and Latin America are showing accelerated growth rates as their regulatory systems mature and industrial quality standards rise.
Current Challenges in ICP-MS Quality Assurance
Despite significant advancements in ICP-MS technology, quality assurance remains a persistent challenge for laboratories implementing this powerful analytical technique. One of the primary obstacles is the inherent complexity of the instrumentation itself, which requires meticulous calibration and maintenance. The high sensitivity of ICP-MS makes it particularly susceptible to various forms of interference, including spectral overlaps, matrix effects, and polyatomic ion formation, all of which can compromise data quality if not properly addressed.
Sample preparation presents another significant hurdle, as inconsistencies in digestion procedures, contamination, or analyte loss can introduce substantial errors before samples even reach the instrument. The variability in sample matrices across different applications—from environmental monitoring to pharmaceutical analysis—further complicates the establishment of standardized quality control protocols that can be universally applied.
Regulatory compliance adds another layer of complexity, with different industries subject to varying standards and requirements. For instance, pharmaceutical laboratories must adhere to GMP guidelines, while environmental testing facilities follow EPA methods, each with distinct quality control expectations and documentation requirements. This regulatory diversity often necessitates customized QC approaches rather than one-size-fits-all solutions.
Data management and interpretation challenges have intensified as ICP-MS applications have expanded. The volume of data generated during multi-element analyses can be overwhelming, making it difficult to implement effective real-time quality control measures. Many laboratories struggle with establishing appropriate statistical tools for trend analysis and outlier detection, which are essential components of robust quality assurance programs.
Resource constraints represent a practical challenge for many facilities. Comprehensive quality control requires significant investments in certified reference materials, internal standards, and quality control samples, which can strain laboratory budgets. Additionally, the shortage of skilled personnel with expertise in both ICP-MS operation and quality assurance principles creates operational bottlenecks in many organizations.
Emerging applications in fields such as single-cell analysis, nanoparticle characterization, and speciation studies introduce novel quality control challenges that existing protocols may not adequately address. These cutting-edge applications often operate at the limits of detection and require specialized approaches to validate method performance and ensure data reliability.
The integration of ICP-MS with other analytical techniques, such as chromatographic separation methods, compounds these challenges by introducing additional variables that must be controlled and monitored. These hyphenated techniques demand comprehensive quality assurance strategies that address the entire analytical workflow rather than focusing solely on the ICP-MS measurement step.
Sample preparation presents another significant hurdle, as inconsistencies in digestion procedures, contamination, or analyte loss can introduce substantial errors before samples even reach the instrument. The variability in sample matrices across different applications—from environmental monitoring to pharmaceutical analysis—further complicates the establishment of standardized quality control protocols that can be universally applied.
Regulatory compliance adds another layer of complexity, with different industries subject to varying standards and requirements. For instance, pharmaceutical laboratories must adhere to GMP guidelines, while environmental testing facilities follow EPA methods, each with distinct quality control expectations and documentation requirements. This regulatory diversity often necessitates customized QC approaches rather than one-size-fits-all solutions.
Data management and interpretation challenges have intensified as ICP-MS applications have expanded. The volume of data generated during multi-element analyses can be overwhelming, making it difficult to implement effective real-time quality control measures. Many laboratories struggle with establishing appropriate statistical tools for trend analysis and outlier detection, which are essential components of robust quality assurance programs.
Resource constraints represent a practical challenge for many facilities. Comprehensive quality control requires significant investments in certified reference materials, internal standards, and quality control samples, which can strain laboratory budgets. Additionally, the shortage of skilled personnel with expertise in both ICP-MS operation and quality assurance principles creates operational bottlenecks in many organizations.
Emerging applications in fields such as single-cell analysis, nanoparticle characterization, and speciation studies introduce novel quality control challenges that existing protocols may not adequately address. These cutting-edge applications often operate at the limits of detection and require specialized approaches to validate method performance and ensure data reliability.
The integration of ICP-MS with other analytical techniques, such as chromatographic separation methods, compounds these challenges by introducing additional variables that must be controlled and monitored. These hyphenated techniques demand comprehensive quality assurance strategies that address the entire analytical workflow rather than focusing solely on the ICP-MS measurement step.
