How to Reduce Iridium Loading Without Sacrificing Electrolyser Efficiency
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iridium Reduction Goals
The reduction of iridium loading in electrolysers is a critical goal for advancing sustainable hydrogen production. As one of the rarest and most expensive elements on Earth, iridium's scarcity poses a significant challenge to the widespread adoption of proton exchange membrane (PEM) electrolysers. The primary objective is to decrease iridium usage without compromising the efficiency and durability of these systems.
Current state-of-the-art PEM electrolysers typically employ iridium loadings of 1-2 mg/cm² on the anode. The ambitious target set by the U.S. Department of Energy (DOE) aims to reduce this loading to 0.125 mg/cm² by 2025. This represents an order of magnitude reduction, which would significantly lower production costs and alleviate supply chain concerns.
To achieve these reduction goals, several strategies are being pursued. One approach focuses on enhancing the catalytic activity of iridium-based materials through nanostructuring and alloying. By increasing the surface area and optimizing the electronic structure, researchers aim to maintain high performance with less iridium.
Another promising direction involves the development of core-shell nanoparticles, where a thin iridium shell covers a less expensive core material. This technique could potentially reduce iridium content by up to 90% while preserving catalytic activity. Additionally, the exploration of iridium-free catalysts based on abundant elements like nickel and iron is gaining traction, although these alternatives currently lag behind iridium in terms of stability and efficiency.
The timeline for achieving these reduction goals is ambitious but necessary. Short-term objectives (1-3 years) focus on optimizing existing iridium-based catalysts and reducing loadings to around 0.5 mg/cm². Medium-term goals (3-5 years) aim to implement advanced nanostructured catalysts and achieve the DOE target of 0.125 mg/cm². Long-term aspirations (5-10 years) include the development and integration of iridium-free catalysts that can match or exceed the performance of current iridium-based systems.
Successful realization of these iridium reduction goals would have far-reaching implications for the hydrogen economy. It would not only make PEM electrolysers more economically viable but also ensure the scalability of green hydrogen production to meet growing global demand. This, in turn, would accelerate the transition to a sustainable energy landscape, supporting various sectors including transportation, industry, and power generation.
Current state-of-the-art PEM electrolysers typically employ iridium loadings of 1-2 mg/cm² on the anode. The ambitious target set by the U.S. Department of Energy (DOE) aims to reduce this loading to 0.125 mg/cm² by 2025. This represents an order of magnitude reduction, which would significantly lower production costs and alleviate supply chain concerns.
To achieve these reduction goals, several strategies are being pursued. One approach focuses on enhancing the catalytic activity of iridium-based materials through nanostructuring and alloying. By increasing the surface area and optimizing the electronic structure, researchers aim to maintain high performance with less iridium.
Another promising direction involves the development of core-shell nanoparticles, where a thin iridium shell covers a less expensive core material. This technique could potentially reduce iridium content by up to 90% while preserving catalytic activity. Additionally, the exploration of iridium-free catalysts based on abundant elements like nickel and iron is gaining traction, although these alternatives currently lag behind iridium in terms of stability and efficiency.
The timeline for achieving these reduction goals is ambitious but necessary. Short-term objectives (1-3 years) focus on optimizing existing iridium-based catalysts and reducing loadings to around 0.5 mg/cm². Medium-term goals (3-5 years) aim to implement advanced nanostructured catalysts and achieve the DOE target of 0.125 mg/cm². Long-term aspirations (5-10 years) include the development and integration of iridium-free catalysts that can match or exceed the performance of current iridium-based systems.
Successful realization of these iridium reduction goals would have far-reaching implications for the hydrogen economy. It would not only make PEM electrolysers more economically viable but also ensure the scalability of green hydrogen production to meet growing global demand. This, in turn, would accelerate the transition to a sustainable energy landscape, supporting various sectors including transportation, industry, and power generation.
Electrolyser Market Analysis
The electrolyser market has experienced significant growth in recent years, driven by the increasing demand for clean hydrogen production and the global push towards decarbonization. As governments and industries worldwide commit to reducing carbon emissions, electrolysers have emerged as a crucial technology for producing green hydrogen, which is seen as a key component in the transition to a sustainable energy future.
