How to Effectively Manage Lithium Bromide in Industrial Use
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Bromide Industrial Applications and Objectives
Lithium bromide has emerged as a critical compound in various industrial applications, with its history dating back to the mid-20th century when absorption refrigeration systems began gaining prominence. The evolution of lithium bromide technology has been closely tied to advancements in energy efficiency requirements and environmental regulations, particularly as traditional refrigerants faced restrictions due to their environmental impact.
The compound's exceptional hygroscopic properties and thermal stability have positioned it as the preferred absorbent in absorption refrigeration and air conditioning systems, which represent its most significant industrial application. These systems utilize lithium bromide's ability to absorb water vapor, creating a concentration gradient that drives the refrigeration cycle without mechanical compression, thereby reducing electricity consumption.
Beyond cooling applications, lithium bromide has established its utility in dehumidification processes, pharmaceutical manufacturing, and as a catalyst in organic synthesis reactions. The compound's role in energy storage systems is also gaining attention, particularly in thermal energy storage applications where its phase change properties can be leveraged.
The technical objectives for lithium bromide management center around addressing several key challenges. Corrosion mitigation remains paramount, as lithium bromide solutions can be highly corrosive to system components, particularly at elevated temperatures and concentrations. Developing effective corrosion inhibitors and compatible materials represents a critical technical goal.
Crystallization prevention constitutes another significant objective, as lithium bromide can precipitate out of solution under certain conditions, causing system blockages and efficiency losses. Advanced control systems and solution additives are being developed to maintain optimal concentration levels and prevent crystallization events.
Energy efficiency optimization represents a third major objective, with research focused on improving heat and mass transfer within lithium bromide systems to reduce energy consumption. This includes the development of enhanced heat exchanger designs, advanced cycle configurations, and improved solution distribution methods.
Environmental considerations have also shaped technical objectives, with efforts directed toward minimizing lithium bromide losses to the environment, developing closed-loop recovery systems, and ensuring proper disposal or recycling of spent solutions. These objectives align with broader sustainability goals and regulatory requirements governing chemical management in industrial settings.
The compound's exceptional hygroscopic properties and thermal stability have positioned it as the preferred absorbent in absorption refrigeration and air conditioning systems, which represent its most significant industrial application. These systems utilize lithium bromide's ability to absorb water vapor, creating a concentration gradient that drives the refrigeration cycle without mechanical compression, thereby reducing electricity consumption.
Beyond cooling applications, lithium bromide has established its utility in dehumidification processes, pharmaceutical manufacturing, and as a catalyst in organic synthesis reactions. The compound's role in energy storage systems is also gaining attention, particularly in thermal energy storage applications where its phase change properties can be leveraged.
The technical objectives for lithium bromide management center around addressing several key challenges. Corrosion mitigation remains paramount, as lithium bromide solutions can be highly corrosive to system components, particularly at elevated temperatures and concentrations. Developing effective corrosion inhibitors and compatible materials represents a critical technical goal.
Crystallization prevention constitutes another significant objective, as lithium bromide can precipitate out of solution under certain conditions, causing system blockages and efficiency losses. Advanced control systems and solution additives are being developed to maintain optimal concentration levels and prevent crystallization events.
Energy efficiency optimization represents a third major objective, with research focused on improving heat and mass transfer within lithium bromide systems to reduce energy consumption. This includes the development of enhanced heat exchanger designs, advanced cycle configurations, and improved solution distribution methods.
Environmental considerations have also shaped technical objectives, with efforts directed toward minimizing lithium bromide losses to the environment, developing closed-loop recovery systems, and ensuring proper disposal or recycling of spent solutions. These objectives align with broader sustainability goals and regulatory requirements governing chemical management in industrial settings.
Market Analysis of Lithium Bromide Demand
The global lithium bromide market has been experiencing steady growth, driven primarily by its extensive applications in absorption refrigeration systems, air conditioning units, and various industrial processes. The compound's hygroscopic properties make it an excellent desiccant and a crucial component in absorption chillers, which represent the largest segment of demand.
