How to Tackle ICP-MS Sample Preparation Challenges
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Technology Evolution and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer to detect and quantify trace elements at concentrations as low as one part per trillion. The evolution of ICP-MS technology has been driven by the increasing demand for more sensitive, accurate, and efficient elemental analysis across various industries including environmental monitoring, pharmaceutical research, food safety, and materials science.
The initial ICP-MS systems faced considerable limitations in terms of sample preparation requirements, interference management, and detection capabilities. Early instruments required extensive sample preparation protocols to minimize matrix effects and spectral interferences, which significantly limited throughput and increased the risk of contamination. The evolution trajectory has been marked by progressive improvements in ionization efficiency, interface design, and mass analyzer technology.
A significant milestone in ICP-MS development was the introduction of collision/reaction cell technology in the late 1990s, which revolutionized the approach to handling polyatomic interferences. This innovation substantially improved the accuracy of measurements for previously problematic elements such as iron, arsenic, and selenium in complex matrices. Subsequently, the development of high-resolution ICP-MS systems further enhanced the ability to separate analytes from spectral interferences.
Sample preparation has consistently remained one of the most challenging aspects of ICP-MS analysis. Traditional methods often involve time-consuming digestion procedures using concentrated acids at elevated temperatures, presenting safety concerns and potential for contamination. The evolution of sample preparation techniques has paralleled instrument development, with innovations focusing on minimizing sample handling, reducing reagent consumption, and increasing automation.
The primary objectives of current ICP-MS technology development include simplifying sample preparation workflows while maintaining or improving analytical performance. This encompasses the development of more efficient digestion methods, novel sample introduction systems, and integrated automation solutions. Additionally, there is a growing emphasis on developing techniques that can handle increasingly complex sample matrices without compromising detection limits or precision.
Another key objective is to enhance the accessibility of ICP-MS technology by reducing operational complexity and maintenance requirements. This includes the development of more intuitive software interfaces, self-diagnostic capabilities, and robust hardware components that can withstand challenging laboratory environments. The ultimate goal is to transform ICP-MS from a specialized analytical technique requiring extensive expertise into a more routine analytical tool accessible to a broader range of laboratories and applications.
The initial ICP-MS systems faced considerable limitations in terms of sample preparation requirements, interference management, and detection capabilities. Early instruments required extensive sample preparation protocols to minimize matrix effects and spectral interferences, which significantly limited throughput and increased the risk of contamination. The evolution trajectory has been marked by progressive improvements in ionization efficiency, interface design, and mass analyzer technology.
A significant milestone in ICP-MS development was the introduction of collision/reaction cell technology in the late 1990s, which revolutionized the approach to handling polyatomic interferences. This innovation substantially improved the accuracy of measurements for previously problematic elements such as iron, arsenic, and selenium in complex matrices. Subsequently, the development of high-resolution ICP-MS systems further enhanced the ability to separate analytes from spectral interferences.
Sample preparation has consistently remained one of the most challenging aspects of ICP-MS analysis. Traditional methods often involve time-consuming digestion procedures using concentrated acids at elevated temperatures, presenting safety concerns and potential for contamination. The evolution of sample preparation techniques has paralleled instrument development, with innovations focusing on minimizing sample handling, reducing reagent consumption, and increasing automation.
The primary objectives of current ICP-MS technology development include simplifying sample preparation workflows while maintaining or improving analytical performance. This encompasses the development of more efficient digestion methods, novel sample introduction systems, and integrated automation solutions. Additionally, there is a growing emphasis on developing techniques that can handle increasingly complex sample matrices without compromising detection limits or precision.
Another key objective is to enhance the accessibility of ICP-MS technology by reducing operational complexity and maintenance requirements. This includes the development of more intuitive software interfaces, self-diagnostic capabilities, and robust hardware components that can withstand challenging laboratory environments. The ultimate goal is to transform ICP-MS from a specialized analytical technique requiring extensive expertise into a more routine analytical tool accessible to a broader range of laboratories and applications.
