How to Optimize Heat Generation Using Sodium Acetate?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Heat Generation Background
Sodium acetate, a chemical compound with the formula CH3COONa, has gained significant attention in the field of heat generation due to its unique properties. This crystalline substance, also known as sodium ethanoate, is widely recognized for its ability to store and release thermal energy through a process called supercooling and crystallization.
The concept of using sodium acetate for heat generation dates back to the early 20th century, but it wasn't until recent decades that its potential for practical applications was fully realized. The compound's ability to remain in a liquid state below its melting point, and then rapidly crystallize when triggered, releasing latent heat, forms the basis of its heat generation mechanism.
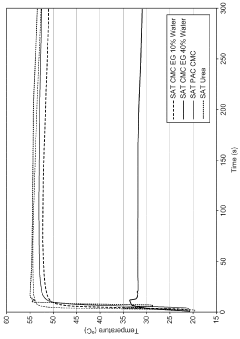
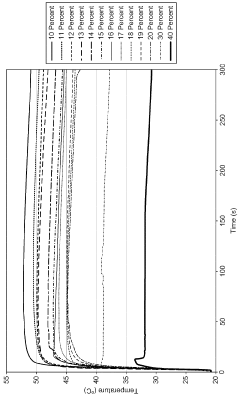
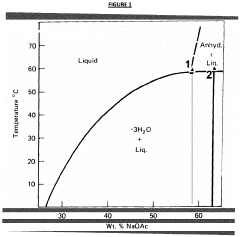
Sodium acetate's heat-generating properties are rooted in its phase change behavior. When heated above its melting point of 58°C (136.4°F), sodium acetate trihydrate dissolves in its own water of crystallization. As it cools, it can remain in a liquid state even below its freezing point, creating a supersaturated solution. This metastable state can be maintained until an external stimulus, such as a mechanical shock or the introduction of a seed crystal, triggers rapid crystallization.
The crystallization process is exothermic, releasing the latent heat of fusion stored during the melting process. This heat release can raise the temperature of the surrounding environment, making sodium acetate an effective heat source for various applications. The amount of heat released during crystallization is substantial, with sodium acetate trihydrate capable of storing up to 264–289 kJ/kg of thermal energy.
Over the years, researchers and engineers have explored various ways to optimize the heat generation process using sodium acetate. These efforts have focused on enhancing the supercooling stability, improving the triggering mechanism, and maximizing the heat output. Innovations in packaging and containment have also played a crucial role in making sodium acetate-based heat generation systems more practical and user-friendly.
The growing interest in sustainable and renewable energy sources has further propelled research into sodium acetate heat generation. Its potential as a thermal energy storage medium aligns well with the global push towards more efficient and environmentally friendly heating solutions. The compound's non-toxic nature and relatively low cost have also contributed to its appeal in both industrial and consumer applications.
As we delve deeper into the optimization of heat generation using sodium acetate, it is essential to consider the historical context and fundamental principles that underpin this technology. Understanding the background of sodium acetate heat generation provides a solid foundation for exploring innovative approaches to enhance its efficiency and expand its applications in various fields.
The concept of using sodium acetate for heat generation dates back to the early 20th century, but it wasn't until recent decades that its potential for practical applications was fully realized. The compound's ability to remain in a liquid state below its melting point, and then rapidly crystallize when triggered, releasing latent heat, forms the basis of its heat generation mechanism.
Sodium acetate's heat-generating properties are rooted in its phase change behavior. When heated above its melting point of 58°C (136.4°F), sodium acetate trihydrate dissolves in its own water of crystallization. As it cools, it can remain in a liquid state even below its freezing point, creating a supersaturated solution. This metastable state can be maintained until an external stimulus, such as a mechanical shock or the introduction of a seed crystal, triggers rapid crystallization.