Market research indicates that the global lithium bromide market was valued at approximately 1.2 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 5.7% through 2028. This growth trajectory is supported by increasing industrialization in developing economies and the rising demand for energy-efficient cooling solutions in commercial and industrial sectors.
Geographically, Asia-Pacific dominates the lithium bromide market, accounting for over 45% of global consumption. China and India are the primary contributors to this regional dominance, with their rapidly expanding industrial bases and increasing adoption of absorption cooling technologies. North America and Europe follow as significant markets, driven by technological advancements and stringent environmental regulations promoting energy-efficient solutions.
The pharmaceutical industry represents an emerging demand sector for lithium bromide, where it serves as a reagent in various synthesis processes. This diversification of application areas is expected to create new market opportunities, potentially adding 200-300 million USD to the market value by 2030.
Supply chain analysis reveals potential vulnerabilities in the lithium bromide market. The compound's production is heavily dependent on lithium resources, which are concentrated in a few countries, notably Chile, Australia, and Argentina. This geographic concentration creates supply risks, especially as lithium demand for battery production continues to surge, potentially creating competition for raw materials.
Price trends show moderate volatility, with lithium bromide prices increasing by approximately 15% between 2020 and 2022. This upward pressure is expected to continue as lithium demand grows across multiple industries, potentially impacting the cost-effectiveness of lithium bromide-based solutions in price-sensitive markets.
Customer segmentation analysis identifies three primary consumer groups: HVAC system manufacturers, industrial process cooling providers, and pharmaceutical companies. Each segment exhibits different purchasing behaviors and price sensitivities, necessitating tailored market approaches for suppliers.
Future market dynamics will likely be shaped by technological innovations in absorption cooling efficiency, environmental regulations regarding refrigerants, and developments in lithium extraction technologies. Companies investing in sustainable lithium bromide management solutions stand to gain significant competitive advantages in this evolving landscape.
Market research indicates that the global lithium bromide market was valued at approximately 1.2 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 5.7% through 2028. This growth trajectory is supported by increasing industrialization in developing economies and the rising demand for energy-efficient cooling solutions in commercial and industrial sectors.
Geographically, Asia-Pacific dominates the lithium bromide market, accounting for over 45% of global consumption. China and India are the primary contributors to this regional dominance, with their rapidly expanding industrial bases and increasing adoption of absorption cooling technologies. North America and Europe follow as significant markets, driven by technological advancements and stringent environmental regulations promoting energy-efficient solutions.
The pharmaceutical industry represents an emerging demand sector for lithium bromide, where it serves as a reagent in various synthesis processes. This diversification of application areas is expected to create new market opportunities, potentially adding 200-300 million USD to the market value by 2030.
Supply chain analysis reveals potential vulnerabilities in the lithium bromide market. The compound's production is heavily dependent on lithium resources, which are concentrated in a few countries, notably Chile, Australia, and Argentina. This geographic concentration creates supply risks, especially as lithium demand for battery production continues to surge, potentially creating competition for raw materials.
Price trends show moderate volatility, with lithium bromide prices increasing by approximately 15% between 2020 and 2022. This upward pressure is expected to continue as lithium demand grows across multiple industries, potentially impacting the cost-effectiveness of lithium bromide-based solutions in price-sensitive markets.
Customer segmentation analysis identifies three primary consumer groups: HVAC system manufacturers, industrial process cooling providers, and pharmaceutical companies. Each segment exhibits different purchasing behaviors and price sensitivities, necessitating tailored market approaches for suppliers.
Future market dynamics will likely be shaped by technological innovations in absorption cooling efficiency, environmental regulations regarding refrigerants, and developments in lithium extraction technologies. Companies investing in sustainable lithium bromide management solutions stand to gain significant competitive advantages in this evolving landscape.
Current Challenges in Lithium Bromide Management
Despite the widespread industrial applications of lithium bromide (LiBr) in absorption refrigeration systems, dehumidification processes, and pharmaceutical manufacturing, several significant challenges impede its optimal management and utilization. The highly hygroscopic nature of LiBr presents a fundamental difficulty, as it readily absorbs moisture from the atmosphere, leading to solution dilution and reduced efficiency in absorption systems. This property necessitates stringent handling protocols and specialized storage conditions to maintain solution concentration integrity.