Market Demand Analysis for Advanced ICP-MS Solutions
The global market for ICP-MS (Inductively Coupled Plasma Mass Spectrometry) solutions is experiencing robust growth driven by increasing demands across multiple sectors. Current market valuations indicate the global ICP-MS market reached approximately 1.2 billion USD in 2022, with projections suggesting a compound annual growth rate of 7.8% through 2028. This growth trajectory is primarily fueled by expanding applications in environmental monitoring, pharmaceutical analysis, food safety testing, and clinical diagnostics.
Environmental regulatory compliance represents a significant market driver, with governmental agencies worldwide implementing stricter monitoring protocols for heavy metals and trace elements in soil, water, and air samples. The EPA, EU Environmental Directives, and similar regulatory bodies in Asia-Pacific regions continue to lower permissible limits for toxic elements, necessitating more sensitive and efficient analytical methods.
The pharmaceutical and biotechnology sectors demonstrate particularly strong demand growth, with requirements for high-throughput sample preparation solutions that can maintain analytical integrity while processing increasingly complex biological matrices. Market research indicates that approximately 65% of pharmaceutical laboratories cite sample preparation as their primary bottleneck in ICP-MS workflows.
Food safety testing represents another rapidly expanding market segment, growing at nearly 9% annually, as consumers and regulatory bodies demand more comprehensive screening for contaminants. The ability to process diverse food matrices efficiently while maintaining detection sensitivity has become a critical requirement for laboratories serving this sector.
Clinical diagnostics applications are emerging as a high-potential growth area, with increasing adoption of ICP-MS for trace element analysis in biological fluids. This segment demands sample preparation solutions capable of handling high-salt matrices while minimizing contamination risks in clinical environments.
Regional analysis reveals the North American market currently holds the largest share at approximately 38%, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region demonstrates the fastest growth rate, driven by expanding industrial activities, environmental concerns, and strengthening regulatory frameworks in China, India, and Southeast Asian countries.
Customer surveys indicate that laboratories are increasingly prioritizing automation capabilities, with over 70% of potential buyers ranking automated sample preparation as "highly important" in purchasing decisions. This trend reflects the growing pressure on analytical laboratories to increase throughput while maintaining analytical quality and reducing labor costs.
Environmental regulatory compliance represents a significant market driver, with governmental agencies worldwide implementing stricter monitoring protocols for heavy metals and trace elements in soil, water, and air samples. The EPA, EU Environmental Directives, and similar regulatory bodies in Asia-Pacific regions continue to lower permissible limits for toxic elements, necessitating more sensitive and efficient analytical methods.
The pharmaceutical and biotechnology sectors demonstrate particularly strong demand growth, with requirements for high-throughput sample preparation solutions that can maintain analytical integrity while processing increasingly complex biological matrices. Market research indicates that approximately 65% of pharmaceutical laboratories cite sample preparation as their primary bottleneck in ICP-MS workflows.
Food safety testing represents another rapidly expanding market segment, growing at nearly 9% annually, as consumers and regulatory bodies demand more comprehensive screening for contaminants. The ability to process diverse food matrices efficiently while maintaining detection sensitivity has become a critical requirement for laboratories serving this sector.
Clinical diagnostics applications are emerging as a high-potential growth area, with increasing adoption of ICP-MS for trace element analysis in biological fluids. This segment demands sample preparation solutions capable of handling high-salt matrices while minimizing contamination risks in clinical environments.
Regional analysis reveals the North American market currently holds the largest share at approximately 38%, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region demonstrates the fastest growth rate, driven by expanding industrial activities, environmental concerns, and strengthening regulatory frameworks in China, India, and Southeast Asian countries.
Customer surveys indicate that laboratories are increasingly prioritizing automation capabilities, with over 70% of potential buyers ranking automated sample preparation as "highly important" in purchasing decisions. This trend reflects the growing pressure on analytical laboratories to increase throughput while maintaining analytical quality and reducing labor costs.
Current Challenges in ICP-MS Sample Preparation
ICP-MS (Inductively Coupled Plasma Mass Spectrometry) sample preparation remains one of the most critical and challenging aspects of the analytical workflow. Despite technological advancements in instrumentation, inadequate sample preparation continues to be the primary source of errors, affecting measurement accuracy, precision, and reliability. The complexity of sample matrices across various industries—from environmental monitoring to pharmaceutical quality control—demands tailored preparation approaches that often require significant expertise.
