How to Implement Sodium Acetate in Green Chemistry Initiatives?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate in Green Chemistry: Background and Objectives
Sodium acetate has emerged as a key compound in the field of green chemistry, offering a sustainable alternative to more harmful chemicals in various industrial processes. The evolution of green chemistry principles has led to increased interest in sodium acetate due to its environmentally friendly properties and versatile applications. This compound, formed by the neutralization of acetic acid with sodium hydroxide, has a long history of use in various industries, but its potential in green chemistry initiatives has only recently been fully recognized.
The primary objective of implementing sodium acetate in green chemistry initiatives is to reduce the environmental impact of chemical processes while maintaining or improving their efficiency. This aligns with the core principles of green chemistry, which aim to design chemical products and processes that minimize the use and generation of hazardous substances. Sodium acetate's biodegradability, low toxicity, and ability to be derived from renewable resources make it an ideal candidate for such initiatives.
In recent years, the global push towards sustainability has accelerated research and development efforts focused on sodium acetate applications. The compound's unique properties, including its high solubility in water and its ability to act as a buffer, have opened up new possibilities in fields ranging from pharmaceuticals to food preservation and textile manufacturing. As industries seek to reduce their carbon footprint and comply with increasingly stringent environmental regulations, sodium acetate has gained prominence as a green alternative to traditional chemicals.
The technical evolution of sodium acetate implementation in green chemistry has been marked by several key developments. These include the optimization of production methods to reduce energy consumption and waste, the exploration of novel applications that leverage its eco-friendly characteristics, and the integration of sodium acetate into existing industrial processes as a replacement for less sustainable compounds. Researchers and industry professionals are continuously working to expand the scope of sodium acetate's applications, driven by the need for more sustainable chemical solutions.
As we look towards the future, the trajectory of sodium acetate in green chemistry initiatives is expected to continue its upward trend. The compound's potential to address environmental concerns while meeting industrial needs positions it as a critical component in the transition towards more sustainable chemical practices. The ongoing research aims to further enhance the efficiency of sodium acetate-based processes, develop new formulations that expand its applicability, and explore synergies with other green chemistry technologies to create comprehensive sustainable solutions.
The primary objective of implementing sodium acetate in green chemistry initiatives is to reduce the environmental impact of chemical processes while maintaining or improving their efficiency. This aligns with the core principles of green chemistry, which aim to design chemical products and processes that minimize the use and generation of hazardous substances. Sodium acetate's biodegradability, low toxicity, and ability to be derived from renewable resources make it an ideal candidate for such initiatives.
In recent years, the global push towards sustainability has accelerated research and development efforts focused on sodium acetate applications. The compound's unique properties, including its high solubility in water and its ability to act as a buffer, have opened up new possibilities in fields ranging from pharmaceuticals to food preservation and textile manufacturing. As industries seek to reduce their carbon footprint and comply with increasingly stringent environmental regulations, sodium acetate has gained prominence as a green alternative to traditional chemicals.
The technical evolution of sodium acetate implementation in green chemistry has been marked by several key developments. These include the optimization of production methods to reduce energy consumption and waste, the exploration of novel applications that leverage its eco-friendly characteristics, and the integration of sodium acetate into existing industrial processes as a replacement for less sustainable compounds. Researchers and industry professionals are continuously working to expand the scope of sodium acetate's applications, driven by the need for more sustainable chemical solutions.
As we look towards the future, the trajectory of sodium acetate in green chemistry initiatives is expected to continue its upward trend. The compound's potential to address environmental concerns while meeting industrial needs positions it as a critical component in the transition towards more sustainable chemical practices. The ongoing research aims to further enhance the efficiency of sodium acetate-based processes, develop new formulations that expand its applicability, and explore synergies with other green chemistry technologies to create comprehensive sustainable solutions.
Market Demand for Sustainable Chemical Processes
The market demand for sustainable chemical processes has been steadily increasing in recent years, driven by growing environmental concerns, stricter regulations, and consumer preferences for eco-friendly products. This trend has created a significant opportunity for green chemistry initiatives, including the implementation of sodium acetate as a sustainable alternative in various chemical processes.
Sodium acetate, a versatile compound with numerous applications, has gained attention in the green chemistry sector due to its potential to replace more harmful chemicals and reduce environmental impact. The global market for sodium acetate is expected to grow substantially, with projections indicating a compound annual growth rate (CAGR) of over 5% in the coming years.