Established ICP-MS QC Protocol Solutions
01 Quality control protocols for ICP-MS sample preparation
Effective quality control protocols for ICP-MS involve standardized sample preparation methods to ensure accurate and reliable results. These protocols include procedures for sample digestion, dilution, and filtration to minimize contamination and matrix effects. Implementing consistent preparation techniques helps maintain the integrity of samples and reduces variability in analytical measurements, which is crucial for achieving reproducible results in trace element analysis.- Quality control protocols for ICP-MS sample preparation: Effective quality control protocols for ICP-MS sample preparation involve standardized procedures for sample digestion, dilution, and filtration to ensure accurate and reproducible results. These protocols typically include the use of certified reference materials, method blanks, and spike recovery tests to validate the sample preparation process. Proper sample handling and contamination prevention measures are essential components of these protocols to maintain the integrity of analytical results.
- Calibration and instrument performance monitoring systems: Advanced calibration and instrument performance monitoring systems are critical for ensuring ICP-MS quality control effectiveness. These systems include automated calibration verification, drift correction algorithms, and real-time monitoring of key performance indicators such as sensitivity, oxide formation rates, and doubly-charged ion ratios. Regular performance checks using tuning solutions and quality control standards help maintain optimal instrument conditions and detect potential issues before they affect analytical results.
- Data validation and statistical quality control methods: Robust data validation and statistical quality control methods enhance the reliability of ICP-MS analyses. These methods include the application of statistical tools for outlier detection, uncertainty estimation, and trend analysis of quality control samples. Implementation of control charts, measurement uncertainty budgets, and automated data flagging systems helps analysts identify and address analytical issues promptly. Regular proficiency testing and inter-laboratory comparisons further validate the effectiveness of quality control protocols.
- Interference management and correction techniques: Effective interference management and correction techniques are essential components of ICP-MS quality control protocols. These techniques include the use of collision/reaction cell technology, mathematical correction equations, and alternative isotope selection strategies to minimize spectral and non-spectral interferences. Implementation of internal standardization, isotope dilution methods, and matrix-matched calibration approaches helps compensate for matrix effects and signal suppression or enhancement, ensuring accurate quantification across diverse sample types.
- Automated quality control systems and software solutions: Automated quality control systems and software solutions streamline ICP-MS quality control processes and improve protocol effectiveness. These systems feature integrated software for automated quality control checks, real-time data evaluation, and customizable alert thresholds for quality control failures. Advanced data management platforms enable comprehensive audit trails, automated reporting of quality metrics, and integration with laboratory information management systems. Machine learning algorithms can be employed to predict instrument maintenance needs and optimize quality control sampling frequencies.
02 Calibration and standardization methods for ICP-MS
Calibration and standardization are essential components of ICP-MS quality control protocols. This includes the use of certified reference materials, internal standards, and multi-point calibration curves to ensure measurement accuracy. Regular verification of instrument performance using quality control standards helps detect drift and maintain consistent analytical performance. Effective calibration protocols enable reliable quantification of elements across different concentration ranges and sample matrices.Expand Specific Solutions03 Automated quality control systems for ICP-MS
Automated quality control systems enhance the effectiveness of ICP-MS protocols by reducing human error and increasing throughput. These systems incorporate real-time monitoring of instrument parameters, automated data processing, and intelligent error detection algorithms. Integration with laboratory information management systems allows for continuous tracking of quality metrics and immediate flagging of anomalous results, improving overall reliability and efficiency of analytical processes.Expand Specific Solutions04 Interference reduction techniques in ICP-MS analysis
Effective quality control protocols for ICP-MS include methods to identify and mitigate spectral and non-spectral interferences. Techniques such as collision/reaction cell technology, mathematical correction models, and optimized plasma conditions help improve measurement accuracy. Implementing systematic approaches to interference management ensures reliable quantification of target elements even in complex matrices, which is critical for applications requiring high sensitivity and selectivity.Expand Specific Solutions05 Statistical methods for ICP-MS data validation
Statistical approaches are integral to validating ICP-MS quality control protocols. These include the application of statistical process control charts, outlier detection algorithms, and uncertainty estimation methods to evaluate data quality. Regular analysis of quality control samples, method blanks, and duplicates provides metrics for assessing precision and accuracy. Implementing robust statistical frameworks enables laboratories to demonstrate the reliability of their analytical results and identify opportunities for method improvement.Expand Specific Solutions
Leading Manufacturers and Laboratory Standards
The ICP-MS quality control protocol market is in a growth phase, characterized by increasing adoption across pharmaceutical, environmental, and research sectors. The market size is expanding steadily, driven by stringent regulatory requirements and growing analytical testing needs. Technologically, the field shows moderate maturity with established protocols, but continuous innovation in automation and data management. Leading players include Thermo Fisher Scientific (Bremen) GmbH, which dominates with comprehensive solutions, alongside Revvity Health Sciences offering specialized protocols. Elemental Scientific has carved a niche in sample automation, while Applied Materials and Keysight Technologies contribute advanced measurement capabilities. Academic institutions like Central South University and Huazhong University are advancing fundamental research, creating a competitive landscape balanced between established corporations and specialized providers.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed a specialized ICP-MS quality control protocol tailored for semiconductor manufacturing environments, where ultra-trace elemental analysis is critical for process control. Their approach integrates ICP-MS analysis with their comprehensive fab management systems, creating closed-loop feedback for process optimization. The protocol implements cleanroom-compatible automated sampling systems that maintain sample integrity from process tools to analysis. Their system features specialized sample preparation techniques for semiconductor materials including automated digestion procedures for silicon wafers, photoresists, and process chemicals that maintain ultra-low backgrounds for critical elements. The QC protocol incorporates multi-level contamination control measures including HEPA-filtered enclosures, automated clean chemistry dispensing systems, and specialized labware cleaning procedures[9]. Their approach includes statistical process control integration that automatically correlates ICP-MS results with semiconductor yield data, enabling real-time process adjustments. The protocol features specialized calibration procedures for semiconductor-specific matrices and implements dedicated monitoring of memory effects for elements critical to semiconductor performance such as Na, Fe, Cu, and Au at sub-ppt levels[10].