The crystallization process is exothermic, releasing the latent heat of fusion stored during the melting process. This heat release can raise the temperature of the surrounding environment, making sodium acetate an effective heat source for various applications. The amount of heat released during crystallization is substantial, with sodium acetate trihydrate capable of storing up to 264–289 kJ/kg of thermal energy.
Over the years, researchers and engineers have explored various ways to optimize the heat generation process using sodium acetate. These efforts have focused on enhancing the supercooling stability, improving the triggering mechanism, and maximizing the heat output. Innovations in packaging and containment have also played a crucial role in making sodium acetate-based heat generation systems more practical and user-friendly.
The growing interest in sustainable and renewable energy sources has further propelled research into sodium acetate heat generation. Its potential as a thermal energy storage medium aligns well with the global push towards more efficient and environmentally friendly heating solutions. The compound's non-toxic nature and relatively low cost have also contributed to its appeal in both industrial and consumer applications.
As we delve deeper into the optimization of heat generation using sodium acetate, it is essential to consider the historical context and fundamental principles that underpin this technology. Understanding the background of sodium acetate heat generation provides a solid foundation for exploring innovative approaches to enhance its efficiency and expand its applications in various fields.
Market Analysis for Heat Generation Solutions
The market for heat generation solutions has been experiencing significant growth due to increasing energy demands and a shift towards more sustainable heating methods. Sodium acetate-based heat generation systems are emerging as a promising technology in this landscape, offering unique advantages over traditional heating solutions.
The global heat generation market is primarily driven by the residential and commercial sectors, with industrial applications also contributing substantially. As of recent estimates, the market size for heat generation solutions is projected to reach several billion dollars by 2025, with a compound annual growth rate (CAGR) exceeding 5%. This growth is attributed to factors such as urbanization, industrialization, and the need for energy-efficient heating systems.
Sodium acetate-based heat generation solutions are positioned to capture a growing share of this market. These systems leverage the exothermic crystallization process of sodium acetate trihydrate, offering advantages such as reusability, non-toxicity, and high energy density. The market potential for sodium acetate heat generation is particularly strong in applications requiring portable or on-demand heat sources, such as hand warmers, therapeutic devices, and emergency heating solutions.
Consumer awareness and demand for eco-friendly heating alternatives are driving the adoption of sodium acetate-based systems. The technology aligns well with the global push for sustainable energy solutions, as it does not produce harmful emissions and can be recharged using various energy sources, including renewable ones.
In the commercial sector, sodium acetate heat generation systems are finding applications in food service, where they can be used to keep meals warm during transport or in buffet settings. The healthcare industry is another promising market, with potential uses in medical devices and patient care products that require controlled heat application.
The industrial sector presents opportunities for large-scale sodium acetate heat generation systems, particularly in processes that require consistent, low-grade heat. These could include applications in chemical processing, waste heat recovery, and thermal energy storage systems.
However, the market for sodium acetate heat generation faces competition from other emerging technologies, such as phase change materials (PCMs) and advanced thermoelectric devices. To gain a competitive edge, manufacturers of sodium acetate-based systems must focus on optimizing heat generation efficiency, improving the activation mechanism, and enhancing the overall user experience.
Geographically, North America and Europe are expected to be the leading markets for advanced heat generation solutions, including those based on sodium acetate. These regions have stringent energy efficiency regulations and a high adoption rate of innovative technologies. Asia-Pacific is anticipated to be the fastest-growing market, driven by rapid industrialization and increasing energy demands in countries like China and India.
The global heat generation market is primarily driven by the residential and commercial sectors, with industrial applications also contributing substantially. As of recent estimates, the market size for heat generation solutions is projected to reach several billion dollars by 2025, with a compound annual growth rate (CAGR) exceeding 5%. This growth is attributed to factors such as urbanization, industrialization, and the need for energy-efficient heating systems.
Sodium acetate-based heat generation solutions are positioned to capture a growing share of this market. These systems leverage the exothermic crystallization process of sodium acetate trihydrate, offering advantages such as reusability, non-toxicity, and high energy density. The market potential for sodium acetate heat generation is particularly strong in applications requiring portable or on-demand heat sources, such as hand warmers, therapeutic devices, and emergency heating solutions.
