Hydrofluoric Acid Applications in Pharmaceutical Processing
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Acid in Pharma: Background and Objectives
Hydrofluoric acid (HF) has emerged as a critical reagent in pharmaceutical processing, with its applications dating back to the early 20th century. Initially utilized primarily in inorganic chemistry, HF's unique properties have gradually found specialized applications in pharmaceutical synthesis and manufacturing processes. The evolution of HF usage in pharmaceuticals has been marked by significant advancements in safety protocols, handling techniques, and application methodologies.
The pharmaceutical industry has witnessed a growing trend toward the utilization of HF in selective fluorination reactions, which are essential for developing novel drug compounds with enhanced bioavailability and metabolic stability. This trend aligns with the broader movement toward more efficient and targeted drug development strategies that have characterized pharmaceutical research over the past two decades.
Current technical objectives for HF applications in pharmaceutical processing center around three primary areas: enhancing reaction selectivity, improving safety measures, and developing more environmentally sustainable processes. Researchers aim to optimize HF-mediated reactions to achieve higher yields and greater stereoselectivity, particularly in the synthesis of fluorinated pharmaceutical intermediates that form the backbone of many modern medications.
The development of buffered HF systems and polymer-supported HF reagents represents a significant advancement in addressing safety concerns while maintaining reactivity. These innovations have enabled more controlled fluorination processes with reduced risks to personnel and equipment, thereby expanding the potential applications of HF in pharmaceutical manufacturing environments.
Another important technical objective involves the integration of HF-based processes with continuous flow chemistry systems. This approach offers advantages in terms of reaction control, safety, and scalability, which are particularly valuable in pharmaceutical production settings where consistency and quality assurance are paramount.
The pharmaceutical industry's increasing focus on green chemistry principles has also driven research into more sustainable HF applications. This includes the development of recyclable HF reagent systems, reduced HF concentration requirements, and alternative fluorination methodologies that maintain efficacy while minimizing environmental impact.
Looking forward, the technical trajectory for HF in pharmaceutical applications is expected to focus on further refinement of selective fluorination techniques, development of novel catalyst systems that enhance HF reactivity under milder conditions, and the creation of more sophisticated containment and handling technologies that address the inherent challenges associated with this powerful but hazardous reagent.
The pharmaceutical industry has witnessed a growing trend toward the utilization of HF in selective fluorination reactions, which are essential for developing novel drug compounds with enhanced bioavailability and metabolic stability. This trend aligns with the broader movement toward more efficient and targeted drug development strategies that have characterized pharmaceutical research over the past two decades.
Current technical objectives for HF applications in pharmaceutical processing center around three primary areas: enhancing reaction selectivity, improving safety measures, and developing more environmentally sustainable processes. Researchers aim to optimize HF-mediated reactions to achieve higher yields and greater stereoselectivity, particularly in the synthesis of fluorinated pharmaceutical intermediates that form the backbone of many modern medications.
The development of buffered HF systems and polymer-supported HF reagents represents a significant advancement in addressing safety concerns while maintaining reactivity. These innovations have enabled more controlled fluorination processes with reduced risks to personnel and equipment, thereby expanding the potential applications of HF in pharmaceutical manufacturing environments.
Another important technical objective involves the integration of HF-based processes with continuous flow chemistry systems. This approach offers advantages in terms of reaction control, safety, and scalability, which are particularly valuable in pharmaceutical production settings where consistency and quality assurance are paramount.
The pharmaceutical industry's increasing focus on green chemistry principles has also driven research into more sustainable HF applications. This includes the development of recyclable HF reagent systems, reduced HF concentration requirements, and alternative fluorination methodologies that maintain efficacy while minimizing environmental impact.
Looking forward, the technical trajectory for HF in pharmaceutical applications is expected to focus on further refinement of selective fluorination techniques, development of novel catalyst systems that enhance HF reactivity under milder conditions, and the creation of more sophisticated containment and handling technologies that address the inherent challenges associated with this powerful but hazardous reagent.