Corrosion management represents perhaps the most persistent challenge in LiBr systems. The salt's aggressive corrosive properties, particularly at high concentrations and elevated temperatures, severely impact system components including copper, steel, and aluminum materials. This corrosion not only compromises system integrity but also introduces metal ions that can catalyze unwanted side reactions, further degrading system performance and accelerating material deterioration.
Crystallization and precipitation issues frequently plague LiBr systems, especially during concentration fluctuations or temperature variations. When LiBr solution exceeds its solubility limit, crystal formation occurs, leading to flow restrictions, heat transfer inefficiencies, and potential mechanical damage to pumps and valves. This phenomenon, known as "crystallization fouling," can necessitate costly system shutdowns and maintenance procedures.
Environmental and safety concerns constitute another significant challenge. While LiBr itself has relatively low toxicity compared to alternative working fluids, its handling requires appropriate safety measures due to its irritant properties and potential for causing chemical burns. Disposal of spent LiBr solutions presents environmental challenges, as improper discharge can impact aquatic ecosystems and water quality.
Economic considerations further complicate LiBr management. The fluctuating global market price of lithium compounds, driven by increasing demand for lithium-ion batteries, has created price volatility for industrial LiBr users. Additionally, the energy-intensive nature of LiBr regeneration processes in absorption systems contributes significantly to operational costs, particularly in regions with high energy prices.
Technical limitations in monitoring and control systems represent another challenge. Real-time measurement of LiBr concentration, especially in operating systems, remains difficult despite technological advances. This monitoring gap complicates precise control of solution properties, potentially leading to suboptimal system performance or unexpected operational issues.
Cross-contamination with other process chemicals or impurities can degrade LiBr solution performance and accelerate corrosion processes. Maintaining solution purity requires sophisticated filtration systems and regular quality monitoring, adding complexity to industrial operations utilizing this versatile but demanding chemical compound.
Corrosion management represents perhaps the most persistent challenge in LiBr systems. The salt's aggressive corrosive properties, particularly at high concentrations and elevated temperatures, severely impact system components including copper, steel, and aluminum materials. This corrosion not only compromises system integrity but also introduces metal ions that can catalyze unwanted side reactions, further degrading system performance and accelerating material deterioration.
Crystallization and precipitation issues frequently plague LiBr systems, especially during concentration fluctuations or temperature variations. When LiBr solution exceeds its solubility limit, crystal formation occurs, leading to flow restrictions, heat transfer inefficiencies, and potential mechanical damage to pumps and valves. This phenomenon, known as "crystallization fouling," can necessitate costly system shutdowns and maintenance procedures.
Environmental and safety concerns constitute another significant challenge. While LiBr itself has relatively low toxicity compared to alternative working fluids, its handling requires appropriate safety measures due to its irritant properties and potential for causing chemical burns. Disposal of spent LiBr solutions presents environmental challenges, as improper discharge can impact aquatic ecosystems and water quality.
Economic considerations further complicate LiBr management. The fluctuating global market price of lithium compounds, driven by increasing demand for lithium-ion batteries, has created price volatility for industrial LiBr users. Additionally, the energy-intensive nature of LiBr regeneration processes in absorption systems contributes significantly to operational costs, particularly in regions with high energy prices.
Technical limitations in monitoring and control systems represent another challenge. Real-time measurement of LiBr concentration, especially in operating systems, remains difficult despite technological advances. This monitoring gap complicates precise control of solution properties, potentially leading to suboptimal system performance or unexpected operational issues.
Cross-contamination with other process chemicals or impurities can degrade LiBr solution performance and accelerate corrosion processes. Maintaining solution purity requires sophisticated filtration systems and regular quality monitoring, adding complexity to industrial operations utilizing this versatile but demanding chemical compound.