Consumer awareness and demand for eco-friendly heating alternatives are driving the adoption of sodium acetate-based systems. The technology aligns well with the global push for sustainable energy solutions, as it does not produce harmful emissions and can be recharged using various energy sources, including renewable ones.
In the commercial sector, sodium acetate heat generation systems are finding applications in food service, where they can be used to keep meals warm during transport or in buffet settings. The healthcare industry is another promising market, with potential uses in medical devices and patient care products that require controlled heat application.
The industrial sector presents opportunities for large-scale sodium acetate heat generation systems, particularly in processes that require consistent, low-grade heat. These could include applications in chemical processing, waste heat recovery, and thermal energy storage systems.
However, the market for sodium acetate heat generation faces competition from other emerging technologies, such as phase change materials (PCMs) and advanced thermoelectric devices. To gain a competitive edge, manufacturers of sodium acetate-based systems must focus on optimizing heat generation efficiency, improving the activation mechanism, and enhancing the overall user experience.
Geographically, North America and Europe are expected to be the leading markets for advanced heat generation solutions, including those based on sodium acetate. These regions have stringent energy efficiency regulations and a high adoption rate of innovative technologies. Asia-Pacific is anticipated to be the fastest-growing market, driven by rapid industrialization and increasing energy demands in countries like China and India.
Current Challenges in Sodium Acetate Heat Technology
Despite the promising potential of sodium acetate as a heat storage medium, several challenges currently hinder its widespread adoption and optimal utilization. One of the primary issues is the inconsistent crystallization process, which can lead to unpredictable heat release patterns. This inconsistency affects the reliability and efficiency of heat generation systems, making it difficult to design and implement large-scale applications.
Another significant challenge is the limited heat storage capacity of sodium acetate compared to other phase change materials. While sodium acetate offers advantages in terms of safety and cost-effectiveness, its energy density is relatively low. This limitation necessitates larger storage volumes, which can be problematic in space-constrained applications or when trying to achieve high power outputs.
The supercooling phenomenon associated with sodium acetate presents both opportunities and challenges. While it allows for long-term heat storage without insulation, initiating the crystallization process on demand can be problematic. Current triggering mechanisms are not always reliable, leading to potential delays or failures in heat generation when needed.
Thermal cycling stability is another area of concern. Repeated melting and crystallization cycles can lead to phase separation and degradation of the sodium acetate mixture over time. This degradation affects the long-term performance and reliability of heat storage systems, potentially increasing maintenance requirements and reducing overall system lifespan.
Heat transfer efficiency during both the charging and discharging phases remains a challenge. The low thermal conductivity of sodium acetate can result in slow heat transfer rates, limiting the speed at which heat can be stored or released. This issue is particularly problematic in applications requiring rapid heat generation or absorption.
Containment and packaging of sodium acetate heat storage systems also present technical difficulties. The corrosive nature of the material when in its liquid state can lead to container degradation over time. Additionally, ensuring proper sealing to prevent moisture ingress, which can affect the supercooling properties, is crucial but challenging to maintain over extended periods.
Scaling up sodium acetate heat storage systems for industrial or large-scale applications faces several engineering challenges. These include designing efficient heat exchangers, managing large volumes of the phase change material, and integrating the systems with existing infrastructure. The lack of standardized design principles and best practices for large-scale implementations further complicates the scaling process.
Another significant challenge is the limited heat storage capacity of sodium acetate compared to other phase change materials. While sodium acetate offers advantages in terms of safety and cost-effectiveness, its energy density is relatively low. This limitation necessitates larger storage volumes, which can be problematic in space-constrained applications or when trying to achieve high power outputs.