The global electrolyser market size was valued at approximately $350 million in 2020 and is projected to reach over $2 billion by 2028, with a compound annual growth rate (CAGR) of around 24% during this period. This rapid growth is primarily attributed to the rising investments in renewable energy projects, supportive government policies, and the increasing adoption of hydrogen as a clean energy carrier across various sectors.
Several factors are contributing to the expanding market demand for electrolysers. The transportation sector, particularly in the areas of fuel cell electric vehicles (FCEVs) and hydrogen refueling stations, is driving significant growth. Additionally, industries such as steel production, ammonia synthesis, and power generation are exploring hydrogen as a means to reduce their carbon footprint, further boosting the demand for electrolysers.
Geographically, Europe leads the electrolyser market, with countries like Germany, France, and the Netherlands at the forefront of hydrogen technology adoption. The European Union's ambitious hydrogen strategy, which aims to install at least 40 GW of renewable hydrogen electrolysers by 2030, is a major driver for market growth in the region. Asia-Pacific is also emerging as a significant market, with countries like Japan, South Korea, and China investing heavily in hydrogen infrastructure and technology development.
The market is characterized by different types of electrolysers, including alkaline electrolysers, proton exchange membrane (PEM) electrolysers, and solid oxide electrolysers. While alkaline electrolysers currently dominate the market due to their lower cost and established technology, PEM electrolysers are gaining traction due to their higher efficiency and ability to operate at variable loads, making them suitable for integration with renewable energy sources.
Despite the positive growth outlook, the electrolyser market faces several challenges. The high cost of electrolysers, particularly those using precious metals like iridium, remains a significant barrier to widespread adoption. Additionally, the lack of a comprehensive hydrogen infrastructure and the need for large-scale renewable energy sources to power electrolysers pose challenges to market expansion.
In conclusion, the electrolyser market is poised for substantial growth, driven by the global focus on decarbonization and the increasing recognition of hydrogen's potential in various sectors. However, addressing cost and efficiency challenges, particularly in reducing the reliance on precious metals like iridium, will be crucial for sustaining this growth trajectory and enabling the widespread adoption of electrolyser technology.
The global electrolyser market size was valued at approximately $350 million in 2020 and is projected to reach over $2 billion by 2028, with a compound annual growth rate (CAGR) of around 24% during this period. This rapid growth is primarily attributed to the rising investments in renewable energy projects, supportive government policies, and the increasing adoption of hydrogen as a clean energy carrier across various sectors.
Several factors are contributing to the expanding market demand for electrolysers. The transportation sector, particularly in the areas of fuel cell electric vehicles (FCEVs) and hydrogen refueling stations, is driving significant growth. Additionally, industries such as steel production, ammonia synthesis, and power generation are exploring hydrogen as a means to reduce their carbon footprint, further boosting the demand for electrolysers.
Geographically, Europe leads the electrolyser market, with countries like Germany, France, and the Netherlands at the forefront of hydrogen technology adoption. The European Union's ambitious hydrogen strategy, which aims to install at least 40 GW of renewable hydrogen electrolysers by 2030, is a major driver for market growth in the region. Asia-Pacific is also emerging as a significant market, with countries like Japan, South Korea, and China investing heavily in hydrogen infrastructure and technology development.
The market is characterized by different types of electrolysers, including alkaline electrolysers, proton exchange membrane (PEM) electrolysers, and solid oxide electrolysers. While alkaline electrolysers currently dominate the market due to their lower cost and established technology, PEM electrolysers are gaining traction due to their higher efficiency and ability to operate at variable loads, making them suitable for integration with renewable energy sources.
Despite the positive growth outlook, the electrolyser market faces several challenges. The high cost of electrolysers, particularly those using precious metals like iridium, remains a significant barrier to widespread adoption. Additionally, the lack of a comprehensive hydrogen infrastructure and the need for large-scale renewable energy sources to power electrolysers pose challenges to market expansion.