A fundamental challenge lies in achieving complete dissolution of solid samples, particularly those containing refractory elements or complex mineral structures. Traditional acid digestion methods using HNO3, HCl, or HF often prove insufficient for certain matrices, necessitating more aggressive approaches like high-pressure microwave digestion or fusion techniques. However, these advanced methods introduce additional complications, including potential contamination, loss of volatile elements, and increased procedural blank values.
Matrix effects represent another significant hurdle in ICP-MS analysis. High dissolved solid content can cause signal suppression or enhancement, leading to inaccurate quantification. While internal standardization and matrix-matched calibration offer partial solutions, they cannot fully compensate for complex matrix interferences in many real-world samples. The presence of polyatomic interferences, particularly in environmental and biological samples, further complicates accurate determination of certain elements.
Sample heterogeneity presents persistent difficulties, especially when dealing with limited sample quantities or materials with non-uniform elemental distribution. Achieving representative sampling while maintaining analytical sensitivity requires careful consideration of homogenization techniques, which must be tailored to specific sample types without introducing contamination or analyte loss.
Time constraints in high-throughput laboratories create additional pressure on sample preparation workflows. Many current protocols are labor-intensive and time-consuming, with some digestion procedures requiring several hours to complete. This bottleneck significantly impacts laboratory productivity and increases per-sample analysis costs, creating demand for more efficient preparation methods.
Contamination control remains a persistent challenge, particularly when analyzing trace and ultra-trace elements. Common sources include reagent impurities, laboratory environment, sampling tools, and storage containers. Establishing and maintaining clean laboratory conditions requires substantial investment and rigorous protocols that may be difficult to implement in all analytical settings.
Automation of sample preparation workflows presents both opportunities and challenges. While automated systems can improve reproducibility and throughput, they often lack the flexibility to handle diverse sample types and may require significant method development for each new matrix. The high capital investment for advanced automated systems also presents a barrier for many laboratories, particularly in academic or small-scale industrial settings.
A fundamental challenge lies in achieving complete dissolution of solid samples, particularly those containing refractory elements or complex mineral structures. Traditional acid digestion methods using HNO3, HCl, or HF often prove insufficient for certain matrices, necessitating more aggressive approaches like high-pressure microwave digestion or fusion techniques. However, these advanced methods introduce additional complications, including potential contamination, loss of volatile elements, and increased procedural blank values.
Matrix effects represent another significant hurdle in ICP-MS analysis. High dissolved solid content can cause signal suppression or enhancement, leading to inaccurate quantification. While internal standardization and matrix-matched calibration offer partial solutions, they cannot fully compensate for complex matrix interferences in many real-world samples. The presence of polyatomic interferences, particularly in environmental and biological samples, further complicates accurate determination of certain elements.
Sample heterogeneity presents persistent difficulties, especially when dealing with limited sample quantities or materials with non-uniform elemental distribution. Achieving representative sampling while maintaining analytical sensitivity requires careful consideration of homogenization techniques, which must be tailored to specific sample types without introducing contamination or analyte loss.
Time constraints in high-throughput laboratories create additional pressure on sample preparation workflows. Many current protocols are labor-intensive and time-consuming, with some digestion procedures requiring several hours to complete. This bottleneck significantly impacts laboratory productivity and increases per-sample analysis costs, creating demand for more efficient preparation methods.
Contamination control remains a persistent challenge, particularly when analyzing trace and ultra-trace elements. Common sources include reagent impurities, laboratory environment, sampling tools, and storage containers. Establishing and maintaining clean laboratory conditions requires substantial investment and rigorous protocols that may be difficult to implement in all analytical settings.
Automation of sample preparation workflows presents both opportunities and challenges. While automated systems can improve reproducibility and throughput, they often lack the flexibility to handle diverse sample types and may require significant method development for each new matrix. The high capital investment for advanced automated systems also presents a barrier for many laboratories, particularly in academic or small-scale industrial settings.
