How To Quantify Lithium Hydroxide Purity Using Titration
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Titration Background and Objectives
Lithium hydroxide (LiOH) has emerged as a critical material in the global energy transition, particularly in the production of high-performance lithium-ion batteries for electric vehicles and renewable energy storage systems. The evolution of lithium hydroxide quantification techniques spans several decades, with titration methods representing one of the most established and reliable approaches for purity determination. Initially developed in the mid-20th century, these analytical methods have undergone significant refinement to meet the increasingly stringent quality requirements of modern industrial applications.
The technological trajectory of lithium hydroxide analysis reflects broader trends in analytical chemistry, moving from basic acid-base titrations to more sophisticated potentiometric and colorimetric techniques. This evolution has been driven by the growing demand for higher purity lithium compounds, as even minor impurities can significantly impact battery performance, safety, and longevity. The industry has progressively raised purity standards from 99.0% to 99.9% and beyond, necessitating more precise quantification methods.
Current titration techniques for lithium hydroxide purity assessment typically involve neutralization reactions with standardized acids, often employing indicators or potentiometric endpoints to determine the equivalence point. These methods leverage the fundamental chemical properties of lithium hydroxide as a strong base that reacts quantitatively with acids. The technological objective in this field is to develop titration protocols that offer enhanced precision, reproducibility, and sensitivity while minimizing interference from common impurities such as sodium, potassium, and calcium compounds.
Recent advancements in titration technology have incorporated automated systems, improved endpoint detection algorithms, and specialized electrode configurations designed specifically for lithium compound analysis. These innovations aim to address the challenges of accurately quantifying lithium hydroxide in the presence of carbonate contamination—a persistent issue in industrial settings due to the hygroscopic nature of lithium hydroxide and its tendency to absorb atmospheric carbon dioxide.
The technical goals for lithium hydroxide titration methods include achieving detection limits below 0.05% for impurities, ensuring reproducibility within ±0.1% across different operators and laboratories, and developing protocols that can be readily implemented in both research and production environments. Additionally, there is growing interest in developing rapid titration methods that can support high-throughput quality control processes in battery material manufacturing facilities, where timely purity verification is essential for maintaining production efficiency.
The technological trajectory of lithium hydroxide analysis reflects broader trends in analytical chemistry, moving from basic acid-base titrations to more sophisticated potentiometric and colorimetric techniques. This evolution has been driven by the growing demand for higher purity lithium compounds, as even minor impurities can significantly impact battery performance, safety, and longevity. The industry has progressively raised purity standards from 99.0% to 99.9% and beyond, necessitating more precise quantification methods.
Current titration techniques for lithium hydroxide purity assessment typically involve neutralization reactions with standardized acids, often employing indicators or potentiometric endpoints to determine the equivalence point. These methods leverage the fundamental chemical properties of lithium hydroxide as a strong base that reacts quantitatively with acids. The technological objective in this field is to develop titration protocols that offer enhanced precision, reproducibility, and sensitivity while minimizing interference from common impurities such as sodium, potassium, and calcium compounds.
Recent advancements in titration technology have incorporated automated systems, improved endpoint detection algorithms, and specialized electrode configurations designed specifically for lithium compound analysis. These innovations aim to address the challenges of accurately quantifying lithium hydroxide in the presence of carbonate contamination—a persistent issue in industrial settings due to the hygroscopic nature of lithium hydroxide and its tendency to absorb atmospheric carbon dioxide.
The technical goals for lithium hydroxide titration methods include achieving detection limits below 0.05% for impurities, ensuring reproducibility within ±0.1% across different operators and laboratories, and developing protocols that can be readily implemented in both research and production environments. Additionally, there is growing interest in developing rapid titration methods that can support high-throughput quality control processes in battery material manufacturing facilities, where timely purity verification is essential for maintaining production efficiency.
Market Demand for High-Purity Lithium Hydroxide Analysis
The global lithium market has witnessed unprecedented growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry and renewable energy storage systems. Within this ecosystem, high-purity lithium hydroxide has emerged as a critical material, particularly for the production of high-performance cathode materials used in lithium-ion batteries. Market research indicates that the global demand for battery-grade lithium hydroxide (≥99.5% purity) is expected to grow at a compound annual growth rate of 18-20% through 2030.