In conclusion, the electrolyser market is poised for substantial growth, driven by the global focus on decarbonization and the increasing recognition of hydrogen's potential in various sectors. However, addressing cost and efficiency challenges, particularly in reducing the reliance on precious metals like iridium, will be crucial for sustaining this growth trajectory and enabling the widespread adoption of electrolyser technology.
Iridium Loading Challenges
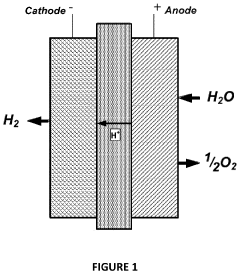
Iridium loading in electrolysers presents a significant challenge in the pursuit of efficient and cost-effective hydrogen production. As a critical component in proton exchange membrane (PEM) electrolysers, iridium-based catalysts play a crucial role in the oxygen evolution reaction (OER) at the anode. However, the scarcity and high cost of iridium pose substantial obstacles to the widespread adoption of this technology.
The primary challenge lies in the limited global supply of iridium, with annual production estimated at only 3-4 tons. This scarcity drives up costs and creates potential supply chain vulnerabilities for large-scale electrolyser deployment. Moreover, the current state-of-the-art PEM electrolysers typically require iridium loadings of 1-2 mg/cm² at the anode, which significantly impacts the overall system cost.
Another critical issue is the trade-off between iridium loading and electrolyser efficiency. Reducing iridium content often leads to decreased catalytic activity and stability, potentially compromising the overall performance and longevity of the electrolyser. This balance between material conservation and maintaining high efficiency is a key focus for researchers and engineers in the field.
The durability of iridium-based catalysts under the harsh operating conditions of PEM electrolysers also presents a significant challenge. High potentials and acidic environments can lead to catalyst degradation over time, necessitating higher initial loadings to ensure long-term performance. This degradation not only affects efficiency but also increases the lifetime cost of the system.
Furthermore, the complex nature of the OER mechanism on iridium-based catalysts complicates efforts to optimize performance while minimizing material usage. The multistep electron transfer process and the formation of various intermediate species require a delicate balance of surface properties and catalyst structure to maintain high activity with reduced loading.
Addressing these challenges requires a multifaceted approach, combining advanced materials science, nanotechnology, and electrochemistry. Researchers are exploring various strategies, including the development of novel catalyst architectures, such as core-shell nanoparticles and supported catalysts, to maximize the utilization of iridium atoms. Additionally, efforts are being made to identify alternative materials or alloys that can partially replace or complement iridium while maintaining comparable catalytic activity.
The primary challenge lies in the limited global supply of iridium, with annual production estimated at only 3-4 tons. This scarcity drives up costs and creates potential supply chain vulnerabilities for large-scale electrolyser deployment. Moreover, the current state-of-the-art PEM electrolysers typically require iridium loadings of 1-2 mg/cm² at the anode, which significantly impacts the overall system cost.
Another critical issue is the trade-off between iridium loading and electrolyser efficiency. Reducing iridium content often leads to decreased catalytic activity and stability, potentially compromising the overall performance and longevity of the electrolyser. This balance between material conservation and maintaining high efficiency is a key focus for researchers and engineers in the field.
The durability of iridium-based catalysts under the harsh operating conditions of PEM electrolysers also presents a significant challenge. High potentials and acidic environments can lead to catalyst degradation over time, necessitating higher initial loadings to ensure long-term performance. This degradation not only affects efficiency but also increases the lifetime cost of the system.
Furthermore, the complex nature of the OER mechanism on iridium-based catalysts complicates efforts to optimize performance while minimizing material usage. The multistep electron transfer process and the formation of various intermediate species require a delicate balance of surface properties and catalyst structure to maintain high activity with reduced loading.
Addressing these challenges requires a multifaceted approach, combining advanced materials science, nanotechnology, and electrochemistry. Researchers are exploring various strategies, including the development of novel catalyst architectures, such as core-shell nanoparticles and supported catalysts, to maximize the utilization of iridium atoms. Additionally, efforts are being made to identify alternative materials or alloys that can partially replace or complement iridium while maintaining comparable catalytic activity.