Market Analysis of HF Acid in Pharmaceutical Industry
The global market for hydrofluoric acid (HF) in the pharmaceutical industry has been experiencing steady growth, driven primarily by its critical applications in drug synthesis and manufacturing processes. Currently valued at approximately 320 million USD, this specialized segment of the HF market is projected to grow at a compound annual growth rate of 4.7% through 2028, outpacing the broader industrial chemicals sector.
Demand patterns reveal significant regional variations, with North America and Europe collectively accounting for over 60% of global consumption. This concentration stems from the established pharmaceutical manufacturing bases in these regions. However, Asia-Pacific markets, particularly China and India, are demonstrating the most rapid growth rates, exceeding 7% annually as pharmaceutical manufacturing increasingly shifts to these regions.
The pharmaceutical-grade HF market exhibits distinct characteristics compared to industrial-grade applications. While industrial uses prioritize bulk volume and cost efficiency, pharmaceutical applications demand ultra-high purity (typically 99.99%+), specialized packaging, and rigorous quality control documentation. This specialization commands premium pricing, with pharmaceutical-grade HF typically selling at 3-4 times the price of industrial grades.
Market segmentation analysis reveals that approximately 45% of pharmaceutical HF consumption occurs in active pharmaceutical ingredient (API) synthesis, 30% in drug formulation processes, and the remainder distributed across analytical testing, cleaning validation, and other specialized applications. The growing trend toward complex small molecule drugs has particularly driven demand, as these often require fluorination steps where HF serves as a critical reagent.
Supply chain dynamics present notable challenges, with only a limited number of suppliers globally certified to provide pharmaceutical-grade HF. This concentration creates potential vulnerability to supply disruptions. Recent years have witnessed several supply shortages, particularly following regulatory changes in major producing countries and pandemic-related logistics constraints.
Regulatory factors significantly influence market dynamics, with increasingly stringent environmental and safety regulations impacting both production costs and handling requirements. The pharmaceutical industry's adoption of green chemistry principles is gradually reshaping demand patterns, with some manufacturers seeking alternatives to HF where technically feasible, though complete substitution remains challenging for many critical processes.
Customer segmentation reveals that large pharmaceutical manufacturers account for approximately 65% of market volume, with contract manufacturing organizations (CMOs) representing 25%, and research institutions and smaller specialty pharmaceutical companies comprising the remainder. This distribution underscores the importance of established supply relationships and quality assurance in this specialized market.
Demand patterns reveal significant regional variations, with North America and Europe collectively accounting for over 60% of global consumption. This concentration stems from the established pharmaceutical manufacturing bases in these regions. However, Asia-Pacific markets, particularly China and India, are demonstrating the most rapid growth rates, exceeding 7% annually as pharmaceutical manufacturing increasingly shifts to these regions.
The pharmaceutical-grade HF market exhibits distinct characteristics compared to industrial-grade applications. While industrial uses prioritize bulk volume and cost efficiency, pharmaceutical applications demand ultra-high purity (typically 99.99%+), specialized packaging, and rigorous quality control documentation. This specialization commands premium pricing, with pharmaceutical-grade HF typically selling at 3-4 times the price of industrial grades.
Market segmentation analysis reveals that approximately 45% of pharmaceutical HF consumption occurs in active pharmaceutical ingredient (API) synthesis, 30% in drug formulation processes, and the remainder distributed across analytical testing, cleaning validation, and other specialized applications. The growing trend toward complex small molecule drugs has particularly driven demand, as these often require fluorination steps where HF serves as a critical reagent.
Supply chain dynamics present notable challenges, with only a limited number of suppliers globally certified to provide pharmaceutical-grade HF. This concentration creates potential vulnerability to supply disruptions. Recent years have witnessed several supply shortages, particularly following regulatory changes in major producing countries and pandemic-related logistics constraints.
Regulatory factors significantly influence market dynamics, with increasingly stringent environmental and safety regulations impacting both production costs and handling requirements. The pharmaceutical industry's adoption of green chemistry principles is gradually reshaping demand patterns, with some manufacturers seeking alternatives to HF where technically feasible, though complete substitution remains challenging for many critical processes.
Customer segmentation reveals that large pharmaceutical manufacturers account for approximately 65% of market volume, with contract manufacturing organizations (CMOs) representing 25%, and research institutions and smaller specialty pharmaceutical companies comprising the remainder. This distribution underscores the importance of established supply relationships and quality assurance in this specialized market.