One of the key drivers for this market growth is the increasing demand for sustainable de-icing solutions. Sodium acetate-based de-icers are becoming increasingly popular as alternatives to traditional chloride-based products, which can be corrosive and environmentally damaging. Airports, in particular, are adopting sodium acetate de-icers to meet stringent environmental regulations while maintaining operational efficiency.
In the textile industry, there is a growing demand for eco-friendly dyeing processes. Sodium acetate plays a crucial role in these sustainable dyeing methods, acting as a buffer and pH regulator. As more textile manufacturers commit to reducing their environmental footprint, the demand for sodium acetate in this sector is expected to rise significantly.
The food industry is another major market for sodium acetate, where it is used as a preservative and acidity regulator. With consumers increasingly seeking clean-label products, sodium acetate's natural origin and safety profile make it an attractive option for food manufacturers looking to replace synthetic additives.
In the pharmaceutical sector, sodium acetate is gaining traction as a buffering agent in various formulations. The increasing focus on green chemistry principles in drug development and manufacturing is driving the adoption of more sustainable ingredients like sodium acetate.
The construction industry is also showing interest in sodium acetate for its potential use in sustainable concrete admixtures. Research into sodium acetate-based phase change materials for thermal energy storage in buildings is opening up new market opportunities in the green construction sector.
As industries continue to prioritize sustainability and seek alternatives to traditional chemical processes, the demand for sodium acetate in green chemistry initiatives is expected to grow. This trend is further supported by government regulations promoting the use of environmentally friendly chemicals and processes across various sectors.
Sodium acetate, a versatile compound with numerous applications, has gained attention in the green chemistry sector due to its potential to replace more harmful chemicals and reduce environmental impact. The global market for sodium acetate is expected to grow substantially, with projections indicating a compound annual growth rate (CAGR) of over 5% in the coming years.
One of the key drivers for this market growth is the increasing demand for sustainable de-icing solutions. Sodium acetate-based de-icers are becoming increasingly popular as alternatives to traditional chloride-based products, which can be corrosive and environmentally damaging. Airports, in particular, are adopting sodium acetate de-icers to meet stringent environmental regulations while maintaining operational efficiency.
In the textile industry, there is a growing demand for eco-friendly dyeing processes. Sodium acetate plays a crucial role in these sustainable dyeing methods, acting as a buffer and pH regulator. As more textile manufacturers commit to reducing their environmental footprint, the demand for sodium acetate in this sector is expected to rise significantly.
The food industry is another major market for sodium acetate, where it is used as a preservative and acidity regulator. With consumers increasingly seeking clean-label products, sodium acetate's natural origin and safety profile make it an attractive option for food manufacturers looking to replace synthetic additives.
In the pharmaceutical sector, sodium acetate is gaining traction as a buffering agent in various formulations. The increasing focus on green chemistry principles in drug development and manufacturing is driving the adoption of more sustainable ingredients like sodium acetate.
The construction industry is also showing interest in sodium acetate for its potential use in sustainable concrete admixtures. Research into sodium acetate-based phase change materials for thermal energy storage in buildings is opening up new market opportunities in the green construction sector.
As industries continue to prioritize sustainability and seek alternatives to traditional chemical processes, the demand for sodium acetate in green chemistry initiatives is expected to grow. This trend is further supported by government regulations promoting the use of environmentally friendly chemicals and processes across various sectors.
Current State and Challenges in Sodium Acetate Implementation
The implementation of sodium acetate in green chemistry initiatives has gained significant traction in recent years, driven by the growing demand for sustainable chemical processes. Currently, sodium acetate is widely used as a buffering agent, food preservative, and in various industrial applications. However, its integration into green chemistry practices faces several challenges and opportunities.
One of the primary challenges in implementing sodium acetate in green chemistry is the sourcing of raw materials. While sodium acetate can be produced through the reaction of acetic acid and sodium hydroxide, both of these precursors are often derived from non-renewable resources. The shift towards more sustainable feedstocks, such as bio-based acetic acid, is ongoing but faces hurdles in terms of cost-effectiveness and scalability.