The EV sector represents the largest consumer of high-purity lithium hydroxide, accounting for approximately 65% of total demand. This is attributed to the industry's shift toward nickel-rich cathode chemistries (NMC 811, NCA) which require lithium hydroxide rather than lithium carbonate due to lower processing temperatures and superior performance characteristics. Major automakers have announced ambitious electrification targets, with several planning to achieve 50-100% electric vehicle production by 2030-2035.
Energy storage systems constitute the second-largest market segment, representing about 20% of high-purity lithium hydroxide demand. Grid-scale storage installations grew by 62% in 2022 alone, with projections indicating continued robust growth as renewable energy integration accelerates globally.
The pharmaceutical and industrial sectors collectively account for the remaining 15% of market demand, utilizing high-purity lithium hydroxide in applications ranging from specialized lubricants to air purification systems and certain pharmaceutical formulations.
Quality control has become increasingly stringent across all these sectors, with manufacturers demanding lithium hydroxide that meets or exceeds 99.5% purity, with precisely quantified impurity profiles. This trend has intensified the need for accurate, reliable analytical methods for purity determination, with titration remaining the industry standard despite advances in instrumental techniques.
Regional analysis reveals that Asia-Pacific dominates the consumption landscape, accounting for over 70% of global high-purity lithium hydroxide demand, primarily due to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America and Europe are experiencing the fastest growth rates as they establish domestic battery supply chains to reduce dependence on Asian imports.
Price sensitivity analysis indicates that while high-purity lithium hydroxide commands a premium of 15-25% over standard grades, manufacturers are willing to absorb these costs due to the performance benefits and quality assurance they provide. This premium pricing structure has created strong economic incentives for producers to invest in advanced purification technologies and precise analytical methods.
The EV sector represents the largest consumer of high-purity lithium hydroxide, accounting for approximately 65% of total demand. This is attributed to the industry's shift toward nickel-rich cathode chemistries (NMC 811, NCA) which require lithium hydroxide rather than lithium carbonate due to lower processing temperatures and superior performance characteristics. Major automakers have announced ambitious electrification targets, with several planning to achieve 50-100% electric vehicle production by 2030-2035.
Energy storage systems constitute the second-largest market segment, representing about 20% of high-purity lithium hydroxide demand. Grid-scale storage installations grew by 62% in 2022 alone, with projections indicating continued robust growth as renewable energy integration accelerates globally.
The pharmaceutical and industrial sectors collectively account for the remaining 15% of market demand, utilizing high-purity lithium hydroxide in applications ranging from specialized lubricants to air purification systems and certain pharmaceutical formulations.
Quality control has become increasingly stringent across all these sectors, with manufacturers demanding lithium hydroxide that meets or exceeds 99.5% purity, with precisely quantified impurity profiles. This trend has intensified the need for accurate, reliable analytical methods for purity determination, with titration remaining the industry standard despite advances in instrumental techniques.
Regional analysis reveals that Asia-Pacific dominates the consumption landscape, accounting for over 70% of global high-purity lithium hydroxide demand, primarily due to the concentration of battery manufacturing facilities in China, South Korea, and Japan. North America and Europe are experiencing the fastest growth rates as they establish domestic battery supply chains to reduce dependence on Asian imports.
Price sensitivity analysis indicates that while high-purity lithium hydroxide commands a premium of 15-25% over standard grades, manufacturers are willing to absorb these costs due to the performance benefits and quality assurance they provide. This premium pricing structure has created strong economic incentives for producers to invest in advanced purification technologies and precise analytical methods.
Current Titration Methods and Technical Limitations
Titration remains the predominant analytical method for quantifying lithium hydroxide purity in industrial settings. The most widely employed technique is acid-base titration, where a standardized acid solution (typically hydrochloric acid or sulfuric acid) is used to neutralize the lithium hydroxide sample. The endpoint is determined using indicators such as phenolphthalein, which changes from pink to colorless as the solution transitions from basic to acidic, or through potentiometric measurements that track pH changes during titration.
Automated titrators have significantly improved the precision and reproducibility of these measurements, reducing human error and increasing throughput. Modern systems incorporate features like automatic endpoint detection, data logging, and statistical analysis capabilities, making them valuable tools in quality control laboratories across the lithium production industry.