Current Applications and Technical Challenges
Hydrofluoric acid (HF) has established itself as a critical reagent in pharmaceutical processing, with applications spanning multiple stages of drug development and manufacturing. Currently, its primary use centers on the synthesis of fluorinated compounds, which represent approximately 20% of all pharmaceutical products on the market. The strategic incorporation of fluorine atoms into drug molecules enhances their metabolic stability, bioavailability, and binding affinity to target proteins.
In API (Active Pharmaceutical Ingredient) synthesis, HF serves as both a catalyst and a fluorinating agent in various reactions, including nucleophilic fluorination, electrophilic fluorination, and deoxofluorination processes. The pharmaceutical industry particularly values HF for its ability to facilitate the formation of carbon-fluorine bonds under controlled conditions, which is essential for developing drugs targeting conditions such as cancer, cardiovascular diseases, and central nervous system disorders.
Beyond direct synthesis applications, HF finds utility in glass etching processes for pharmaceutical equipment, particularly in the preparation of specialized laboratory glassware and microfluidic devices used in drug discovery. Its ability to dissolve silica makes it valuable for cleaning and preparing surfaces in pharmaceutical manufacturing environments where precision is paramount.
Despite its versatility, the implementation of HF in pharmaceutical processing faces significant technical challenges. Foremost among these is the extreme corrosivity and toxicity of HF, which necessitates specialized handling protocols, dedicated containment systems, and extensive safety measures. This substantially increases operational costs and requires specialized training for personnel.
Equipment compatibility presents another major hurdle, as HF rapidly deteriorates standard processing equipment. Manufacturers must invest in specialized materials such as fluoropolymers (PTFE, PFA), certain grades of stainless steel, or monel alloys, significantly elevating capital expenditures for HF-based processes.
Process control and reaction selectivity remain challenging when working with HF. The high reactivity of the acid often leads to unwanted side reactions and potential degradation of sensitive pharmaceutical intermediates. Achieving consistent quality in fluorinated products requires sophisticated monitoring systems and precise reaction parameters.
Environmental and regulatory constraints further complicate HF utilization. Stringent emission controls, waste management protocols, and increasingly restrictive regulations regarding fluorinated compounds create compliance challenges. The pharmaceutical industry faces growing pressure to develop greener alternatives to traditional HF-based processes, particularly as environmental concerns about persistent fluorinated compounds continue to mount.
In API (Active Pharmaceutical Ingredient) synthesis, HF serves as both a catalyst and a fluorinating agent in various reactions, including nucleophilic fluorination, electrophilic fluorination, and deoxofluorination processes. The pharmaceutical industry particularly values HF for its ability to facilitate the formation of carbon-fluorine bonds under controlled conditions, which is essential for developing drugs targeting conditions such as cancer, cardiovascular diseases, and central nervous system disorders.
Beyond direct synthesis applications, HF finds utility in glass etching processes for pharmaceutical equipment, particularly in the preparation of specialized laboratory glassware and microfluidic devices used in drug discovery. Its ability to dissolve silica makes it valuable for cleaning and preparing surfaces in pharmaceutical manufacturing environments where precision is paramount.
Despite its versatility, the implementation of HF in pharmaceutical processing faces significant technical challenges. Foremost among these is the extreme corrosivity and toxicity of HF, which necessitates specialized handling protocols, dedicated containment systems, and extensive safety measures. This substantially increases operational costs and requires specialized training for personnel.
Equipment compatibility presents another major hurdle, as HF rapidly deteriorates standard processing equipment. Manufacturers must invest in specialized materials such as fluoropolymers (PTFE, PFA), certain grades of stainless steel, or monel alloys, significantly elevating capital expenditures for HF-based processes.
Process control and reaction selectivity remain challenging when working with HF. The high reactivity of the acid often leads to unwanted side reactions and potential degradation of sensitive pharmaceutical intermediates. Achieving consistent quality in fluorinated products requires sophisticated monitoring systems and precise reaction parameters.