Strengths: Highly specialized for semiconductor applications; superior contamination control; integrated with manufacturing process control systems. Weaknesses: Limited applicability outside semiconductor industry; extremely high implementation costs; requires specialized cleanroom infrastructure and training.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed a comprehensive ICP-MS quality control protocol centered around their iCAP TQ ICP-MS system with triple quadrupole technology. Their approach integrates automated daily performance checks, intelligent QA/QC software, and advanced interference removal capabilities. The protocol includes automated tuning procedures that optimize instrument parameters based on daily performance metrics, ensuring consistent sensitivity and minimal oxide formation. Their QA/QC software automatically tracks internal standards, calibration curves, and QC samples against user-defined acceptance criteria, flagging any deviations for immediate attention. The system employs a triple quadrupole design that enables selective reaction monitoring for interference removal, particularly beneficial for complex matrices in environmental and clinical samples[1]. Their protocol incorporates automated drift correction using internal standardization and implements regular verification of detection limits and quantification ranges to ensure data reliability across analytical runs[2].
Strengths: Superior interference management through triple quadrupole technology; comprehensive automated QC workflows that reduce operator intervention; robust drift correction algorithms. Weaknesses: Higher initial investment compared to single quadrupole systems; requires more specialized training for operators; proprietary software ecosystem may limit integration with third-party laboratory information management systems.
Critical Technical Innovations in ICP-MS Validation
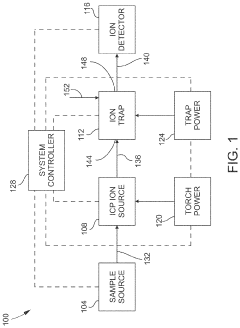
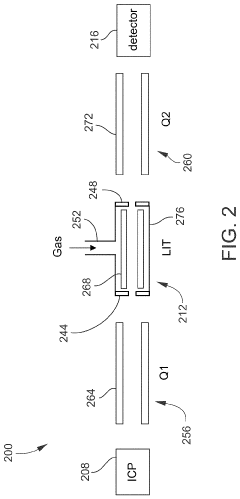
Inductively coupled plasma mass spectrometry (ICP-MS) with ion trapping
PatentActiveUS11443933B1
Innovation
- Incorporating an ion trap, such as a linear ion trap, into the ICP-MS system to confine and mass-selectively eject ions, allowing for the simultaneous analysis of multiple elements from transient signals by preventing ion exit and entry during a confinement period and transmitting selected ions to a detector for measurement.