The supercooling phenomenon associated with sodium acetate presents both opportunities and challenges. While it allows for long-term heat storage without insulation, initiating the crystallization process on demand can be problematic. Current triggering mechanisms are not always reliable, leading to potential delays or failures in heat generation when needed.
Thermal cycling stability is another area of concern. Repeated melting and crystallization cycles can lead to phase separation and degradation of the sodium acetate mixture over time. This degradation affects the long-term performance and reliability of heat storage systems, potentially increasing maintenance requirements and reducing overall system lifespan.
Heat transfer efficiency during both the charging and discharging phases remains a challenge. The low thermal conductivity of sodium acetate can result in slow heat transfer rates, limiting the speed at which heat can be stored or released. This issue is particularly problematic in applications requiring rapid heat generation or absorption.
Containment and packaging of sodium acetate heat storage systems also present technical difficulties. The corrosive nature of the material when in its liquid state can lead to container degradation over time. Additionally, ensuring proper sealing to prevent moisture ingress, which can affect the supercooling properties, is crucial but challenging to maintain over extended periods.
Scaling up sodium acetate heat storage systems for industrial or large-scale applications faces several engineering challenges. These include designing efficient heat exchangers, managing large volumes of the phase change material, and integrating the systems with existing infrastructure. The lack of standardized design principles and best practices for large-scale implementations further complicates the scaling process.
Existing Sodium Acetate Heat Generation Methods
01 Heat generation mechanism of sodium acetate
Sodium acetate trihydrate undergoes an exothermic crystallization process when triggered, releasing heat. This process involves the transition from a supersaturated liquid state to a crystalline solid state, which is the basis for its use in heat packs and thermal energy storage applications.- Heat generation mechanisms using sodium acetate: Sodium acetate is used in heat generation devices due to its ability to release heat when crystallizing from a supersaturated solution. This process, known as crystallization or solidification, is triggered by activating a metal disc or introducing a seed crystal, resulting in an exothermic reaction that produces heat.
- Reusable heat packs incorporating sodium acetate: Reusable heat packs often utilize sodium acetate trihydrate as the heat-generating material. These packs can be reset by heating to dissolve the crystals and then cooled to create a supersaturated solution. When activated, they provide instant warmth and can be used multiple times, making them efficient for various applications.
- Sodium acetate heat generation in medical applications: Sodium acetate-based heat generation is employed in medical devices and treatments. These include thermal therapy packs, warming blankets, and temperature-controlled medical equipment. The controlled and consistent heat release properties of sodium acetate make it suitable for maintaining specific temperatures in medical settings.
- Industrial and commercial uses of sodium acetate heat generation: Sodium acetate heat generation finds applications in various industrial and commercial sectors. It is used in food warming systems, hand warmers, automotive defrosting devices, and even in certain types of energy storage systems. The versatility of sodium acetate allows for its integration into diverse heat-requiring processes.
- Innovations in sodium acetate heat generation technology: Recent innovations focus on improving the efficiency and controllability of sodium acetate heat generation. These advancements include developing new activation methods, enhancing heat retention, improving the reusability of the solutions, and creating composite materials that combine sodium acetate with other heat-generating substances for optimized performance.