Despite these advancements, current titration methods face several technical limitations. The accuracy of titration results is highly dependent on sample preparation techniques, particularly in handling hygroscopic lithium hydroxide that readily absorbs atmospheric moisture and carbon dioxide. This absorption can lead to the formation of lithium carbonate, which affects the titration results and creates systematic errors in purity assessments.
Another significant challenge is the difficulty in distinguishing between lithium hydroxide and other alkaline impurities present in the sample. Traditional acid-base titration measures total alkalinity rather than specifically quantifying lithium hydroxide content. This limitation becomes particularly problematic when analyzing technical-grade lithium hydroxide, which may contain varying levels of sodium, potassium, and calcium compounds that contribute to the overall alkalinity measurement.
The detection and quantification of trace impurities present another technical hurdle. While titration excels at determining major components, it lacks the sensitivity required for detecting low-concentration impurities that can significantly impact the performance of lithium hydroxide in battery applications. This limitation necessitates complementary analytical techniques for comprehensive purity assessment.
Endpoint detection accuracy represents another challenge, particularly in colored or turbid samples where visual indicators may be difficult to observe. While potentiometric titration addresses this issue to some extent, it introduces complexity and requires careful calibration and maintenance of pH electrodes to ensure reliable results.
Standardization of titration procedures across the industry remains inconsistent, with variations in methodology leading to discrepancies in reported purity values between different laboratories. This lack of standardization complicates quality assurance efforts and creates challenges in establishing universal specifications for lithium hydroxide products in the rapidly expanding battery market.
Automated titrators have significantly improved the precision and reproducibility of these measurements, reducing human error and increasing throughput. Modern systems incorporate features like automatic endpoint detection, data logging, and statistical analysis capabilities, making them valuable tools in quality control laboratories across the lithium production industry.
Despite these advancements, current titration methods face several technical limitations. The accuracy of titration results is highly dependent on sample preparation techniques, particularly in handling hygroscopic lithium hydroxide that readily absorbs atmospheric moisture and carbon dioxide. This absorption can lead to the formation of lithium carbonate, which affects the titration results and creates systematic errors in purity assessments.
Another significant challenge is the difficulty in distinguishing between lithium hydroxide and other alkaline impurities present in the sample. Traditional acid-base titration measures total alkalinity rather than specifically quantifying lithium hydroxide content. This limitation becomes particularly problematic when analyzing technical-grade lithium hydroxide, which may contain varying levels of sodium, potassium, and calcium compounds that contribute to the overall alkalinity measurement.
The detection and quantification of trace impurities present another technical hurdle. While titration excels at determining major components, it lacks the sensitivity required for detecting low-concentration impurities that can significantly impact the performance of lithium hydroxide in battery applications. This limitation necessitates complementary analytical techniques for comprehensive purity assessment.
Endpoint detection accuracy represents another challenge, particularly in colored or turbid samples where visual indicators may be difficult to observe. While potentiometric titration addresses this issue to some extent, it introduces complexity and requires careful calibration and maintenance of pH electrodes to ensure reliable results.
Standardization of titration procedures across the industry remains inconsistent, with variations in methodology leading to discrepancies in reported purity values between different laboratories. This lack of standardization complicates quality assurance efforts and creates challenges in establishing universal specifications for lithium hydroxide products in the rapidly expanding battery market.
Standard Titration Protocols for Lithium Hydroxide Purity Assessment
01 Acid-base titration methods for lithium hydroxide purity determination
Acid-base titration is a common analytical method for determining the purity of lithium hydroxide. This typically involves using standardized acids such as hydrochloric acid or sulfuric acid as titrants, with indicators like phenolphthalein to detect the endpoint. The method allows for precise quantification of lithium hydroxide content by measuring the amount of acid required to neutralize the base, with results typically expressed as percentage purity. These methods are widely used in quality control processes for lithium hydroxide production.- Acid-base titration methods for lithium hydroxide purity determination: Acid-base titration is a common analytical method used to determine the purity of lithium hydroxide. This typically involves titrating a lithium hydroxide sample with a standardized acid solution (such as hydrochloric acid or sulfuric acid) to a specific endpoint, often using indicators like phenolphthalein. The titration allows for the quantitative determination of hydroxide content, which directly correlates to the purity of the lithium hydroxide sample. This method is widely used in quality control processes for lithium hydroxide production.