Environmental and regulatory constraints further complicate HF utilization. Stringent emission controls, waste management protocols, and increasingly restrictive regulations regarding fluorinated compounds create compliance challenges. The pharmaceutical industry faces growing pressure to develop greener alternatives to traditional HF-based processes, particularly as environmental concerns about persistent fluorinated compounds continue to mount.
Current HF Acid Processing Methods
01 Etching applications of hydrofluoric acid
Hydrofluoric acid is widely used as an etching agent in semiconductor manufacturing and glass processing. It effectively removes silicon dioxide layers and can be formulated with other chemicals to control etching rates and selectivity. Various compositions and methods have been developed to optimize etching performance while minimizing damage to underlying materials and reducing environmental impact.- Etching and cleaning applications in semiconductor manufacturing: Hydrofluoric acid is widely used in semiconductor manufacturing processes for etching silicon dioxide and cleaning silicon wafers. It effectively removes oxide layers, contaminants, and residues from semiconductor surfaces, which is crucial for producing high-quality electronic components. Various formulations and concentrations of hydrofluoric acid are employed depending on the specific etching or cleaning requirements in the fabrication process.
- Recovery and purification methods for hydrofluoric acid: Various techniques have been developed to recover and purify hydrofluoric acid from industrial waste streams. These methods include distillation, adsorption, membrane separation, and chemical precipitation processes. Recovering hydrofluoric acid not only reduces environmental pollution but also provides economic benefits by allowing the reuse of this valuable chemical in various industrial applications.
- Safety measures and neutralization techniques: Due to the highly corrosive and toxic nature of hydrofluoric acid, specialized safety measures and neutralization techniques have been developed. These include the use of specific neutralizing agents like calcium compounds, specialized containment systems, personal protective equipment, and emergency response protocols. Effective neutralization methods are crucial for handling spills and waste disposal to prevent environmental contamination and protect worker safety.
- Production methods of hydrofluoric acid: Various methods for producing hydrofluoric acid have been developed, including the reaction of calcium fluoride (fluorspar) with sulfuric acid, electrochemical processes, and alternative synthesis routes using different fluoride compounds. These production methods focus on improving yield, purity, energy efficiency, and reducing environmental impact. Innovations in production technology aim to meet the growing industrial demand while addressing safety and sustainability concerns.
- Industrial applications beyond semiconductors: Hydrofluoric acid has numerous applications beyond semiconductor manufacturing, including glass etching, metal surface treatment, fluoropolymer production, and as a catalyst in alkylation processes in petroleum refining. It is also used in the production of refrigerants, pharmaceuticals, and various fluorine-containing compounds. Different formulations and concentrations are employed depending on the specific industrial application requirements.
02 Production and purification methods of hydrofluoric acid
Various methods have been developed for the production and purification of hydrofluoric acid. These include processes for manufacturing high-purity hydrofluoric acid from fluoride-containing raw materials, separation techniques to remove impurities, and recycling methods to recover hydrofluoric acid from waste streams. Advanced purification techniques help meet the stringent requirements for semiconductor and electronics applications.Expand Specific Solutions03 Safety measures and handling of hydrofluoric acid
Due to its highly corrosive and toxic nature, specialized safety measures and handling procedures have been developed for hydrofluoric acid. These include containment systems, neutralization methods, personal protective equipment, and emergency response protocols. Various compositions and devices have been designed to detect leaks, neutralize spills, and treat exposure to minimize health risks associated with this dangerous chemical.Expand Specific Solutions04 Waste treatment and environmental applications
Environmental concerns have led to the development of methods for treating hydrofluoric acid waste and reducing its environmental impact. These include neutralization processes, recovery systems, and conversion to less harmful compounds. Some applications focus on using controlled amounts of hydrofluoric acid for environmental remediation, such as removing contaminants from industrial waste or treating polluted soils and water systems.Expand Specific Solutions05 Industrial applications beyond etching