Another significant challenge lies in the energy-intensive nature of sodium acetate production. Traditional manufacturing processes often require high temperatures and pressures, leading to substantial energy consumption and associated carbon emissions. Green chemistry initiatives are focusing on developing low-energy alternatives, such as room-temperature synthesis methods and the use of catalysts to reduce reaction temperatures.
Water consumption and waste generation in sodium acetate production also pose environmental concerns. Current production methods may involve significant water usage and generate aqueous waste streams that require treatment. Implementing closed-loop systems and developing water-efficient processes are key areas of focus for improving the sustainability of sodium acetate manufacturing.
The purity requirements for sodium acetate in certain applications, particularly in the pharmaceutical and food industries, present another challenge. Green chemistry approaches must ensure that environmentally friendly production methods do not compromise product quality or introduce impurities that could affect end-use performance.
Despite these challenges, there are promising developments in the field. Research into bio-based production methods, such as the use of waste biomass as a feedstock for acetic acid, shows potential for reducing the reliance on petrochemical sources. Additionally, advancements in process intensification and continuous flow chemistry are enabling more efficient and less resource-intensive production of sodium acetate.
The current state of sodium acetate implementation in green chemistry also reflects a growing interest in its use as a phase change material for thermal energy storage. This application aligns well with sustainable energy initiatives, but requires further research to optimize performance and address long-term stability issues.
Regulatory frameworks and industry standards play a crucial role in driving the adoption of green chemistry practices for sodium acetate. While there is increasing support for sustainable chemical processes, the lack of uniform global standards can hinder widespread implementation. Harmonizing regulations and providing incentives for green chemistry adoption could accelerate progress in this area.
One of the primary challenges in implementing sodium acetate in green chemistry is the sourcing of raw materials. While sodium acetate can be produced through the reaction of acetic acid and sodium hydroxide, both of these precursors are often derived from non-renewable resources. The shift towards more sustainable feedstocks, such as bio-based acetic acid, is ongoing but faces hurdles in terms of cost-effectiveness and scalability.
Another significant challenge lies in the energy-intensive nature of sodium acetate production. Traditional manufacturing processes often require high temperatures and pressures, leading to substantial energy consumption and associated carbon emissions. Green chemistry initiatives are focusing on developing low-energy alternatives, such as room-temperature synthesis methods and the use of catalysts to reduce reaction temperatures.
Water consumption and waste generation in sodium acetate production also pose environmental concerns. Current production methods may involve significant water usage and generate aqueous waste streams that require treatment. Implementing closed-loop systems and developing water-efficient processes are key areas of focus for improving the sustainability of sodium acetate manufacturing.
The purity requirements for sodium acetate in certain applications, particularly in the pharmaceutical and food industries, present another challenge. Green chemistry approaches must ensure that environmentally friendly production methods do not compromise product quality or introduce impurities that could affect end-use performance.
Despite these challenges, there are promising developments in the field. Research into bio-based production methods, such as the use of waste biomass as a feedstock for acetic acid, shows potential for reducing the reliance on petrochemical sources. Additionally, advancements in process intensification and continuous flow chemistry are enabling more efficient and less resource-intensive production of sodium acetate.
The current state of sodium acetate implementation in green chemistry also reflects a growing interest in its use as a phase change material for thermal energy storage. This application aligns well with sustainable energy initiatives, but requires further research to optimize performance and address long-term stability issues.
Regulatory frameworks and industry standards play a crucial role in driving the adoption of green chemistry practices for sodium acetate. While there is increasing support for sustainable chemical processes, the lack of uniform global standards can hinder widespread implementation. Harmonizing regulations and providing incentives for green chemistry adoption could accelerate progress in this area.
Existing Green Chemistry Solutions Using Sodium Acetate
01 Use of sodium acetate in chemical processes
Sodium acetate is widely used in various chemical processes as a reagent, catalyst, or buffer. It plays a role in reactions such as acetylation, esterification, and pH control. Its properties make it valuable in industrial applications and laboratory settings.- Use of sodium acetate in heat storage materials: Sodium acetate is utilized in heat storage materials due to its phase change properties. It can absorb and release heat during phase transitions, making it suitable for thermal energy storage applications. These materials can be used in various heating and cooling systems to improve energy efficiency.
- Sodium acetate in food preservation and packaging: Sodium acetate is employed in food preservation and packaging applications. It acts as a preservative and pH regulator, helping to extend the shelf life of various food products. Additionally, it can be incorporated into packaging materials to create active packaging systems that help maintain food quality.