- Automated titration systems for lithium hydroxide analysis: Advanced automated titration systems have been developed specifically for lithium hydroxide purity analysis. These systems incorporate precise dispensing mechanisms, potentiometric detection, and specialized software for endpoint determination. Automated titration provides higher accuracy, better reproducibility, and reduced operator error compared to manual methods. These systems can also perform multiple analyses in sequence, improving laboratory efficiency and throughput for quality control in lithium compound manufacturing.
- Potentiometric titration techniques for lithium hydroxide: Potentiometric titration techniques utilize electrode potential measurements to determine the endpoint in lithium hydroxide purity analysis. This method offers greater precision than visual indicators, especially for samples with impurities that might interfere with color changes. The technique involves monitoring the potential difference between reference and indicator electrodes during acid addition, with the endpoint identified by a sharp inflection in the potential curve. This approach is particularly valuable for high-purity lithium hydroxide assessment where precise purity determination is critical.
- Lithium hydroxide purity analysis for battery applications: Specialized titration methods have been developed for determining lithium hydroxide purity specifically for battery-grade applications. These methods focus on detecting trace impurities that could affect battery performance while accurately quantifying lithium hydroxide content. The techniques often incorporate additional purification steps before titration and may use multiple analytical approaches to verify results. These methods are critical for ensuring that lithium hydroxide meets the stringent purity requirements (typically >99.5%) necessary for high-performance lithium-ion battery production.
- Combined analytical approaches for comprehensive purity assessment: Comprehensive lithium hydroxide purity assessment often combines titration with complementary analytical techniques. While titration provides the primary quantitative measurement of hydroxide content, techniques such as ICP-MS, atomic absorption spectroscopy, or ion chromatography are used to identify and quantify specific impurities. This multi-method approach provides a complete purity profile of lithium hydroxide samples, ensuring both the main component content and impurity levels meet specifications. Such comprehensive analysis is particularly important for high-value applications where consistent performance is critical.
02 Automated titration systems for lithium hydroxide analysis
Advanced automated titration systems have been developed specifically for lithium hydroxide purity analysis. These systems incorporate precise dispensing mechanisms, potentiometric endpoint detection, and computerized data processing to improve accuracy and reproducibility. Automated titrators can perform multiple analyses in sequence, reducing human error and increasing throughput. Some systems include temperature compensation and can detect multiple endpoints, making them suitable for complex lithium compound mixtures analysis.Expand Specific Solutions03 Impurity detection and quantification in lithium hydroxide
Methods for detecting and quantifying impurities in lithium hydroxide samples are essential for determining overall purity. These techniques can identify and measure contaminants such as sodium, calcium, magnesium, and other metal ions that may be present. Complementary analytical methods including ICP-MS (Inductively Coupled Plasma Mass Spectrometry), atomic absorption spectroscopy, and ion chromatography are often used alongside titration to provide a comprehensive purity profile. These methods are particularly important for battery-grade lithium hydroxide where impurity levels must be strictly controlled.Expand Specific Solutions04 Lithium hydroxide purity standards for battery applications
Specific purity standards have been established for lithium hydroxide used in battery manufacturing. These standards typically require high purity levels (often >99.5%) with strict limits on specific impurities that can negatively impact battery performance. Titration methods are adapted to meet these standards, with particular attention to precision and accuracy at high purity levels. The methods must be capable of detecting minute variations in purity that could affect battery efficiency, capacity, and lifespan when the lithium hydroxide is used in cathode material production.Expand Specific Solutions05 Sample preparation techniques for lithium hydroxide titration
Proper sample preparation is crucial for accurate lithium hydroxide purity determination by titration. Techniques include careful weighing, dissolution in carbon dioxide-free water, filtration to remove insoluble impurities, and protection from atmospheric carbon dioxide which can react with the sample. Some methods incorporate pre-treatments to address specific interferences or to separate lithium hydroxide from other components in complex mixtures. Standardized procedures often specify sample size, dissolution parameters, and environmental controls to ensure consistent and reliable results across different laboratories.Expand Specific Solutions
Key Industry Players in Lithium Analysis Instrumentation
The lithium hydroxide purity quantification market is currently in a growth phase, driven by expanding electric vehicle battery production requiring high-purity materials. The global market size is estimated at $350-400 million annually with projected CAGR of 12-15% through 2030. Technologically, titration methods have reached maturity but continue evolving with automation and precision improvements. Leading players include established chemical companies like BASF, LG Chem, and Sumitomo Metal Mining offering comprehensive analytical solutions, while specialized firms such as Terralithium and Water Lens focus on innovative titration techniques with enhanced accuracy. Research institutions like Naval Research Laboratory and Korea Atomic Energy Research Institute contribute significant advancements in standardization and reference materials development, creating a competitive landscape balanced between established industrial players and emerging technology specialists.