02 Design of sodium acetate heat packs
Heat packs utilizing sodium acetate often incorporate a metal trigger or activation mechanism to initiate crystallization. The design may include flexible containers, insulation layers, and specific shapes to optimize heat distribution and user comfort. Some designs feature reusable elements or integrate with wearable items for convenience.Expand Specific Solutions03 Applications in thermal management systems
Sodium acetate heat generation is applied in various thermal management systems, including temperature-controlled packaging, building heating systems, and industrial process heat recovery. These applications leverage the material's phase change properties for efficient heat storage and release, contributing to energy conservation efforts.Expand Specific Solutions04 Enhancement of sodium acetate heat generation efficiency
Research focuses on improving the efficiency of sodium acetate heat generation through various methods. These include the addition of nucleating agents to promote faster crystallization, modification of the chemical composition to adjust phase change temperatures, and development of composite materials to enhance thermal conductivity and heat storage capacity.Expand Specific Solutions05 Integration with renewable energy systems
Sodium acetate heat generation is being integrated with renewable energy systems for thermal energy storage. This integration allows for the storage of excess heat from solar or wind energy sources, which can be released on demand. Such systems contribute to the overall efficiency and reliability of renewable energy installations by addressing intermittency issues.Expand Specific Solutions
Key Players in Chemical Heat Generation Industry
The optimization of heat generation using sodium acetate is in a developing stage, with a growing market driven by increasing demand for sustainable energy solutions. The technology's maturity is moderate, with ongoing research and development efforts. Key players like Sunamp Ltd. and BSH Hausgeräte GmbH are leading innovation in thermal energy storage using phase change materials. Companies such as LG Chem Ltd. and LOTTE Chemical Corp. are contributing to the advancement of materials science in this field. The competitive landscape is diverse, with both established corporations and specialized firms like Anhui Ruibai New Material Co., Ltd. actively participating in research and commercialization efforts.
Dow Global Technologies LLC
Technical Solution: Dow has developed innovative encapsulation techniques for sodium acetate-based phase change materials (PCMs) to optimize heat generation and storage. Their approach involves microencapsulating sodium acetate within a polymer shell, creating a stable and easily handled form of the PCM. This encapsulation prevents leakage and improves thermal conductivity, allowing for more efficient heat transfer. Dow's technology enables the integration of sodium acetate PCMs into various building materials and textiles, expanding their application range. The company has also focused on enhancing the thermal cycling stability of sodium acetate, reportedly achieving over 10,000 melt-freeze cycles without significant performance loss[4][5].
Strengths: Versatile application in various materials, improved handling, and enhanced thermal stability. Weaknesses: Potential reduction in overall heat storage capacity due to encapsulation material volume.
Panasonic Holdings Corp.
Technical Solution: Panasonic has developed a sodium acetate-based heat storage system for residential and commercial applications. Their technology focuses on optimizing heat generation through precise control of the crystallization process. By using a combination of mechanical and thermal triggers, Panasonic's system can initiate crystallization on demand, allowing for efficient heat release when needed. The company has also implemented a cascading design, where multiple sodium acetate modules with slightly different melting points are used to provide a more consistent heat output over a broader temperature range. Panasonic's heat storage units can reportedly store up to 5 times more thermal energy than water-based systems of the same volume[6][7].
Strengths: High energy density, on-demand heat release, and consistent output across a wide temperature range. Weaknesses: Complexity of the control system and potential higher maintenance requirements.
Innovative Approaches in Sodium Acetate Heat Optimization
Sodium acetate trihydrate formulations
PatentWO2015001101A1
Innovation
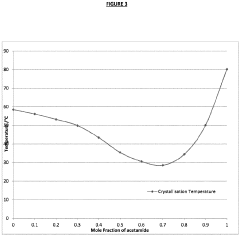
- Incorporating a kinetic inhibitor like sodium carboxymethyl cellulose and a solvent like ethylene glycol into the SAT formulations to enhance stability and maintain the phase change reaction temperature, with specific ratios of these additives to achieve increased stability and thermal output.
Improved phase change compositions
PatentPendingEP4023732A1
Innovation
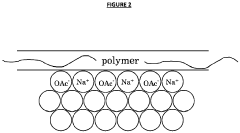
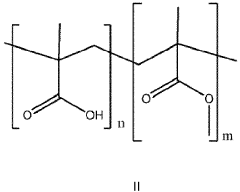
- The development of aqueous compositions containing sodium acetate trihydrate, combined with alkali soluble polymers to inhibit sodium acetate anhydrous crystal formation and nucleation promoters to promote stable nucleation, which enhances homogeneity and thermodynamic stability, preventing solid sodium acetate formation and maintaining a fully liquid state at 58°C.