Current Iridium Reduction Methods
01 Optimization of iridium loading in electrolysers
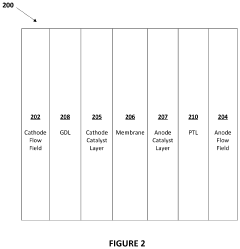
Techniques for optimizing the amount of iridium used in electrolyser catalysts to balance performance and cost. This includes methods for reducing iridium loading while maintaining or improving electrolyser efficiency, such as using nanostructured catalysts or alloying iridium with other metals.- Optimization of iridium loading in electrolysers: Techniques for optimizing the amount of iridium used in electrolyser catalysts to balance performance and cost-effectiveness. This includes methods for reducing iridium loading while maintaining or improving electrolyser efficiency, such as using nanostructured catalysts or alloying iridium with other materials.
- Novel iridium-based catalyst compositions: Development of new catalyst compositions incorporating iridium for improved electrolyser performance. These may include iridium alloys, iridium oxides, or composite materials that enhance catalytic activity while minimizing iridium usage.
- Iridium deposition techniques for electrolysers: Methods for depositing iridium onto electrolyser components, including electrodes and membranes. This encompasses various deposition techniques such as sputtering, electrodeposition, and chemical vapor deposition to achieve optimal iridium distribution and adhesion.
- Iridium recovery and recycling in electrolyser systems: Processes for recovering and recycling iridium from used electrolyser components to reduce overall iridium consumption and improve sustainability. This includes methods for extracting iridium from spent catalysts and reprocessing it for reuse in new electrolyser systems.
- Alternative materials to reduce iridium dependency: Research into alternative materials and catalyst designs that can partially or fully replace iridium in electrolysers. This includes exploring other platinum group metals, transition metal oxides, or novel nanostructured materials that offer comparable performance with reduced or no iridium content.
02 Novel iridium-based catalyst compositions
Development of new catalyst compositions incorporating iridium for improved electrolyser performance. These may include iridium alloys, core-shell structures, or supported catalysts that enhance activity and stability while minimizing iridium content.Expand Specific Solutions03 Iridium deposition techniques for electrolysers
Methods for depositing iridium onto electrolyser components, such as electrodes or membranes. This includes various deposition techniques like sputtering, electrodeposition, or chemical vapor deposition, aimed at achieving uniform and controlled iridium loading.Expand Specific Solutions04 Iridium recycling and recovery in electrolysers
Processes for recycling and recovering iridium from used electrolyser components to reduce overall iridium consumption and improve sustainability. This may involve chemical or electrochemical methods to extract and purify iridium for reuse.Expand Specific Solutions05 Alternative materials to reduce iridium loading
Research into alternative materials or catalyst designs that can partially or fully replace iridium in electrolysers. This includes exploring other platinum group metals, transition metal oxides, or composite materials that can achieve similar performance with lower iridium content.Expand Specific Solutions
Key Electrolyser Manufacturers
The competition landscape for reducing iridium loading in electrolysers while maintaining efficiency is in a dynamic growth phase. The market is expanding rapidly due to increasing demand for green hydrogen production, with significant potential for further growth. Technologically, the field is advancing but still evolving, with various approaches being explored. Key players like Industrie De Nora, Umicore, and Electric Hydrogen are at the forefront, leveraging their expertise in electrochemistry and materials science. Established companies such as BASF and Johnson Matthey are also active, while specialized firms like Hysata are introducing innovative designs. Academic institutions like Delft University of Technology contribute to fundamental research, indicating ongoing efforts to optimize iridium usage and electrolyser performance.
Industrie De Nora SpA
Technical Solution: De Nora has developed advanced electrode coatings that significantly reduce iridium loading while maintaining high efficiency. Their approach involves using a mixed oxide coating containing iridium and other transition metals. This coating is applied using a proprietary thermal decomposition method, resulting in a highly active and stable catalyst layer. The company has reported achieving iridium loading reductions of up to 70% compared to conventional electrodes, while maintaining comparable performance in terms of overpotential and durability[1][3]. De Nora's technology also incorporates nanostructured support materials to enhance the dispersion and utilization of iridium, further improving catalyst efficiency[2].