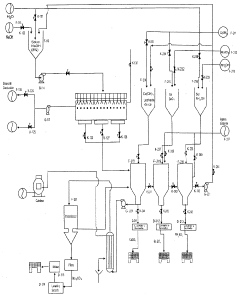
- Production methods for sodium acetate: Various methods are used to produce sodium acetate, including the reaction of acetic acid with sodium hydroxide or sodium carbonate. Some processes involve the use of catalysts or specific reaction conditions to improve yield and purity. These production methods aim to optimize efficiency and reduce costs in industrial-scale manufacturing.
- Sodium acetate in textile and fiber treatment: Sodium acetate finds applications in textile and fiber treatment processes. It can be used as a buffering agent in dyeing and finishing operations, helping to control pH levels and improve color fastness. Additionally, it may be employed in fiber modification techniques to enhance certain properties of textiles.
- Use of sodium acetate in environmental applications: Sodium acetate is utilized in various environmental applications, including wastewater treatment and air pollution control. It can serve as a carbon source for biological processes in wastewater treatment systems. In air pollution control, it may be used in scrubbing solutions to remove certain pollutants from gas streams.
02 Application in heat storage and thermal management
Sodium acetate trihydrate is utilized in heat storage systems and thermal management applications. It can absorb and release heat through phase change, making it useful in heat packs, building materials for temperature regulation, and energy storage solutions.Expand Specific Solutions03 Use in food and beverage industry
Sodium acetate serves as a food additive, acting as a preservative, acidity regulator, and flavoring agent. It is used in various food products to enhance shelf life, control pH, and improve taste profiles.Expand Specific Solutions04 Application in textile and paper industries
Sodium acetate finds applications in textile and paper industries for processes such as dyeing, printing, and paper sizing. It helps improve color fastness, ink absorption, and overall product quality in these sectors.Expand Specific Solutions05 Use in environmental and waste treatment
Sodium acetate is employed in environmental applications and waste treatment processes. It can be used for neutralizing acidic waste, as a deicer for roads, and in certain bioremediation techniques for contaminated soil and water.Expand Specific Solutions
Key Players in Sustainable Chemical Industry
The implementation of sodium acetate in green chemistry initiatives is currently in a growth phase, with increasing market size driven by the rising demand for sustainable chemical processes. The global market for green chemistry is projected to expand significantly in the coming years, with sodium acetate playing a crucial role in various applications. Technologically, the field is advancing rapidly, with companies like Ecolab USA, Inc. and Solvay SA leading the way in developing innovative solutions. Research institutions such as the Council of Scientific & Industrial Research and universities like the National University of Singapore are contributing to the advancement of sodium acetate applications in green chemistry. While the technology is relatively mature, ongoing research and development efforts are focused on improving efficiency and expanding its use across different industries.
Ecolab USA, Inc.
Technical Solution: Ecolab has incorporated sodium acetate into their green chemistry initiatives, particularly in their water treatment solutions. They have developed a proprietary technology called 3D TRASAR™ that uses sodium acetate as part of a smart water management system. This system optimizes water usage and reduces chemical consumption in industrial processes. Sodium acetate is used as a pH buffer and corrosion inhibitor, replacing more harmful chemicals. Additionally, Ecolab has implemented sodium acetate in their cleaning and sanitizing solutions for the food and beverage industry, where it serves as an environmentally friendly alternative to traditional chemicals[4][5].
Strengths: Wide-ranging applications across multiple industries; proven track record in water treatment and industrial cleaning. Weaknesses: May face challenges in scaling up production of sodium acetate-based solutions; potential regulatory hurdles in certain markets.
National University of Singapore
Technical Solution: Researchers at the National University of Singapore have developed a novel green chemistry approach using sodium acetate in the synthesis of metal-organic frameworks (MOFs). Their method employs sodium acetate as both a pH regulator and a structure-directing agent in the synthesis of highly porous MOFs. This approach significantly reduces the use of harmful solvents and improves the overall sustainability of MOF production. The resulting MOFs have shown promising applications in gas storage, catalysis, and environmental remediation. Additionally, the research team has explored the use of sodium acetate in the development of bio-based polymers, where it serves as a crosslinking agent, enhancing the mechanical properties of biodegradable materials[8][9].