Current Sample Preparation Methodologies and Protocols
01 Sample digestion and dissolution techniques
Various methods for breaking down complex samples into solutions suitable for ICP-MS analysis. These techniques include acid digestion, microwave-assisted digestion, and specialized dissolution procedures for difficult matrices. Proper digestion ensures complete extraction of target elements while minimizing contamination and loss of volatile components, which is critical for accurate quantitative analysis.- Sample preparation techniques for ICP-MS analysis: Various techniques are employed for preparing samples for ICP-MS analysis, including digestion methods, dilution protocols, and extraction procedures. These techniques aim to convert solid samples into solutions suitable for analysis while maintaining the integrity of the analytes. Proper sample preparation is crucial for accurate and reliable ICP-MS results, as it helps to eliminate matrix effects and reduce interferences.
- Automated sample preparation systems: Automated systems have been developed to streamline the sample preparation process for ICP-MS analysis. These systems can handle multiple samples simultaneously, reducing human error and increasing throughput. Automation helps to ensure consistency in sample preparation procedures, which is essential for obtaining reproducible results. These systems often incorporate features such as precise temperature control, automated reagent addition, and programmable digestion protocols.
- Handling challenging sample matrices: Certain sample matrices present specific challenges for ICP-MS analysis, requiring specialized preparation techniques. These challenging matrices include high-salt samples, organic-rich materials, and samples with high dissolved solids content. Techniques such as matrix matching, internal standardization, and specialized digestion protocols have been developed to address these challenges and improve the accuracy of ICP-MS measurements for complex samples.
- Microwave-assisted digestion methods: Microwave-assisted digestion has emerged as an efficient method for preparing samples for ICP-MS analysis. This technique uses microwave energy to rapidly heat samples in closed vessels, accelerating the digestion process. Compared to conventional heating methods, microwave digestion offers advantages such as shorter digestion times, reduced contamination risk, and improved recovery of volatile elements. Various protocols have been developed for different sample types, optimizing parameters such as temperature, pressure, and acid combinations.
- Quality control in sample preparation: Quality control measures are essential in sample preparation for ICP-MS to ensure reliable analytical results. These measures include the use of certified reference materials, method blanks, and duplicate samples. Proper cleaning procedures for labware, careful selection of reagents, and contamination control strategies are also critical aspects of quality control in sample preparation. Implementing robust quality control protocols helps to identify and minimize sources of error in the analytical process.
02 Matrix interference reduction strategies
Approaches to minimize or eliminate matrix effects that can compromise ICP-MS measurement accuracy. These include sample dilution, matrix matching, internal standardization, and specialized separation techniques. Matrix components can suppress or enhance analyte signals, affecting quantification, so these strategies are essential for obtaining reliable analytical results across diverse sample types.Expand Specific Solutions03 Automated sample preparation systems
Integrated systems and devices designed to automate the sample preparation workflow for ICP-MS analysis. These systems can handle tasks such as weighing, dilution, digestion, and introduction to the instrument with minimal human intervention. Automation improves throughput, reduces operator exposure to hazardous chemicals, enhances reproducibility, and minimizes contamination risks in the analytical process.Expand Specific Solutions04 Specialized sample introduction devices
Custom equipment designed to efficiently introduce prepared samples into ICP-MS instruments. These include nebulizers, spray chambers, and sample introduction interfaces optimized for different sample types and analytical requirements. Specialized introduction systems can improve sensitivity, stability, and tolerance to challenging matrices while reducing memory effects between samples.Expand Specific Solutions05 Trace element preconcentration methods
Techniques to increase the concentration of target analytes prior to ICP-MS analysis, particularly for ultra-trace level determinations. These include solid-phase extraction, co-precipitation, chelation, and other separation approaches that can selectively isolate analytes from the sample matrix. Preconcentration improves detection limits and can simultaneously remove interfering matrix components, enhancing measurement sensitivity and accuracy.Expand Specific Solutions