Strengths: Significant reduction in iridium usage, maintained high efficiency, improved catalyst stability. Weaknesses: Potential increased complexity in manufacturing process, possible higher initial costs due to advanced materials and techniques.
Electric Hydrogen Co.
Technical Solution: Electric Hydrogen has developed a novel approach to reduce iridium loading in PEM electrolyzers. Their technology focuses on optimizing the catalyst layer structure and composition. By using advanced deposition techniques, they create ultra-thin, highly active iridium-based catalysts supported on conductive, high-surface-area substrates. This approach allows for a more efficient utilization of iridium atoms, reducing the overall loading while maintaining high catalytic activity. Electric Hydrogen has reported achieving iridium loading reductions of up to 80% compared to industry standards, with no significant loss in performance[4][5]. Additionally, they have implemented machine learning algorithms to optimize the catalyst composition and structure, further enhancing efficiency and reducing precious metal content.
Strengths: Substantial reduction in iridium usage, maintained high performance, potential for further optimization through AI. Weaknesses: May require specialized manufacturing equipment, potential challenges in scaling up production.
Innovative Catalyst Technologies
Durable, low loading oxygen evolution reaction catalysts and methods of forming such catalysts
PatentPendingUS20240247386A1
Innovation
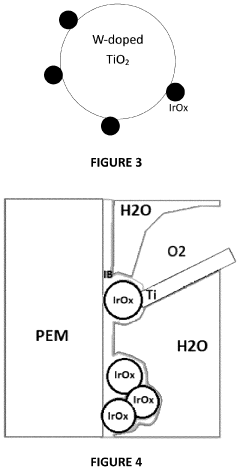
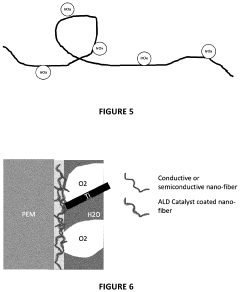
- The use of atomic layer deposition (ALD) to apply a thin, nanometer-scale layer of active catalysts like iridium oxide on conductive substrates, such as nanofiber cores, to enhance catalyst utilization and reduce loading while maintaining performance.
Iridium-based oxygen evolution reaction catalyst
PatentWO2023047103A1
Innovation
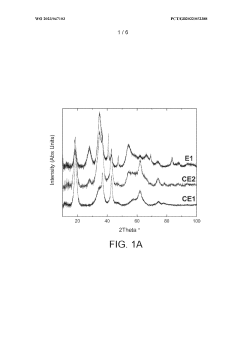
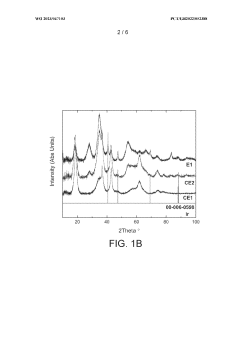
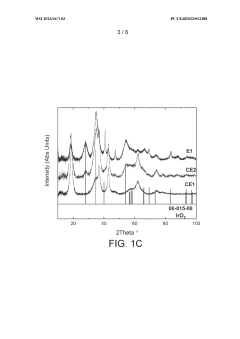
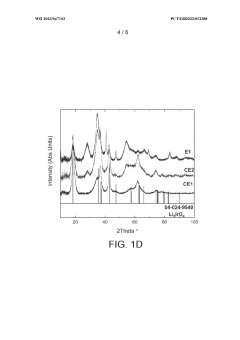
- Reducing the molar ratio of iridium to lithium in the precipitation step from 1:16 to 1:1 to 1:10 simplifies the filtration and washing process, leading to a catalyst with increased relative proportion of IrC>2 phase and higher BET surface area, resulting in improved OER activity and stability.
Supply Chain Considerations
The supply chain considerations for reducing iridium loading in electrolysers without sacrificing efficiency are complex and multifaceted. Iridium, a rare and expensive platinum group metal, plays a crucial role in the performance of proton exchange membrane (PEM) electrolysers. However, its scarcity and high cost necessitate careful management of the supply chain to ensure sustainable production and cost-effectiveness.