BASF Corp.
Technical Solution: BASF has developed a sophisticated titration methodology for lithium hydroxide purity assessment that integrates into their broader battery materials quality control framework. Their approach utilizes automated potentiometric titration with standardized acid, complemented by ion chromatography for impurity profiling. The titration protocol employs specialized glass electrodes with lithium-selective membranes to minimize interference from other cations. BASF's method incorporates a pre-titration carbon dioxide removal step using nitrogen purging to eliminate carbonate formation that would otherwise affect accuracy. Their system features proprietary software algorithms that analyze the titration curve inflection points to distinguish between lithium hydroxide and other basic species present in technical-grade samples. The methodology includes standardized procedures for sample dissolution that account for the varying solubility of lithium hydroxide monohydrate versus anhydrous forms, ensuring consistent results regardless of the starting material's hydration state.
Strengths: Comprehensive approach that quantifies both lithium hydroxide content and impurity profiles. Integration with broader quality control systems provides contextual data interpretation. Weaknesses: Complex methodology requires significant analytical expertise. Multiple analytical techniques increase analysis time and cost compared to simpler methods.
LG Chem Ltd.
Technical Solution: LG Chem has engineered a precision titration methodology for lithium hydroxide purity determination tailored specifically for battery-grade material quality control. Their approach employs automated potentiometric titration with hydrochloric acid as the primary titrant, utilizing specialized pH electrodes with enhanced stability in highly alkaline environments. The system incorporates a controlled-atmosphere sample preparation chamber to prevent atmospheric carbon dioxide contamination, which can significantly impact accuracy through carbonate formation. LG Chem's methodology features a multi-point calibration protocol using NIST-traceable lithium hydroxide standards across the relevant concentration range. Their titration procedure includes temperature-controlled sample dissolution and equilibration steps to ensure consistent hydration states and solution properties. The analytical system employs proprietary algorithms for endpoint detection that can distinguish between the primary lithium hydroxide endpoint and secondary endpoints from impurities, allowing for both purity determination and impurity profiling in a single analytical run.
Strengths: Highly optimized for battery-grade lithium hydroxide specifications with detection limits suitable for identifying trace impurities. Controlled-atmosphere methodology minimizes carbonate interference. Weaknesses: Specialized equipment requirements increase implementation costs. Method optimization focuses on battery applications, potentially limiting versatility for other lithium hydroxide applications.
Critical Analytical Parameters and Method Validation
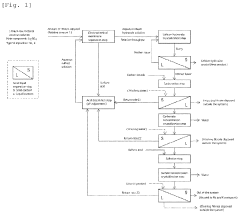
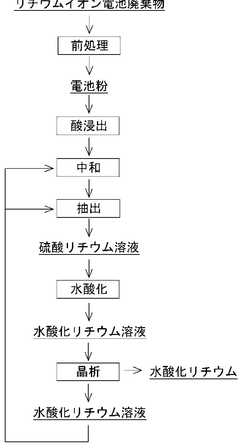
Process for producing lithium hydroxide
PatentPendingUS20240240330A1
Innovation
- A process involving electrochemical membrane separation, crystallization, carbonation, and sulfation steps is employed to efficiently remove alkali metal impurities, recycle lithium, and optimize the yield of high-purity lithium hydroxide, utilizing carbon dioxide and sulfuric acid to convert lithium-containing carbonate compounds back into a sulfate solution for reuse, thereby minimizing waste and increasing lithium recovery.
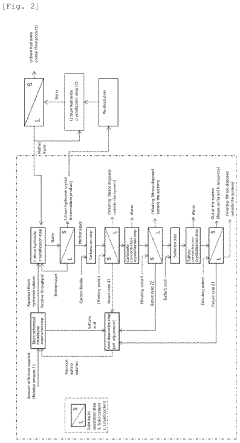
Method for producing lithium hydroxide
PatentWO2025013610A1
Innovation
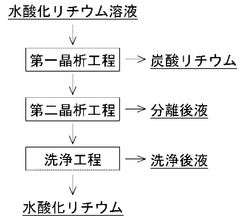
- A two-step crystallization process is employed, where the first step involves concentrating a lithium hydroxide solution containing carbonate ions to precipitate and separate lithium carbonate crystals, followed by a second step under reduced pressure to further remove carbonate ions, ensuring high purity lithium hydroxide is obtained.