Environmental Impact of Sodium Acetate Heat Solutions
The environmental impact of sodium acetate heat solutions is a crucial consideration in the optimization of heat generation using this compound. Sodium acetate, when used in heat packs and other thermal energy storage applications, offers several environmental advantages over traditional heating methods. However, it also presents some challenges that need to be addressed for sustainable implementation.
One of the primary environmental benefits of sodium acetate heat solutions is their reusability. Unlike disposable heat packs, sodium acetate-based systems can be recharged and reused multiple times, significantly reducing waste generation. This characteristic aligns with circular economy principles and helps minimize the overall environmental footprint of heat generation processes.
Furthermore, sodium acetate is non-toxic and biodegradable, making it an environmentally friendly alternative to many other heat storage materials. In the event of accidental release, it poses minimal risk to ecosystems and does not contribute to long-term environmental pollution. This property is particularly valuable in applications where the risk of leakage or disposal in natural environments is a concern.
The production of sodium acetate itself has a relatively low environmental impact compared to some other heat storage materials. It can be synthesized from readily available and renewable resources, such as acetic acid derived from biomass fermentation. This aspect contributes to the overall sustainability of sodium acetate heat solutions.
However, the environmental impact of sodium acetate heat solutions is not entirely benign. The energy required for the initial production of sodium acetate and the manufacturing of heat packs or other containment systems must be considered in the overall environmental assessment. Efforts to optimize these production processes and utilize renewable energy sources can further improve the environmental profile of these solutions.
Another potential environmental concern is the disposal of sodium acetate solutions at the end of their useful life. While biodegradable, improper disposal in large quantities could potentially affect local water systems by altering pH levels or nutrient balances. Implementing proper recycling and disposal protocols is essential to mitigate these risks.
The use of sodium acetate heat solutions can also indirectly contribute to environmental benefits by reducing reliance on fossil fuel-based heating methods. In applications where sodium acetate replaces traditional heating systems, it can lead to decreased greenhouse gas emissions and reduced consumption of non-renewable resources.
In conclusion, while sodium acetate heat solutions offer significant environmental advantages, their implementation must be carefully managed to maximize benefits and minimize potential negative impacts. Continued research and development in this area should focus on improving production efficiency, enhancing reusability, and developing comprehensive lifecycle management strategies to ensure the most environmentally sustainable use of this technology.
One of the primary environmental benefits of sodium acetate heat solutions is their reusability. Unlike disposable heat packs, sodium acetate-based systems can be recharged and reused multiple times, significantly reducing waste generation. This characteristic aligns with circular economy principles and helps minimize the overall environmental footprint of heat generation processes.
Furthermore, sodium acetate is non-toxic and biodegradable, making it an environmentally friendly alternative to many other heat storage materials. In the event of accidental release, it poses minimal risk to ecosystems and does not contribute to long-term environmental pollution. This property is particularly valuable in applications where the risk of leakage or disposal in natural environments is a concern.
The production of sodium acetate itself has a relatively low environmental impact compared to some other heat storage materials. It can be synthesized from readily available and renewable resources, such as acetic acid derived from biomass fermentation. This aspect contributes to the overall sustainability of sodium acetate heat solutions.
However, the environmental impact of sodium acetate heat solutions is not entirely benign. The energy required for the initial production of sodium acetate and the manufacturing of heat packs or other containment systems must be considered in the overall environmental assessment. Efforts to optimize these production processes and utilize renewable energy sources can further improve the environmental profile of these solutions.
Another potential environmental concern is the disposal of sodium acetate solutions at the end of their useful life. While biodegradable, improper disposal in large quantities could potentially affect local water systems by altering pH levels or nutrient balances. Implementing proper recycling and disposal protocols is essential to mitigate these risks.
The use of sodium acetate heat solutions can also indirectly contribute to environmental benefits by reducing reliance on fossil fuel-based heating methods. In applications where sodium acetate replaces traditional heating systems, it can lead to decreased greenhouse gas emissions and reduced consumption of non-renewable resources.