Beyond its well-known etching applications, hydrofluoric acid serves various industrial purposes. It is used in metal surface treatment, cleaning formulations, catalyst preparation, and as a reactant in chemical synthesis. Modified formulations with additives control reactivity and enhance performance for specific applications such as scale removal in oil wells, metal pickling, and as a component in specialized cleaning solutions for industrial equipment.Expand Specific Solutions
Key Pharmaceutical Companies Using HF Acid
The hydrofluoric acid applications in pharmaceutical processing market is currently in a growth phase, with increasing demand driven by pharmaceutical manufacturing expansion. The global market size is estimated at approximately $300-350 million, growing at 5-7% annually due to rising API production requirements. From a technological maturity perspective, established players like DuPont, Honeywell, and Daikin Industries lead with advanced purification technologies and pharmaceutical-grade products, while emerging companies such as Do-Fluoride New Materials and Jiangyin Runma are developing specialized formulations for pharmaceutical applications. The Chemours Co. and Arkema have strengthened their positions through strategic partnerships with pharmaceutical manufacturers, creating a competitive landscape where technical expertise in ultra-high purity acid production and regulatory compliance capabilities determine market leadership.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed a comprehensive HF management system for pharmaceutical applications called "HF-PharmaSafe," which integrates advanced monitoring technology with specialized handling equipment. Their system employs proprietary vapor suppression technology that reduces HF emissions by up to 95% during pharmaceutical processing operations. Honeywell's solution includes patented fluoropolymer-lined equipment specifically designed for pharmaceutical-grade purity requirements, preventing metal contamination in API production. The company's integrated safety platform incorporates real-time HF detection systems with sensitivity down to sub-ppm levels, crucial for maintaining GMP compliance in pharmaceutical facilities. Honeywell also offers specialized training programs and certification for pharmaceutical personnel working with HF, addressing both regulatory compliance and operational safety concerns in API synthesis and drug manufacturing processes.
Strengths: Comprehensive end-to-end HF management solutions; advanced monitoring and detection systems; strong focus on regulatory compliance for pharmaceutical applications. Weaknesses: Complex implementation requiring significant facility modifications; higher initial investment compared to conventional systems; ongoing maintenance requirements for specialized equipment.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has pioneered pharmaceutical-specific HF handling technology through their "PharmaFluor" system, which provides controlled fluorination capabilities for complex API synthesis. Their technology employs specialized fluoropolymer containment systems that maintain HF purity while minimizing exposure risks in pharmaceutical manufacturing environments. DAIKIN's proprietary HF delivery systems feature precision flow control with accuracy to ±0.1%, critical for consistent pharmaceutical reactions and quality control. The company has developed specialized catalytic systems that enable lower-temperature HF-based reactions, reducing energy requirements and improving safety profiles in pharmaceutical applications. DAIKIN also offers customized HF recovery and recycling systems specifically designed for pharmaceutical facilities, addressing both environmental concerns and operational cost efficiency while maintaining strict pharmaceutical purity standards.
Strengths: Specialized fluoropolymer technology optimized for pharmaceutical applications; precision control systems for consistent API production; integrated recycling capabilities. Weaknesses: Limited flexibility for process modifications once installed; higher specialized maintenance requirements; significant technical expertise needed for operation.
Critical Patents and Technical Literature
Method for producing diluted hydrofluoric acid
PatentActiveUS20180148332A1
Innovation
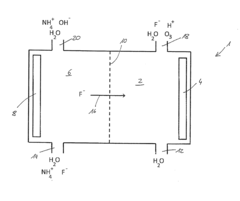
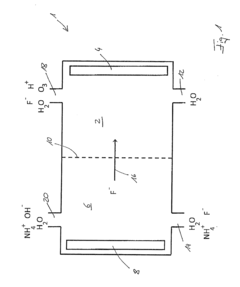
- A method using an electrode arrangement with an anode and cathode chambers separated by an anion exchange membrane, where pure water and an electrolyte solution containing fluoride ions are electrolyzed to produce dilute hydrofluoric acid with precise concentration control, and ozone can be simultaneously produced, with the ability to adjust concentrations independently using electrical current and electrolyte concentration.
Acid dermatological composition
PatentInactiveEP0048443A1
Innovation
- A pharmaceutical preparation using hydrochloric acid with a pH of 1.5 or less is employed, which effectively kills microorganisms by damaging their metabolism without harming treated tissue, leveraging the skin's biological buffering capabilities.