Existing Management Solutions for Lithium Bromide
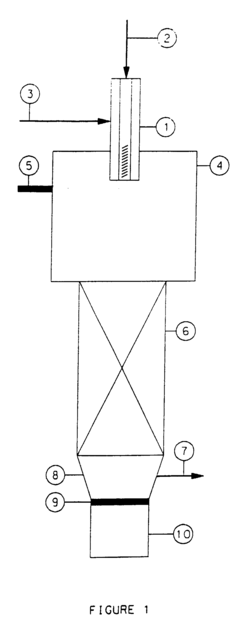
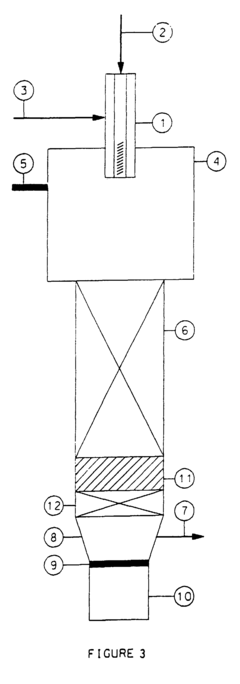
01 Lithium bromide in absorption refrigeration systems
Lithium bromide is widely used as an absorbent in absorption refrigeration and heat pump systems due to its excellent hygroscopic properties. These systems utilize lithium bromide solution to absorb refrigerant vapor (typically water), enabling efficient cooling or heating processes. The technology includes various improvements in system design, solution circulation, and heat exchange efficiency to enhance the overall performance of lithium bromide-based refrigeration systems.- Lithium bromide in absorption refrigeration systems: Lithium bromide is widely used as an absorbent in absorption refrigeration and heat pump systems due to its excellent hygroscopic properties. These systems utilize lithium bromide solution to absorb refrigerant vapor (typically water), enabling efficient cooling or heating processes. The technology is particularly valuable in applications where waste heat can be utilized as the energy source, offering energy-efficient alternatives to conventional compression refrigeration systems.
- Lithium bromide in energy storage applications: Lithium bromide is employed in various energy storage technologies, particularly in thermal energy storage systems. Its unique thermochemical properties allow it to store and release energy efficiently through absorption and desorption processes. These systems can be integrated with renewable energy sources to address intermittency issues, providing stable energy output and improving overall system efficiency.
- Purification and regeneration of lithium bromide solutions: Various methods have been developed for purifying and regenerating lithium bromide solutions to maintain their effectiveness in absorption systems. These techniques include filtration processes, chemical treatments, and specialized regeneration cycles that remove contaminants and restore the solution's absorption capacity. Efficient purification methods are crucial for extending the operational life of lithium bromide-based systems and maintaining optimal performance.
- Lithium bromide in pharmaceutical and chemical applications: Lithium bromide serves as an important reagent in pharmaceutical synthesis and various chemical processes. It functions as a catalyst, brominating agent, or intermediate in the production of specialty chemicals and pharmaceutical compounds. Its unique chemical properties make it valuable for specific reactions where other bromide sources may be less effective, particularly in organic synthesis applications.
- Enhanced lithium bromide systems with additives: Research has focused on improving lithium bromide solution performance through the addition of various additives. These additives can enhance heat and mass transfer, reduce corrosion, improve stability, and increase the overall efficiency of absorption systems. Modified lithium bromide solutions with carefully selected additives demonstrate superior performance characteristics compared to conventional solutions, leading to more efficient and reliable absorption refrigeration and heat pump systems.