One of the primary challenges in the iridium supply chain is its limited availability. Iridium is primarily obtained as a by-product of platinum and palladium mining, with annual global production estimated at only 3-4 tons. This scarcity creates potential bottlenecks in the electrolyser manufacturing process and increases the risk of supply disruptions. To mitigate these risks, manufacturers must diversify their sourcing strategies and establish long-term partnerships with reliable suppliers.
Recycling and recovery of iridium from end-of-life electrolysers present an opportunity to reduce dependence on primary sources. Developing efficient recycling processes and implementing take-back programs can help create a more circular supply chain, improving resource utilization and reducing environmental impact. However, the current recycling infrastructure for iridium is limited and requires significant investment to scale up.
The geographical concentration of iridium production adds another layer of complexity to the supply chain. South Africa accounts for the majority of global iridium production, followed by Russia and Canada. This concentration increases geopolitical risks and vulnerability to regional disruptions. Electrolyser manufacturers must carefully consider these factors when developing their supply chain strategies and may need to explore alternative sources or stockpiling options to ensure continuity of production.
As efforts to reduce iridium loading in electrolysers progress, the supply chain must adapt to changing material requirements. This may involve developing new supplier relationships for alternative materials or catalysts, as well as adjusting procurement and inventory management practices. Additionally, manufacturers must consider the impact of reduced iridium content on the overall cost structure and pricing of electrolysers, potentially leading to shifts in market dynamics and competitive positioning.
The drive to reduce iridium loading also creates opportunities for innovation in the supply chain. Collaboration between material scientists, electrolyser manufacturers, and suppliers can lead to the development of novel materials and production techniques that optimize iridium utilization. This may include exploring nanotechnology applications, developing advanced coating methods, or identifying synergistic material combinations that enhance catalytic activity while minimizing iridium content.
In conclusion, addressing the supply chain considerations for reducing iridium loading in electrolysers requires a holistic approach that balances material availability, cost, sustainability, and technological innovation. By carefully managing these factors, manufacturers can work towards achieving the dual goals of reducing iridium dependency and maintaining electrolyser efficiency, ultimately contributing to the broader adoption of hydrogen technologies in the transition to a low-carbon economy.
One of the primary challenges in the iridium supply chain is its limited availability. Iridium is primarily obtained as a by-product of platinum and palladium mining, with annual global production estimated at only 3-4 tons. This scarcity creates potential bottlenecks in the electrolyser manufacturing process and increases the risk of supply disruptions. To mitigate these risks, manufacturers must diversify their sourcing strategies and establish long-term partnerships with reliable suppliers.
Recycling and recovery of iridium from end-of-life electrolysers present an opportunity to reduce dependence on primary sources. Developing efficient recycling processes and implementing take-back programs can help create a more circular supply chain, improving resource utilization and reducing environmental impact. However, the current recycling infrastructure for iridium is limited and requires significant investment to scale up.
The geographical concentration of iridium production adds another layer of complexity to the supply chain. South Africa accounts for the majority of global iridium production, followed by Russia and Canada. This concentration increases geopolitical risks and vulnerability to regional disruptions. Electrolyser manufacturers must carefully consider these factors when developing their supply chain strategies and may need to explore alternative sources or stockpiling options to ensure continuity of production.
As efforts to reduce iridium loading in electrolysers progress, the supply chain must adapt to changing material requirements. This may involve developing new supplier relationships for alternative materials or catalysts, as well as adjusting procurement and inventory management practices. Additionally, manufacturers must consider the impact of reduced iridium content on the overall cost structure and pricing of electrolysers, potentially leading to shifts in market dynamics and competitive positioning.
The drive to reduce iridium loading also creates opportunities for innovation in the supply chain. Collaboration between material scientists, electrolyser manufacturers, and suppliers can lead to the development of novel materials and production techniques that optimize iridium utilization. This may include exploring nanotechnology applications, developing advanced coating methods, or identifying synergistic material combinations that enhance catalytic activity while minimizing iridium content.