Quality Control Standards and Certification Requirements
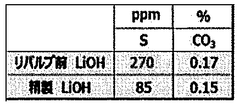
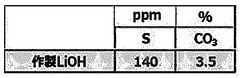
The quality control of lithium hydroxide requires adherence to stringent international and industry-specific standards to ensure product reliability and safety. ASTM International has established the ASTM E1069 standard specifically for the analysis of lithium hydroxide, which outlines precise titration methodologies and acceptable purity thresholds. This standard serves as a cornerstone for manufacturers and testing laboratories worldwide, providing a unified approach to quality assessment.
ISO certification, particularly ISO 9001 for quality management systems, plays a crucial role in validating the consistency of lithium hydroxide production processes. For battery-grade lithium hydroxide, additional specifications such as those defined by the International Electrotechnical Commission (IEC) become relevant, especially for applications in electric vehicle batteries and energy storage systems.
The pharmaceutical and food industries impose their own regulatory frameworks, with the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) specifying rigorous purity requirements for lithium compounds used in medicinal applications. These standards typically mandate titration accuracy within ±0.1% and traceability of all measurement procedures.
Certification requirements extend beyond the analytical methods themselves to encompass laboratory accreditation. Laboratories conducting lithium hydroxide purity assessments often need ISO/IEC 17025 accreditation, demonstrating their technical competence and ability to produce precise and accurate test results. This accreditation involves regular proficiency testing and external audits to verify continued compliance.
Regional variations in quality standards present challenges for global suppliers. The Chinese standard GB/T 8766 specifies requirements for industrial-grade lithium hydroxide that differ slightly from American and European counterparts, necessitating adaptable quality control protocols for manufacturers serving multiple markets. Japanese standards (JIS) similarly impose market-specific requirements that must be accommodated.
Documentation requirements constitute another critical aspect of quality control certification. Complete records of titration procedures, including calibration data, reagent specifications, and environmental conditions during testing, must be maintained. Many standards mandate specific reporting formats and uncertainty calculations to ensure transparency and comparability of results across different laboratories.
Emerging sustainability certifications are increasingly influencing lithium hydroxide quality control frameworks. The Initiative for Responsible Mining Assurance (IRMA) and other sustainability-focused certification programs are beginning to incorporate environmental impact considerations alongside traditional purity metrics, reflecting the growing importance of sustainable production practices in the lithium supply chain.
ISO certification, particularly ISO 9001 for quality management systems, plays a crucial role in validating the consistency of lithium hydroxide production processes. For battery-grade lithium hydroxide, additional specifications such as those defined by the International Electrotechnical Commission (IEC) become relevant, especially for applications in electric vehicle batteries and energy storage systems.
The pharmaceutical and food industries impose their own regulatory frameworks, with the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) specifying rigorous purity requirements for lithium compounds used in medicinal applications. These standards typically mandate titration accuracy within ±0.1% and traceability of all measurement procedures.
Certification requirements extend beyond the analytical methods themselves to encompass laboratory accreditation. Laboratories conducting lithium hydroxide purity assessments often need ISO/IEC 17025 accreditation, demonstrating their technical competence and ability to produce precise and accurate test results. This accreditation involves regular proficiency testing and external audits to verify continued compliance.
Regional variations in quality standards present challenges for global suppliers. The Chinese standard GB/T 8766 specifies requirements for industrial-grade lithium hydroxide that differ slightly from American and European counterparts, necessitating adaptable quality control protocols for manufacturers serving multiple markets. Japanese standards (JIS) similarly impose market-specific requirements that must be accommodated.
Documentation requirements constitute another critical aspect of quality control certification. Complete records of titration procedures, including calibration data, reagent specifications, and environmental conditions during testing, must be maintained. Many standards mandate specific reporting formats and uncertainty calculations to ensure transparency and comparability of results across different laboratories.
Emerging sustainability certifications are increasingly influencing lithium hydroxide quality control frameworks. The Initiative for Responsible Mining Assurance (IRMA) and other sustainability-focused certification programs are beginning to incorporate environmental impact considerations alongside traditional purity metrics, reflecting the growing importance of sustainable production practices in the lithium supply chain.