Safety Protocols and Regulatory Compliance
The implementation of hydrofluoric acid (HF) in pharmaceutical processing necessitates rigorous safety protocols and strict adherence to regulatory frameworks due to its highly corrosive and toxic nature. Pharmaceutical manufacturers utilizing HF must establish comprehensive safety management systems that include detailed standard operating procedures (SOPs), regular risk assessments, and emergency response plans specifically tailored for HF-related incidents.
Personal protective equipment (PPE) requirements for handling HF are exceptionally stringent, mandating chemical-resistant full-body protection, face shields, specialized gloves, and respiratory protection. Facilities must install specialized ventilation systems with acid-resistant components, emergency eyewash stations, and safety showers positioned strategically throughout processing areas. Continuous air monitoring systems that can detect HF at concentrations well below permissible exposure limits are essential for early warning.
Regulatory compliance for HF usage spans multiple frameworks. In the United States, the FDA's Current Good Manufacturing Practices (cGMP) regulations govern HF applications in pharmaceutical production, while OSHA standards (particularly 29 CFR 1910.1200) address worker safety requirements. The EPA regulates waste disposal through the Resource Conservation and Recovery Act (RCRA), classifying HF waste as hazardous. Internationally, organizations must navigate region-specific regulations such as the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and GMP requirements.
Employee training represents a critical compliance component, requiring documented certification programs covering HF properties, handling procedures, exposure symptoms, first-aid measures, and emergency protocols. Medical surveillance programs must be implemented for workers regularly exposed to HF, including baseline and periodic health assessments focused on respiratory function and potential bone density impacts from chronic low-level exposure.
Documentation and record-keeping systems must track all aspects of HF management, including usage quantities, exposure monitoring results, equipment maintenance, training records, and incident reports. These records are subject to regulatory inspection and serve as evidence of compliance during audits.
Waste management protocols for HF require specialized neutralization procedures before disposal, with strict documentation of the entire waste stream. Many jurisdictions mandate third-party certified disposal services with appropriate permits for handling hazardous chemical waste.
Industry best practices increasingly emphasize substitution strategies where feasible, exploring alternative processing methods that can achieve similar pharmaceutical outcomes without HF's inherent hazards. When substitution is not possible, process containment through closed systems and automation technologies can significantly reduce exposure risks while maintaining regulatory compliance.
Personal protective equipment (PPE) requirements for handling HF are exceptionally stringent, mandating chemical-resistant full-body protection, face shields, specialized gloves, and respiratory protection. Facilities must install specialized ventilation systems with acid-resistant components, emergency eyewash stations, and safety showers positioned strategically throughout processing areas. Continuous air monitoring systems that can detect HF at concentrations well below permissible exposure limits are essential for early warning.
Regulatory compliance for HF usage spans multiple frameworks. In the United States, the FDA's Current Good Manufacturing Practices (cGMP) regulations govern HF applications in pharmaceutical production, while OSHA standards (particularly 29 CFR 1910.1200) address worker safety requirements. The EPA regulates waste disposal through the Resource Conservation and Recovery Act (RCRA), classifying HF waste as hazardous. Internationally, organizations must navigate region-specific regulations such as the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and GMP requirements.
Employee training represents a critical compliance component, requiring documented certification programs covering HF properties, handling procedures, exposure symptoms, first-aid measures, and emergency protocols. Medical surveillance programs must be implemented for workers regularly exposed to HF, including baseline and periodic health assessments focused on respiratory function and potential bone density impacts from chronic low-level exposure.
Documentation and record-keeping systems must track all aspects of HF management, including usage quantities, exposure monitoring results, equipment maintenance, training records, and incident reports. These records are subject to regulatory inspection and serve as evidence of compliance during audits.
Waste management protocols for HF require specialized neutralization procedures before disposal, with strict documentation of the entire waste stream. Many jurisdictions mandate third-party certified disposal services with appropriate permits for handling hazardous chemical waste.
Industry best practices increasingly emphasize substitution strategies where feasible, exploring alternative processing methods that can achieve similar pharmaceutical outcomes without HF's inherent hazards. When substitution is not possible, process containment through closed systems and automation technologies can significantly reduce exposure risks while maintaining regulatory compliance.