Strengths: Cutting-edge research in materials science; potential for wide-ranging applications in green technology. Weaknesses: Research still in academic phase; may face challenges in translating to industrial-scale production.
Core Innovations in Sodium Acetate Applications
Drying agent
PatentInactiveUS20130066106A1
Innovation
- A drying agent with the formula [Mg2(BTEC)(H2O)m]·nH2O is produced through a direct mixing method using 1,2,4,5-benzenetetracarboxylic acid and magnesium hydroxide under ambient conditions, eliminating the need for solvents and by-products, and featuring high water sorption capacity.
Process for the removal of acid gases from the air and from combustion gases from burners and internal combustion engines by means of absorption with sodium hydroxide solution and process for obtaining sodium carbonate in order to acquire carbon cred
PatentWO2011122925A1
Innovation
- A chemical process using a 2N sodium hydroxide solution to absorb acid gases in a horizontal absorber, converting CO2 into sodium carbonate, which can be further processed to produce sodium carbonate, thereby accrediting carbon credits through the capture and commercialization of CO2.
Environmental Impact Assessment
The implementation of sodium acetate in green chemistry initiatives necessitates a comprehensive environmental impact assessment to ensure sustainable practices and minimize ecological footprints. This assessment begins with an evaluation of the raw materials used in sodium acetate production, primarily focusing on the sourcing of acetic acid and sodium hydroxide or sodium carbonate. Sustainable procurement strategies and the use of bio-based feedstocks can significantly reduce the environmental burden associated with these precursors.
The production process of sodium acetate itself requires scrutiny, with particular attention paid to energy consumption, water usage, and waste generation. Green chemistry principles advocate for the optimization of reaction conditions to maximize yield and minimize by-products. Implementation of energy-efficient technologies, such as heat recovery systems and advanced process control, can substantially decrease the carbon footprint of sodium acetate manufacturing.
Water management is a critical aspect of the environmental impact assessment. The use of closed-loop water systems and advanced purification technologies can minimize freshwater consumption and reduce the discharge of potentially harmful effluents. Additionally, the assessment should consider the potential for water recycling and the treatment of any wastewater generated during the production process.
Waste reduction and management strategies play a pivotal role in mitigating the environmental impact of sodium acetate production. This includes the implementation of recycling programs for packaging materials, the recovery and reuse of unreacted starting materials, and the exploration of potential applications for any by-products generated during the manufacturing process.
The transportation and distribution of sodium acetate also contribute to its overall environmental impact. Optimizing logistics, utilizing more fuel-efficient vehicles, and exploring alternative transportation methods can help reduce greenhouse gas emissions associated with product distribution. Furthermore, the assessment should consider the potential for localized production to minimize transportation distances and associated environmental costs.
End-of-life considerations for sodium acetate products are equally important in the environmental impact assessment. This includes evaluating the biodegradability of sodium acetate and its derivatives, as well as exploring potential recycling or upcycling opportunities for products containing sodium acetate. The assessment should also address the potential environmental impacts of sodium acetate disposal and its fate in various ecosystems.
Lastly, the environmental impact assessment should consider the broader implications of sodium acetate use in green chemistry initiatives. This includes evaluating its potential to replace more environmentally harmful substances in various applications, such as de-icing agents or pH regulators. By quantifying these potential benefits, a more comprehensive understanding of the net environmental impact of sodium acetate implementation can be achieved.
The production process of sodium acetate itself requires scrutiny, with particular attention paid to energy consumption, water usage, and waste generation. Green chemistry principles advocate for the optimization of reaction conditions to maximize yield and minimize by-products. Implementation of energy-efficient technologies, such as heat recovery systems and advanced process control, can substantially decrease the carbon footprint of sodium acetate manufacturing.
Water management is a critical aspect of the environmental impact assessment. The use of closed-loop water systems and advanced purification technologies can minimize freshwater consumption and reduce the discharge of potentially harmful effluents. Additionally, the assessment should consider the potential for water recycling and the treatment of any wastewater generated during the production process.
Waste reduction and management strategies play a pivotal role in mitigating the environmental impact of sodium acetate production. This includes the implementation of recycling programs for packaging materials, the recovery and reuse of unreacted starting materials, and the exploration of potential applications for any by-products generated during the manufacturing process.