Key Industry Players and Instrument Manufacturers
The ICP-MS sample preparation market is in a growth phase, with increasing demand driven by expanding applications in environmental monitoring, pharmaceuticals, and food safety. The market size is estimated to be over $1 billion globally, growing at 6-8% annually. Technologically, the field shows varying maturity levels, with established players like Thermo Fisher Scientific, Agilent Technologies, and Elemental Scientific leading innovation in automated sample preparation systems. These companies offer comprehensive solutions addressing key challenges such as contamination control and matrix interference. Mid-tier competitors including Shimadzu and Beckman Coulter are expanding their capabilities, while specialized firms like DH Technologies focus on niche applications. Academic institutions such as China University of Geosciences and KAUST contribute to methodological advancements, creating a competitive landscape balanced between established leaders and emerging specialized solution providers.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed comprehensive ICP-MS sample preparation solutions addressing multiple challenges in analytical chemistry. Their PrepFAST automated sample preparation system integrates inline dilution, internal standard addition, and calibration standard preparation to minimize contamination risks and human error. The company's advanced microwave digestion systems (Milestone UltraWAVE) enable high-temperature, high-pressure digestion of complex matrices including organic-rich, refractory, and heterogeneous samples. Thermo Fisher has also pioneered specialized reagent kits designed to minimize background contamination while maximizing dissolution efficiency for specific sample types. Their iCAP RQ and iCAP TQ ICP-MS instruments feature collision/reaction cell technology that works synergistically with their sample preparation protocols to address polyatomic interferences that might otherwise compromise analytical results.
Strengths: Comprehensive ecosystem approach integrating sample preparation with analytical instrumentation; advanced automation capabilities reducing human error; specialized solutions for difficult matrices. Weaknesses: Higher initial investment costs compared to manual methods; proprietary consumables may increase operational costs; complex systems require specialized training.
Elemental Scientific, Inc.
Technical Solution: Elemental Scientific has developed the prepFAST MC system specifically designed to tackle ICP-MS sample preparation challenges. This automated system addresses multiple critical issues in sample preparation through precise micro-volume handling capabilities. The technology employs a dual-syringe system with automated inline dilution that can achieve dilution factors from 2-5000×, significantly reducing contamination risks associated with manual handling. Their proprietary FAST valve technology enables rapid sample switching with minimal carryover (<0.1%), addressing cross-contamination concerns. For challenging matrices, the system incorporates automated matrix removal capabilities using their SC-DX autosampler with integrated column chemistry, allowing for automated separation of analytes from interfering matrix components. The prepFAST MC also features automated internal standard addition with precision better than 2% RSD, ensuring consistent analytical performance across diverse sample types.
Strengths: Superior automation with exceptional precision for micro-volume handling; integrated matrix removal capabilities; minimal carryover between samples; significant reduction in labor and contamination risks. Weaknesses: Requires significant initial capital investment; system complexity necessitates specialized training; potential dependency on proprietary consumables; limited flexibility for extremely unusual sample types.
Critical Innovations in Sample Digestion and Extraction
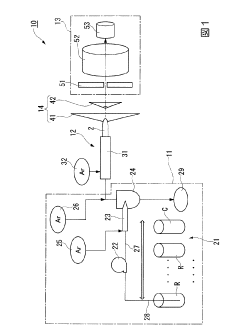
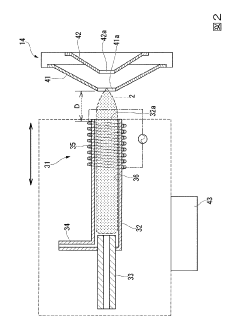
Inductive coupling plasma ms/ms type mass spectroscope
PatentActiveJP2013143196A
Innovation
- A novel differential pumping configuration with independently evacuated vacuum chambers, utilizing quadrupole mass filters and separate turbomolecular pumps to maintain low pressures, reducing ion collisions and extending the mean free path of ions, thereby improving mass selectivity and resolution.
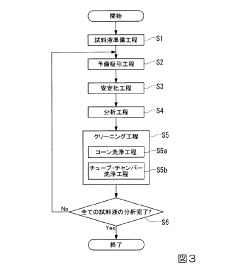
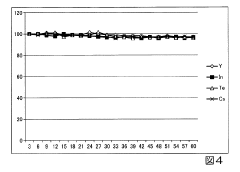
Inductively coupled plasma mass spectrometry method
PatentInactiveJP2016008918A
Innovation
- An inductively coupled plasma mass spectrometry method that allows undiluted high-salt sample analysis, incorporating a cleaning step to remove deposits on the sampler using plasma exposure and a cleaning liquid, maintaining detection sensitivity and accuracy.