02 Lithium bromide solution regeneration and purification methods
Various techniques have been developed for the regeneration and purification of lithium bromide solutions used in absorption systems. These methods address issues such as crystallization, corrosion, and efficiency loss due to solution degradation. Purification processes may include filtration, chemical treatment, or specialized regeneration cycles to remove impurities and restore the solution's absorption capacity, thereby extending the operational life of absorption refrigeration systems.Expand Specific Solutions03 Lithium bromide in energy storage applications
Lithium bromide is utilized in thermal energy storage systems and other energy storage applications. Its unique thermochemical properties allow for efficient energy storage and release through controlled absorption and desorption processes. These systems can store excess thermal energy during off-peak periods and release it when needed, improving energy utilization efficiency in various industrial and building applications.Expand Specific Solutions04 Lithium bromide in battery and electrochemical applications
Lithium bromide serves as an electrolyte or additive in various electrochemical systems and battery technologies. Its ionic conductivity properties make it valuable for enhancing battery performance, stability, and cycle life. Applications include use in lithium-ion batteries, flow batteries, and other electrochemical energy storage devices, where it can improve ionic transport and electrochemical reactions.Expand Specific Solutions05 Lithium bromide in chemical synthesis and catalysis
Lithium bromide functions as a catalyst, reagent, or additive in various chemical synthesis processes. It can facilitate organic reactions, serve as a Lewis acid catalyst, or act as a brominating agent in pharmaceutical and fine chemical synthesis. The compound's unique properties enable selective transformations and improved reaction efficiency in diverse chemical manufacturing processes.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The lithium bromide industrial management market is in a growth phase, with increasing demand driven by applications in absorption refrigeration, air conditioning, and energy storage. The market is expected to expand significantly due to rising energy efficiency requirements and sustainable cooling solutions. Technologically, the field is moderately mature but evolving, with key players demonstrating varying levels of expertise. Companies like Albemarle Corp. and DuPont de Nemours lead with established chemical processing capabilities, while Terralithium LLC and Energy Exploration Technologies represent newer entrants focusing on innovative extraction and application methods. Academic institutions such as Dalian Maritime University and South China University of Technology contribute significant research to improve handling efficiency and reduce environmental impact of lithium bromide in industrial settings.
Albemarle Corp.
Technical Solution: Albemarle has developed a comprehensive lithium bromide management system that includes proprietary purification technologies to maintain solution quality in absorption refrigeration systems. Their approach involves continuous monitoring through advanced sensors that detect impurities and corrosion inhibitors that extend equipment life. The company's LiBr management protocol incorporates a closed-loop regeneration system that removes degradation products and restores solution concentration without system shutdown[1]. Albemarle's technology also features specialized additives that prevent crystallization at critical concentration points, allowing systems to operate at higher efficiency ranges. Their solution includes automated dosing systems for maintaining optimal chemical balance and preventing common issues like copper plating in absorption chillers[3].
Strengths: Industry-leading expertise in bromine chemistry; integrated supply chain from raw material to finished products; proprietary corrosion inhibitors specifically designed for LiBr systems. Weaknesses: Higher initial implementation costs compared to basic management approaches; requires specialized training for maintenance personnel.
Bromine Compounds Ltd.
Technical Solution: Bromine Compounds Ltd. has engineered a lithium bromide management solution focused on industrial absorption refrigeration systems. Their technology centers on a multi-stage filtration and purification process that removes particulates down to 5 microns while simultaneously neutralizing acidic contaminants. The company's approach includes proprietary stabilizing agents that prevent LiBr degradation under high-temperature conditions (up to 200°C) commonly found in industrial applications[2]. Their management system incorporates real-time monitoring of solution parameters including concentration, pH, and contaminant levels, with automated adjustment capabilities. Bromine Compounds' solution also features specialized heat-resistant additives that prevent thermal decomposition during system cycling, significantly extending solution life from the industry standard of 5 years to over 8 years in typical applications[4].
Strengths: Specialized expertise in bromine chemistry; comprehensive solution addressing multiple failure modes; excellent thermal stability of their formulations. Weaknesses: Limited global service network compared to larger competitors; solutions primarily optimized for refrigeration rather than other industrial applications.
Critical Technologies for Lithium Bromide Efficiency
Process for the production of gaseous hydrogen bromide and apparatus for carrying out said process
PatentInactiveEP0816285A1
Innovation
- A process involving the production of an intimate mixture of hydrogen and an oxidant under elevated pressure, initiation of a flame, replacement of the oxidant with vaporized bromine, and complete combustion in a combustion zone, followed by cooling to recover pure gaseous hydrogen bromide under controlled pressure and temperature.