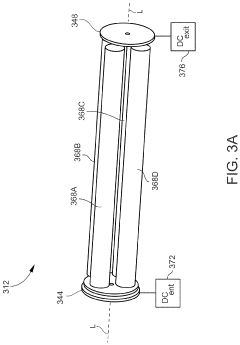
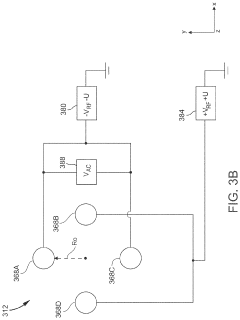
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
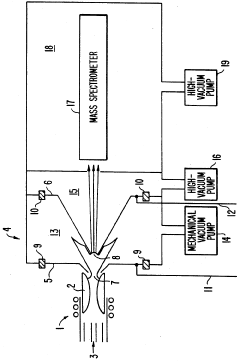
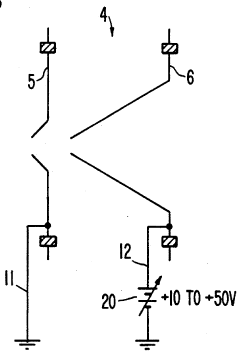
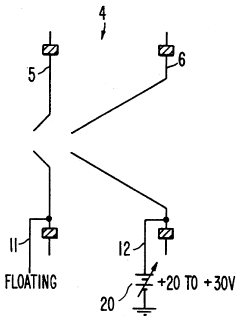
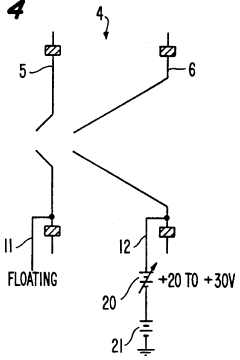
PatentInactiveUS5218204A
Innovation
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Regulatory Compliance Requirements
Regulatory compliance represents a critical foundation for any ICP-MS quality control protocol implementation. Laboratories utilizing ICP-MS technology must navigate a complex landscape of international, national, and industry-specific regulations that govern analytical testing procedures. The primary regulatory frameworks include ISO/IEC 17025 for testing and calibration laboratories, which establishes general requirements for competence, impartiality, and consistent operation. This standard specifically addresses quality management systems, technical requirements, and validation procedures essential for ICP-MS operations.
In the pharmaceutical sector, compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is mandatory, with specific emphasis on USP <232>, <233>, and <2232> chapters that detail elemental impurities testing protocols. These regulations establish permissible daily exposure limits for various elements and specify validation requirements for analytical procedures using ICP-MS.
Environmental testing laboratories must adhere to EPA Method 200.8 for determination of trace elements in waters and wastes, which outlines specific quality control measures including calibration verification, internal standardization, and interference correction methodologies. Similarly, food testing laboratories follow FDA's Elemental Analysis Manual (EAM) and international standards such as AOAC International methods.
The medical device industry faces compliance requirements under ISO 10993-18 for chemical characterization of materials, with specific protocols for extractable and leachable testing using ICP-MS. Additionally, RoHS (Restriction of Hazardous Substances) and REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulations in the EU impose strict limits on heavy metals and require validated analytical methods for compliance verification.
Documentation requirements across these regulatory frameworks include Standard Operating Procedures (SOPs), method validation reports, instrument qualification documentation (IQ/OQ/PQ), training records, and comprehensive audit trails. Regulatory bodies increasingly emphasize data integrity aspects, requiring laboratories to implement systems that ensure raw data security, traceability, and non-manipulability throughout the analytical workflow.
Compliance monitoring necessitates regular participation in proficiency testing programs and inter-laboratory comparison studies to demonstrate analytical competence. Many jurisdictions also mandate periodic regulatory inspections and assessments, requiring laboratories to maintain continuous compliance readiness through internal audit programs and corrective action procedures.
The global nature of modern supply chains has led to increasing harmonization efforts between regulatory bodies, though significant regional variations persist. Laboratories must therefore develop flexible quality control protocols that can adapt to evolving regulatory requirements while maintaining consistent analytical performance across different regulatory jurisdictions.
In the pharmaceutical sector, compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is mandatory, with specific emphasis on USP <232>, <233>, and <2232> chapters that detail elemental impurities testing protocols. These regulations establish permissible daily exposure limits for various elements and specify validation requirements for analytical procedures using ICP-MS.
Environmental testing laboratories must adhere to EPA Method 200.8 for determination of trace elements in waters and wastes, which outlines specific quality control measures including calibration verification, internal standardization, and interference correction methodologies. Similarly, food testing laboratories follow FDA's Elemental Analysis Manual (EAM) and international standards such as AOAC International methods.
The medical device industry faces compliance requirements under ISO 10993-18 for chemical characterization of materials, with specific protocols for extractable and leachable testing using ICP-MS. Additionally, RoHS (Restriction of Hazardous Substances) and REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulations in the EU impose strict limits on heavy metals and require validated analytical methods for compliance verification.
Documentation requirements across these regulatory frameworks include Standard Operating Procedures (SOPs), method validation reports, instrument qualification documentation (IQ/OQ/PQ), training records, and comprehensive audit trails. Regulatory bodies increasingly emphasize data integrity aspects, requiring laboratories to implement systems that ensure raw data security, traceability, and non-manipulability throughout the analytical workflow.