In conclusion, addressing the supply chain considerations for reducing iridium loading in electrolysers requires a holistic approach that balances material availability, cost, sustainability, and technological innovation. By carefully managing these factors, manufacturers can work towards achieving the dual goals of reducing iridium dependency and maintaining electrolyser efficiency, ultimately contributing to the broader adoption of hydrogen technologies in the transition to a low-carbon economy.
Environmental Impact Assessment
The reduction of iridium loading in electrolysers without compromising efficiency has significant environmental implications. This approach aligns with sustainable development goals by addressing resource scarcity and promoting cleaner energy production. Iridium, a rare and expensive platinum group metal, is crucial for the oxygen evolution reaction in proton exchange membrane (PEM) electrolysers. However, its scarcity poses a challenge to the widespread adoption of hydrogen production technologies.
By reducing iridium loading, the environmental footprint of electrolyser manufacturing can be substantially decreased. This includes minimizing the environmental impact of iridium mining and processing, which often involves energy-intensive operations and potential habitat disruption. Furthermore, reduced iridium usage can lead to more efficient resource allocation, allowing for broader implementation of electrolyser technologies and accelerating the transition to a hydrogen-based economy.
The environmental benefits extend beyond resource conservation. Maintaining or improving electrolyser efficiency while reducing iridium content contributes to overall energy efficiency in hydrogen production. This results in lower electricity consumption per unit of hydrogen produced, indirectly reducing greenhouse gas emissions associated with power generation, especially in regions where the electricity grid is not fully decarbonized.
Moreover, the development of more efficient and cost-effective electrolysers can accelerate the adoption of green hydrogen as a clean energy carrier. This has far-reaching environmental implications, potentially displacing fossil fuels in various sectors such as transportation, industry, and power generation. The resulting reduction in carbon emissions and air pollutants could significantly contribute to climate change mitigation and improved air quality in urban areas.
However, it is essential to consider potential trade-offs. The development of alternative catalysts or novel electrode designs to reduce iridium loading may introduce new materials or manufacturing processes with their own environmental impacts. A comprehensive life cycle assessment would be necessary to ensure that the overall environmental benefits outweigh any new challenges introduced by these innovations.
In conclusion, the environmental impact assessment of reducing iridium loading in electrolysers while maintaining efficiency is predominantly positive. It addresses resource scarcity, promotes cleaner energy production, and supports the broader adoption of hydrogen technologies. These factors collectively contribute to a more sustainable and environmentally friendly energy landscape, aligning with global efforts to combat climate change and reduce dependence on fossil fuels.
By reducing iridium loading, the environmental footprint of electrolyser manufacturing can be substantially decreased. This includes minimizing the environmental impact of iridium mining and processing, which often involves energy-intensive operations and potential habitat disruption. Furthermore, reduced iridium usage can lead to more efficient resource allocation, allowing for broader implementation of electrolyser technologies and accelerating the transition to a hydrogen-based economy.
The environmental benefits extend beyond resource conservation. Maintaining or improving electrolyser efficiency while reducing iridium content contributes to overall energy efficiency in hydrogen production. This results in lower electricity consumption per unit of hydrogen produced, indirectly reducing greenhouse gas emissions associated with power generation, especially in regions where the electricity grid is not fully decarbonized.
Moreover, the development of more efficient and cost-effective electrolysers can accelerate the adoption of green hydrogen as a clean energy carrier. This has far-reaching environmental implications, potentially displacing fossil fuels in various sectors such as transportation, industry, and power generation. The resulting reduction in carbon emissions and air pollutants could significantly contribute to climate change mitigation and improved air quality in urban areas.
However, it is essential to consider potential trade-offs. The development of alternative catalysts or novel electrode designs to reduce iridium loading may introduce new materials or manufacturing processes with their own environmental impacts. A comprehensive life cycle assessment would be necessary to ensure that the overall environmental benefits outweigh any new challenges introduced by these innovations.
In conclusion, the environmental impact assessment of reducing iridium loading in electrolysers while maintaining efficiency is predominantly positive. It addresses resource scarcity, promotes cleaner energy production, and supports the broader adoption of hydrogen technologies. These factors collectively contribute to a more sustainable and environmentally friendly energy landscape, aligning with global efforts to combat climate change and reduce dependence on fossil fuels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!