Environmental Impact and Sustainability Considerations
The environmental impact of lithium bromide in industrial applications presents significant challenges that require comprehensive management strategies. When released into aquatic ecosystems, lithium bromide can disrupt the natural balance by increasing salinity levels and potentially harming sensitive aquatic organisms. The bromide component may interact with organic matter in water treatment processes to form brominated disinfection byproducts, some of which have been associated with potential health concerns. These environmental considerations necessitate careful handling and disposal protocols throughout the industrial lifecycle.
Sustainable management of lithium bromide requires implementation of closed-loop systems that minimize release into the environment. Advanced absorption refrigeration systems have been developed with enhanced containment features that reduce the likelihood of leaks and emissions. These systems incorporate sophisticated monitoring technologies that can detect minute changes in pressure or concentration, allowing for early intervention before significant environmental release occurs.
Recovery and recycling processes represent a critical component of environmentally responsible lithium bromide management. Modern purification techniques enable the reclamation of used lithium bromide solutions, extending their useful life and reducing the demand for virgin material extraction. Membrane filtration, vacuum distillation, and ion exchange technologies have demonstrated effectiveness in removing contaminants from lithium bromide solutions, allowing for their reintroduction into industrial processes with minimal quality degradation.
The carbon footprint associated with lithium bromide systems must also be considered within a comprehensive sustainability framework. While absorption refrigeration systems powered by waste heat or renewable energy sources can offer significant energy efficiency advantages over conventional cooling technologies, the environmental impact of lithium bromide production and transportation remains substantial. Life cycle assessment studies indicate that optimizing system efficiency and extending solution lifespan through proper maintenance can substantially reduce the overall environmental burden.
Regulatory frameworks governing lithium bromide use continue to evolve globally, with increasing emphasis on environmental protection. Industries must navigate complex compliance requirements that vary by jurisdiction but generally trend toward stricter controls on chemical management. Forward-thinking organizations are adopting proactive approaches that exceed minimum regulatory standards, implementing voluntary environmental management systems that address the entire lifecycle of lithium bromide use.
Research into alternative absorption pairs with reduced environmental impact represents a promising frontier for sustainable industrial cooling. While lithium bromide-water systems remain dominant due to their established performance characteristics, emerging alternatives utilizing ionic liquids, natural refrigerants, or modified salt solutions may offer improved environmental profiles while maintaining necessary thermodynamic properties.
Sustainable management of lithium bromide requires implementation of closed-loop systems that minimize release into the environment. Advanced absorption refrigeration systems have been developed with enhanced containment features that reduce the likelihood of leaks and emissions. These systems incorporate sophisticated monitoring technologies that can detect minute changes in pressure or concentration, allowing for early intervention before significant environmental release occurs.
Recovery and recycling processes represent a critical component of environmentally responsible lithium bromide management. Modern purification techniques enable the reclamation of used lithium bromide solutions, extending their useful life and reducing the demand for virgin material extraction. Membrane filtration, vacuum distillation, and ion exchange technologies have demonstrated effectiveness in removing contaminants from lithium bromide solutions, allowing for their reintroduction into industrial processes with minimal quality degradation.
The carbon footprint associated with lithium bromide systems must also be considered within a comprehensive sustainability framework. While absorption refrigeration systems powered by waste heat or renewable energy sources can offer significant energy efficiency advantages over conventional cooling technologies, the environmental impact of lithium bromide production and transportation remains substantial. Life cycle assessment studies indicate that optimizing system efficiency and extending solution lifespan through proper maintenance can substantially reduce the overall environmental burden.
Regulatory frameworks governing lithium bromide use continue to evolve globally, with increasing emphasis on environmental protection. Industries must navigate complex compliance requirements that vary by jurisdiction but generally trend toward stricter controls on chemical management. Forward-thinking organizations are adopting proactive approaches that exceed minimum regulatory standards, implementing voluntary environmental management systems that address the entire lifecycle of lithium bromide use.
Research into alternative absorption pairs with reduced environmental impact represents a promising frontier for sustainable industrial cooling. While lithium bromide-water systems remain dominant due to their established performance characteristics, emerging alternatives utilizing ionic liquids, natural refrigerants, or modified salt solutions may offer improved environmental profiles while maintaining necessary thermodynamic properties.