Compliance monitoring necessitates regular participation in proficiency testing programs and inter-laboratory comparison studies to demonstrate analytical competence. Many jurisdictions also mandate periodic regulatory inspections and assessments, requiring laboratories to maintain continuous compliance readiness through internal audit programs and corrective action procedures.
The global nature of modern supply chains has led to increasing harmonization efforts between regulatory bodies, though significant regional variations persist. Laboratories must therefore develop flexible quality control protocols that can adapt to evolving regulatory requirements while maintaining consistent analytical performance across different regulatory jurisdictions.
Cost-Benefit Analysis of QC Implementation
Implementing a comprehensive quality control protocol for ICP-MS analysis requires significant investment in terms of time, personnel, and resources. This cost-benefit analysis examines the financial implications of establishing robust QC procedures against the potential risks and costs associated with inadequate quality control measures.
Initial implementation costs for an effective ICP-MS QC protocol typically include investments in certified reference materials (CRMs), which can range from $300 to $1,500 per standard depending on complexity and certification level. Additional expenses include quality control software ($5,000-$15,000), staff training programs ($2,000-$5,000 per analyst), and potentially dedicated QC personnel ($60,000-$90,000 annual salary for a QC specialist).
Ongoing operational costs encompass regular procurement of QC samples (approximately $10,000-$30,000 annually), instrument time dedicated to QC measurements (estimated at 15-25% of total instrument time), and periodic proficiency testing participation ($1,500-$3,000 per round). These recurring expenses must be factored into laboratory budgets and service pricing structures.
Against these costs, laboratories must consider the substantial financial benefits of robust quality control. Error prevention represents significant savings, as sample re-analysis costs ($150-$300 per sample) and investigation time for questionable results (8-16 hours of analyst time per incident) can quickly accumulate. For commercial laboratories, the reputational damage from reporting inaccurate results may lead to client loss valued at tens to hundreds of thousands of dollars annually.
Regulatory compliance benefits are equally compelling. Non-compliance penalties in regulated environments can range from $10,000 for minor infractions to millions for serious violations. Furthermore, laboratories with demonstrated quality control excellence often secure premium contracts and can command higher service rates (typically 10-15% premium over competitors with less rigorous QC).
The return on investment timeline for comprehensive QC implementation typically shows break-even within 12-18 months, with increasing returns thereafter as error rates decline and operational efficiency improves. Data indicates that laboratories with mature QC protocols experience 30-40% fewer analytical failures and 25% higher client retention rates compared to those with minimal quality control measures.
When properly implemented, the long-term financial benefits of robust ICP-MS quality control protocols substantially outweigh the initial and ongoing investments, making QC implementation not merely a scientific necessity but a sound business decision.
Initial implementation costs for an effective ICP-MS QC protocol typically include investments in certified reference materials (CRMs), which can range from $300 to $1,500 per standard depending on complexity and certification level. Additional expenses include quality control software ($5,000-$15,000), staff training programs ($2,000-$5,000 per analyst), and potentially dedicated QC personnel ($60,000-$90,000 annual salary for a QC specialist).
Ongoing operational costs encompass regular procurement of QC samples (approximately $10,000-$30,000 annually), instrument time dedicated to QC measurements (estimated at 15-25% of total instrument time), and periodic proficiency testing participation ($1,500-$3,000 per round). These recurring expenses must be factored into laboratory budgets and service pricing structures.
Against these costs, laboratories must consider the substantial financial benefits of robust quality control. Error prevention represents significant savings, as sample re-analysis costs ($150-$300 per sample) and investigation time for questionable results (8-16 hours of analyst time per incident) can quickly accumulate. For commercial laboratories, the reputational damage from reporting inaccurate results may lead to client loss valued at tens to hundreds of thousands of dollars annually.
Regulatory compliance benefits are equally compelling. Non-compliance penalties in regulated environments can range from $10,000 for minor infractions to millions for serious violations. Furthermore, laboratories with demonstrated quality control excellence often secure premium contracts and can command higher service rates (typically 10-15% premium over competitors with less rigorous QC).
The return on investment timeline for comprehensive QC implementation typically shows break-even within 12-18 months, with increasing returns thereafter as error rates decline and operational efficiency improves. Data indicates that laboratories with mature QC protocols experience 30-40% fewer analytical failures and 25% higher client retention rates compared to those with minimal quality control measures.
When properly implemented, the long-term financial benefits of robust ICP-MS quality control protocols substantially outweigh the initial and ongoing investments, making QC implementation not merely a scientific necessity but a sound business decision.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!