Environmental Impact Assessment
The environmental impact of hydrofluoric acid (HF) in pharmaceutical processing requires comprehensive assessment due to its significant hazard potential. HF is classified as an extremely corrosive and toxic substance that poses serious risks to both human health and ecological systems when released into the environment. Pharmaceutical manufacturing facilities utilizing HF must implement rigorous containment protocols to prevent atmospheric emissions, which can contribute to air pollution and potentially cause acid rain in surrounding areas.
Water contamination represents one of the most critical environmental concerns associated with HF usage. Even at low concentrations, HF can dramatically alter aquatic pH levels, resulting in devastating effects on aquatic ecosystems and biodiversity. Discharge regulations typically mandate neutralization treatments before any HF-containing wastewater can be released into municipal systems or natural water bodies.
Soil contamination from HF spills presents long-term environmental challenges, as the acid can persist in soil matrices and potentially leach into groundwater supplies. This contamination pathway threatens both terrestrial ecosystems and drinking water sources for nearby communities. The fluoride ions released from HF can accumulate in soil, affecting plant growth and potentially entering the food chain.
Regulatory frameworks worldwide have established increasingly stringent environmental standards for HF handling in pharmaceutical operations. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in Asia-Pacific regions require detailed environmental impact assessments, continuous monitoring programs, and emergency response protocols for facilities utilizing HF.
Modern pharmaceutical facilities have implemented various mitigation technologies to address these environmental concerns. Closed-loop processing systems minimize waste generation, while advanced scrubber technologies can capture HF emissions with efficiency rates exceeding 99%. Wastewater treatment processes specifically designed for fluoride removal, including precipitation methods using calcium compounds, have become standard practice in the industry.
Life cycle assessment (LCA) studies indicate that pharmaceutical processes utilizing HF generate significant environmental footprints compared to alternative approaches. These assessments consider not only direct emissions but also energy consumption, transportation risks, and end-of-life disposal challenges associated with HF-containing waste streams.
The pharmaceutical industry has increasingly explored green chemistry alternatives to reduce or eliminate HF usage where technically feasible. These include the development of less hazardous catalysts, ionic liquids as substitutes, and enzymatic processes that can achieve similar chemical transformations without the environmental liabilities associated with hydrofluoric acid.
Water contamination represents one of the most critical environmental concerns associated with HF usage. Even at low concentrations, HF can dramatically alter aquatic pH levels, resulting in devastating effects on aquatic ecosystems and biodiversity. Discharge regulations typically mandate neutralization treatments before any HF-containing wastewater can be released into municipal systems or natural water bodies.
Soil contamination from HF spills presents long-term environmental challenges, as the acid can persist in soil matrices and potentially leach into groundwater supplies. This contamination pathway threatens both terrestrial ecosystems and drinking water sources for nearby communities. The fluoride ions released from HF can accumulate in soil, affecting plant growth and potentially entering the food chain.
Regulatory frameworks worldwide have established increasingly stringent environmental standards for HF handling in pharmaceutical operations. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in Asia-Pacific regions require detailed environmental impact assessments, continuous monitoring programs, and emergency response protocols for facilities utilizing HF.
Modern pharmaceutical facilities have implemented various mitigation technologies to address these environmental concerns. Closed-loop processing systems minimize waste generation, while advanced scrubber technologies can capture HF emissions with efficiency rates exceeding 99%. Wastewater treatment processes specifically designed for fluoride removal, including precipitation methods using calcium compounds, have become standard practice in the industry.
Life cycle assessment (LCA) studies indicate that pharmaceutical processes utilizing HF generate significant environmental footprints compared to alternative approaches. These assessments consider not only direct emissions but also energy consumption, transportation risks, and end-of-life disposal challenges associated with HF-containing waste streams.
The pharmaceutical industry has increasingly explored green chemistry alternatives to reduce or eliminate HF usage where technically feasible. These include the development of less hazardous catalysts, ionic liquids as substitutes, and enzymatic processes that can achieve similar chemical transformations without the environmental liabilities associated with hydrofluoric acid.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!