In conclusion, while sodium acetate heat solutions offer significant environmental advantages, their implementation must be carefully managed to maximize benefits and minimize potential negative impacts. Continued research and development in this area should focus on improving production efficiency, enhancing reusability, and developing comprehensive lifecycle management strategies to ensure the most environmentally sustainable use of this technology.
Safety Considerations in Chemical Heat Generation
When considering the optimization of heat generation using sodium acetate, safety is paramount. The process involves exothermic crystallization, which can produce significant heat rapidly. Proper handling and storage of sodium acetate are crucial to prevent accidental activation and ensure controlled heat release. The supersaturated solution should be stored in sealed containers to avoid contamination or premature crystallization.
Personal protective equipment (PPE) is essential when working with sodium acetate. This includes safety goggles, gloves, and appropriate clothing to protect against potential splashes or spills. The workspace should be well-ventilated to manage any fumes or vapors that may be produced during the heat generation process.
Temperature control is a critical safety aspect. The heat generated can reach temperatures up to 54°C (130°F), which can cause burns if not properly managed. Implementing temperature monitoring systems and establishing safe handling procedures are necessary to prevent accidents. It's also important to have cooling mechanisms in place to quickly reduce temperatures if needed.
The pH of sodium acetate solutions is typically around 7.5 to 9.0, which is mildly alkaline. While this is generally not hazardous, prolonged skin contact should be avoided. In case of contact, thorough rinsing with water is recommended. Eye exposure requires immediate flushing with water for at least 15 minutes and seeking medical attention.
Fire safety is another consideration. Although sodium acetate itself is not flammable, the heat generated could potentially ignite nearby combustible materials. Proper fire suppression equipment should be readily available, and heat-generating devices should be kept away from flammable substances.
Disposal of sodium acetate solutions must be done responsibly. While it is generally considered non-toxic and biodegradable, large quantities should not be disposed of in regular waste streams. Consulting local regulations and following proper chemical disposal procedures is essential to ensure environmental safety.
Training personnel on the safe handling and use of sodium acetate heat packs is crucial. This includes understanding the activation process, recognizing potential hazards, and knowing emergency procedures. Regular safety audits and updates to protocols based on new research or incidents in the field should be implemented to maintain a high standard of safety.
Personal protective equipment (PPE) is essential when working with sodium acetate. This includes safety goggles, gloves, and appropriate clothing to protect against potential splashes or spills. The workspace should be well-ventilated to manage any fumes or vapors that may be produced during the heat generation process.
Temperature control is a critical safety aspect. The heat generated can reach temperatures up to 54°C (130°F), which can cause burns if not properly managed. Implementing temperature monitoring systems and establishing safe handling procedures are necessary to prevent accidents. It's also important to have cooling mechanisms in place to quickly reduce temperatures if needed.
The pH of sodium acetate solutions is typically around 7.5 to 9.0, which is mildly alkaline. While this is generally not hazardous, prolonged skin contact should be avoided. In case of contact, thorough rinsing with water is recommended. Eye exposure requires immediate flushing with water for at least 15 minutes and seeking medical attention.
Fire safety is another consideration. Although sodium acetate itself is not flammable, the heat generated could potentially ignite nearby combustible materials. Proper fire suppression equipment should be readily available, and heat-generating devices should be kept away from flammable substances.
Disposal of sodium acetate solutions must be done responsibly. While it is generally considered non-toxic and biodegradable, large quantities should not be disposed of in regular waste streams. Consulting local regulations and following proper chemical disposal procedures is essential to ensure environmental safety.
Training personnel on the safe handling and use of sodium acetate heat packs is crucial. This includes understanding the activation process, recognizing potential hazards, and knowing emergency procedures. Regular safety audits and updates to protocols based on new research or incidents in the field should be implemented to maintain a high standard of safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!