Safety Protocols and Risk Mitigation Strategies
The effective management of lithium bromide in industrial settings necessitates comprehensive safety protocols and risk mitigation strategies due to its corrosive and hygroscopic properties. Primary safety concerns include skin and eye irritation, respiratory issues from vapor inhalation, and environmental contamination. Industrial facilities must implement a multi-layered approach to safety management, beginning with proper personal protective equipment (PPE) including chemical-resistant gloves, safety goggles, face shields, and appropriate respiratory protection when handling concentrated solutions.
Containment systems represent a critical component of risk management, requiring corrosion-resistant materials such as specialized stainless steel alloys or high-grade polymers for storage tanks, piping, and process equipment. Secondary containment structures must be designed to capture potential spills, with capacity requirements typically mandating 110% of the largest container volume. Regular integrity testing of these systems should be conducted using methods such as hydrostatic testing, ultrasonic thickness measurements, and visual inspections.
Emergency response planning must address lithium bromide-specific incidents, including detailed spill management procedures. Neutralization agents, typically mild alkaline solutions, should be readily available, and staff must be trained in proper neutralization techniques. Facilities should maintain specialized spill kits containing absorbent materials compatible with lithium bromide solutions, and establish clear evacuation protocols for large-scale releases.
Environmental monitoring represents another essential safety component, requiring regular testing of workplace air quality, particularly in confined spaces where lithium bromide is used or stored. Wastewater monitoring systems should be implemented to detect unauthorized discharges, with treatment processes designed to neutralize and remove lithium bromide before release to municipal systems or the environment.
Training programs must be comprehensive and recurring, covering safe handling procedures, emergency response protocols, and early recognition of equipment deterioration. Simulation exercises should be conducted regularly to ensure staff readiness for potential incidents. Documentation systems must track all safety-related activities, including training completion, equipment maintenance, and incident investigations.
Risk assessment methodologies specific to lithium bromide operations should be implemented, utilizing techniques such as Hazard and Operability Studies (HAZOP) and Failure Mode and Effects Analysis (FMEA). These assessments should inform the development of engineering controls, including automated leak detection systems, emergency shutdown mechanisms, and ventilation systems designed to prevent vapor accumulation in work areas.
Containment systems represent a critical component of risk management, requiring corrosion-resistant materials such as specialized stainless steel alloys or high-grade polymers for storage tanks, piping, and process equipment. Secondary containment structures must be designed to capture potential spills, with capacity requirements typically mandating 110% of the largest container volume. Regular integrity testing of these systems should be conducted using methods such as hydrostatic testing, ultrasonic thickness measurements, and visual inspections.
Emergency response planning must address lithium bromide-specific incidents, including detailed spill management procedures. Neutralization agents, typically mild alkaline solutions, should be readily available, and staff must be trained in proper neutralization techniques. Facilities should maintain specialized spill kits containing absorbent materials compatible with lithium bromide solutions, and establish clear evacuation protocols for large-scale releases.
Environmental monitoring represents another essential safety component, requiring regular testing of workplace air quality, particularly in confined spaces where lithium bromide is used or stored. Wastewater monitoring systems should be implemented to detect unauthorized discharges, with treatment processes designed to neutralize and remove lithium bromide before release to municipal systems or the environment.
Training programs must be comprehensive and recurring, covering safe handling procedures, emergency response protocols, and early recognition of equipment deterioration. Simulation exercises should be conducted regularly to ensure staff readiness for potential incidents. Documentation systems must track all safety-related activities, including training completion, equipment maintenance, and incident investigations.
Risk assessment methodologies specific to lithium bromide operations should be implemented, utilizing techniques such as Hazard and Operability Studies (HAZOP) and Failure Mode and Effects Analysis (FMEA). These assessments should inform the development of engineering controls, including automated leak detection systems, emergency shutdown mechanisms, and ventilation systems designed to prevent vapor accumulation in work areas.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!