Environmental Impact of Titration Reagents and Waste Management
The titration process for quantifying lithium hydroxide purity, while effective, raises significant environmental concerns that must be addressed in laboratory and industrial settings. The primary reagents used in this analytical method include hydrochloric acid, methyl orange or phenolphthalein indicators, and various buffer solutions. These chemicals, particularly strong acids, pose environmental risks when improperly disposed of, potentially causing soil acidification and aquatic ecosystem disruption.
Acid waste from titration procedures can significantly alter pH levels in water bodies, harming aquatic life and disrupting natural biochemical processes. Additionally, indicator dyes like methyl orange contain azo compounds that may persist in the environment and exhibit potential toxicity to aquatic organisms. Research indicates that even at low concentrations, these compounds can bioaccumulate in the food chain.
Modern waste management protocols for titration processes emphasize several key approaches. Neutralization represents the first critical step, where acid waste is carefully treated with appropriate bases to achieve a pH between 6 and 9 before disposal. This process must be conducted under controlled conditions to prevent exothermic reactions and ensure complete neutralization.
Recovery and recycling systems have gained prominence in sustainable laboratory practices. Advanced distillation and membrane filtration technologies now enable the recovery of solvents and some reagents, significantly reducing waste volume. In industrial settings where lithium hydroxide purity testing occurs at scale, closed-loop systems that continuously recycle titration reagents have demonstrated waste reduction of up to 70%.
Green chemistry principles are increasingly being applied to titration methodologies. Micro-titration techniques reduce reagent volumes by up to 90% compared to traditional methods while maintaining analytical precision. Furthermore, the development of biodegradable indicators and less hazardous alternatives to traditional strong acids represents a promising frontier in environmentally responsible analytical chemistry.
Regulatory frameworks worldwide have established strict guidelines for titration waste disposal. The implementation of electronic documentation systems for waste tracking ensures compliance and facilitates continuous improvement in waste management practices. Many facilities now employ specialized waste treatment units that can process laboratory waste on-site, minimizing transportation risks and environmental footprint.
Cost-benefit analyses indicate that while implementing comprehensive waste management systems requires initial investment, long-term savings through reduced disposal costs and regulatory compliance make these systems economically viable. Organizations conducting regular lithium hydroxide purity testing can achieve return on investment within 2-3 years through proper waste management implementation.
Acid waste from titration procedures can significantly alter pH levels in water bodies, harming aquatic life and disrupting natural biochemical processes. Additionally, indicator dyes like methyl orange contain azo compounds that may persist in the environment and exhibit potential toxicity to aquatic organisms. Research indicates that even at low concentrations, these compounds can bioaccumulate in the food chain.
Modern waste management protocols for titration processes emphasize several key approaches. Neutralization represents the first critical step, where acid waste is carefully treated with appropriate bases to achieve a pH between 6 and 9 before disposal. This process must be conducted under controlled conditions to prevent exothermic reactions and ensure complete neutralization.
Recovery and recycling systems have gained prominence in sustainable laboratory practices. Advanced distillation and membrane filtration technologies now enable the recovery of solvents and some reagents, significantly reducing waste volume. In industrial settings where lithium hydroxide purity testing occurs at scale, closed-loop systems that continuously recycle titration reagents have demonstrated waste reduction of up to 70%.
Green chemistry principles are increasingly being applied to titration methodologies. Micro-titration techniques reduce reagent volumes by up to 90% compared to traditional methods while maintaining analytical precision. Furthermore, the development of biodegradable indicators and less hazardous alternatives to traditional strong acids represents a promising frontier in environmentally responsible analytical chemistry.
Regulatory frameworks worldwide have established strict guidelines for titration waste disposal. The implementation of electronic documentation systems for waste tracking ensures compliance and facilitates continuous improvement in waste management practices. Many facilities now employ specialized waste treatment units that can process laboratory waste on-site, minimizing transportation risks and environmental footprint.
Cost-benefit analyses indicate that while implementing comprehensive waste management systems requires initial investment, long-term savings through reduced disposal costs and regulatory compliance make these systems economically viable. Organizations conducting regular lithium hydroxide purity testing can achieve return on investment within 2-3 years through proper waste management implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!