The transportation and distribution of sodium acetate also contribute to its overall environmental impact. Optimizing logistics, utilizing more fuel-efficient vehicles, and exploring alternative transportation methods can help reduce greenhouse gas emissions associated with product distribution. Furthermore, the assessment should consider the potential for localized production to minimize transportation distances and associated environmental costs.
End-of-life considerations for sodium acetate products are equally important in the environmental impact assessment. This includes evaluating the biodegradability of sodium acetate and its derivatives, as well as exploring potential recycling or upcycling opportunities for products containing sodium acetate. The assessment should also address the potential environmental impacts of sodium acetate disposal and its fate in various ecosystems.
Lastly, the environmental impact assessment should consider the broader implications of sodium acetate use in green chemistry initiatives. This includes evaluating its potential to replace more environmentally harmful substances in various applications, such as de-icing agents or pH regulators. By quantifying these potential benefits, a more comprehensive understanding of the net environmental impact of sodium acetate implementation can be achieved.
Regulatory Framework for Green Chemical Processes
The regulatory framework for green chemical processes plays a crucial role in implementing sodium acetate in green chemistry initiatives. Governments and international organizations have established various regulations and guidelines to promote sustainable practices in the chemical industry. These regulations aim to minimize environmental impact, reduce waste generation, and enhance overall safety in chemical processes.
In the United States, the Environmental Protection Agency (EPA) has implemented the Green Chemistry Program, which provides a framework for developing and implementing green chemistry principles. This program encourages the use of alternative chemicals and processes that are environmentally friendly, including the utilization of sodium acetate as a safer alternative in various applications.
The European Union has adopted the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which requires companies to register and evaluate the safety of chemicals they use. This regulation promotes the use of safer alternatives, such as sodium acetate, in chemical processes. Additionally, the EU's Circular Economy Action Plan emphasizes the importance of sustainable chemical production and waste reduction, further supporting the implementation of green chemistry initiatives.
International standards, such as ISO 14001 for environmental management systems, provide guidelines for organizations to implement environmentally responsible practices. These standards can be applied to the use of sodium acetate in green chemistry initiatives, ensuring that its production and application adhere to sustainable principles.
The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) has been adopted by many countries to standardize the classification and labeling of chemicals. This system helps in identifying and communicating the hazards associated with chemicals, including sodium acetate, promoting safer handling and use in green chemistry applications.
Many countries have also implemented specific regulations targeting the reduction of volatile organic compounds (VOCs) and hazardous air pollutants. These regulations indirectly support the use of sodium acetate as a greener alternative in various industrial processes, as it has lower volatility and toxicity compared to some traditional chemicals.
The regulatory landscape for green chemistry is continuously evolving, with increasing emphasis on lifecycle assessments and circular economy principles. This evolution is likely to further support the implementation of sodium acetate in green chemistry initiatives, as it aligns well with these sustainability goals.
In the United States, the Environmental Protection Agency (EPA) has implemented the Green Chemistry Program, which provides a framework for developing and implementing green chemistry principles. This program encourages the use of alternative chemicals and processes that are environmentally friendly, including the utilization of sodium acetate as a safer alternative in various applications.
The European Union has adopted the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which requires companies to register and evaluate the safety of chemicals they use. This regulation promotes the use of safer alternatives, such as sodium acetate, in chemical processes. Additionally, the EU's Circular Economy Action Plan emphasizes the importance of sustainable chemical production and waste reduction, further supporting the implementation of green chemistry initiatives.
International standards, such as ISO 14001 for environmental management systems, provide guidelines for organizations to implement environmentally responsible practices. These standards can be applied to the use of sodium acetate in green chemistry initiatives, ensuring that its production and application adhere to sustainable principles.
The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) has been adopted by many countries to standardize the classification and labeling of chemicals. This system helps in identifying and communicating the hazards associated with chemicals, including sodium acetate, promoting safer handling and use in green chemistry applications.
Many countries have also implemented specific regulations targeting the reduction of volatile organic compounds (VOCs) and hazardous air pollutants. These regulations indirectly support the use of sodium acetate as a greener alternative in various industrial processes, as it has lower volatility and toxicity compared to some traditional chemicals.
The regulatory landscape for green chemistry is continuously evolving, with increasing emphasis on lifecycle assessments and circular economy principles. This evolution is likely to further support the implementation of sodium acetate in green chemistry initiatives, as it aligns well with these sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!