Matrix Interference Mitigation Strategies
Matrix interference represents one of the most significant challenges in ICP-MS analysis, often leading to signal suppression, enhancement, or spectral overlap that compromises analytical accuracy. Effective mitigation strategies have evolved substantially over the past decade, with both chemical and instrumental approaches showing promising results in complex sample matrices.
Chemical separation techniques remain fundamental to matrix interference management. Precipitation methods selectively remove interfering elements while preserving analytes of interest, particularly effective for high-salt matrices. Ion exchange chromatography has demonstrated exceptional utility for separating transition metals from biological samples, with recovery rates exceeding 95% in optimized protocols. Chelation chemistry, employing agents such as EDTA and DTPA, provides another powerful approach for selective analyte isolation from complex environmental samples.
Dilution represents a straightforward yet effective strategy, with optimal dilution factors typically ranging from 10-50× depending on matrix complexity. Studies indicate that a 20× dilution can reduce matrix effects by approximately 85% in most biological samples while maintaining acceptable detection limits for most trace elements. This approach is particularly valuable for high-throughput laboratories where sample preparation time is a critical consideration.
Instrumental modifications have significantly advanced matrix interference management capabilities. Collision/reaction cell technology effectively eliminates polyatomic interferences through kinetic energy discrimination or chemical reactions. Modern collision cells utilizing helium achieve interference reduction efficiencies of 99.9% for common polyatomic species. Dynamic reaction cells employing reactive gases like ammonia or oxygen provide element-specific interference removal, particularly valuable for challenging elements like arsenic and selenium in environmental monitoring.
Mathematical correction algorithms have emerged as complementary approaches to hardware solutions. Internal standardization using elements with similar mass and ionization potential to analytes of interest compensates for matrix-induced signal fluctuations. Advanced multivariate statistical methods, including principal component analysis and partial least squares regression, enable sophisticated matrix effect modeling and correction, improving quantification accuracy by up to 30% compared to traditional calibration methods.
Sample introduction modifications, including aerosol dilution, flow injection, and specialized nebulizers, offer additional matrix tolerance. High-efficiency desolvation systems reduce solvent load to the plasma, minimizing oxide formation and enhancing tolerance for organic matrices by factors of 3-5× compared to conventional sample introduction systems.
Chemical separation techniques remain fundamental to matrix interference management. Precipitation methods selectively remove interfering elements while preserving analytes of interest, particularly effective for high-salt matrices. Ion exchange chromatography has demonstrated exceptional utility for separating transition metals from biological samples, with recovery rates exceeding 95% in optimized protocols. Chelation chemistry, employing agents such as EDTA and DTPA, provides another powerful approach for selective analyte isolation from complex environmental samples.
Dilution represents a straightforward yet effective strategy, with optimal dilution factors typically ranging from 10-50× depending on matrix complexity. Studies indicate that a 20× dilution can reduce matrix effects by approximately 85% in most biological samples while maintaining acceptable detection limits for most trace elements. This approach is particularly valuable for high-throughput laboratories where sample preparation time is a critical consideration.
Instrumental modifications have significantly advanced matrix interference management capabilities. Collision/reaction cell technology effectively eliminates polyatomic interferences through kinetic energy discrimination or chemical reactions. Modern collision cells utilizing helium achieve interference reduction efficiencies of 99.9% for common polyatomic species. Dynamic reaction cells employing reactive gases like ammonia or oxygen provide element-specific interference removal, particularly valuable for challenging elements like arsenic and selenium in environmental monitoring.
Mathematical correction algorithms have emerged as complementary approaches to hardware solutions. Internal standardization using elements with similar mass and ionization potential to analytes of interest compensates for matrix-induced signal fluctuations. Advanced multivariate statistical methods, including principal component analysis and partial least squares regression, enable sophisticated matrix effect modeling and correction, improving quantification accuracy by up to 30% compared to traditional calibration methods.
Sample introduction modifications, including aerosol dilution, flow injection, and specialized nebulizers, offer additional matrix tolerance. High-efficiency desolvation systems reduce solvent load to the plasma, minimizing oxide formation and enhancing tolerance for organic matrices by factors of 3-5× compared to conventional sample introduction systems.
Environmental and Safety Considerations in ICP-MS Analysis
Environmental and safety considerations are paramount in ICP-MS analysis due to the potentially hazardous nature of both the analytical process and the samples being analyzed. The high-energy plasma used in ICP-MS operates at temperatures exceeding 6000K, presenting significant thermal hazards that require proper shielding and ventilation systems. Laboratory personnel must be protected from direct exposure to plasma radiation and heat through appropriate engineering controls and safety protocols.
Sample preparation for ICP-MS introduces additional environmental and safety challenges. Many digestion procedures utilize strong acids such as nitric, hydrochloric, and hydrofluoric acids, which pose serious chemical hazards including burns, respiratory damage, and potential long-term health effects. Proper handling protocols, including the use of fume hoods, appropriate personal protective equipment (PPE), and acid-resistant materials, are essential for minimizing exposure risks.
Waste management represents a significant environmental concern in ICP-MS analysis. The process generates liquid waste containing heavy metals, acids, and other potentially toxic substances that require specialized disposal procedures. Laboratories must implement comprehensive waste management systems that comply with local, national, and international regulations to prevent environmental contamination and ensure regulatory compliance.
The analysis of environmental samples introduces additional considerations regarding cross-contamination and sample integrity. Trace element analysis at parts-per-billion or parts-per-trillion levels demands ultra-clean laboratory conditions to prevent ambient contamination. This necessitates specialized clean room facilities, high-purity reagents, and rigorous quality control procedures to ensure accurate results.
Automation of sample preparation processes can significantly reduce both safety risks and environmental impact. Automated systems minimize direct handling of hazardous materials, reduce reagent consumption, and improve waste management efficiency. The implementation of microwave-assisted digestion and other modern sample preparation techniques further reduces acid consumption and waste generation while improving safety profiles.
Sustainable laboratory practices are increasingly important in ICP-MS analysis. This includes the adoption of green chemistry principles such as reagent minimization, use of less hazardous alternatives when possible, and energy-efficient instrumentation. Many laboratories are now implementing environmental management systems to systematically reduce their environmental footprint while maintaining analytical performance.
Sample preparation for ICP-MS introduces additional environmental and safety challenges. Many digestion procedures utilize strong acids such as nitric, hydrochloric, and hydrofluoric acids, which pose serious chemical hazards including burns, respiratory damage, and potential long-term health effects. Proper handling protocols, including the use of fume hoods, appropriate personal protective equipment (PPE), and acid-resistant materials, are essential for minimizing exposure risks.
Waste management represents a significant environmental concern in ICP-MS analysis. The process generates liquid waste containing heavy metals, acids, and other potentially toxic substances that require specialized disposal procedures. Laboratories must implement comprehensive waste management systems that comply with local, national, and international regulations to prevent environmental contamination and ensure regulatory compliance.
The analysis of environmental samples introduces additional considerations regarding cross-contamination and sample integrity. Trace element analysis at parts-per-billion or parts-per-trillion levels demands ultra-clean laboratory conditions to prevent ambient contamination. This necessitates specialized clean room facilities, high-purity reagents, and rigorous quality control procedures to ensure accurate results.
Automation of sample preparation processes can significantly reduce both safety risks and environmental impact. Automated systems minimize direct handling of hazardous materials, reduce reagent consumption, and improve waste management efficiency. The implementation of microwave-assisted digestion and other modern sample preparation techniques further reduces acid consumption and waste generation while improving safety profiles.
Sustainable laboratory practices are increasingly important in ICP-MS analysis. This includes the adoption of green chemistry principles such as reagent minimization, use of less hazardous alternatives when possible, and energy-efficient instrumentation. Many laboratories are now implementing environmental management systems to systematically reduce their environmental footprint while maintaining analytical performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!