Hydrofluoric Acid in Waste Management: Impact Reduction Methods
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF Acid Waste Management Background and Objectives
Hydrofluoric acid (HF) has been a critical component in various industrial processes for decades, particularly in semiconductor manufacturing, glass etching, and metal surface treatment. The evolution of HF usage in industry can be traced back to the early 20th century, with significant expansion during the technology boom of the 1970s and 1980s. As industrial applications have grown, so too have the challenges associated with HF waste management, creating an urgent need for innovative reduction methods.
The technical landscape surrounding HF waste management has evolved considerably over the past three decades. Initial approaches focused primarily on neutralization techniques, which, while effective at reducing immediate hazards, often resulted in secondary waste streams requiring further treatment. The 1990s saw a shift toward recovery and recycling methodologies, though these were limited by technological constraints and economic feasibility concerns.
Current technical trends indicate a movement toward integrated waste minimization strategies that combine process optimization, substitution technologies, and advanced treatment methods. This holistic approach represents a significant departure from earlier end-of-pipe solutions and aligns with broader sustainability objectives across industries.
The primary technical objective of HF waste management innovation is to develop cost-effective, environmentally sound methods that minimize the volume and toxicity of HF-containing waste while maximizing resource recovery. Specific goals include reducing HF waste generation at source by at least 30%, developing treatment technologies that achieve 99.9% removal efficiency, and creating closed-loop systems that enable the recovery and reuse of fluoride compounds.
Secondary objectives encompass the development of real-time monitoring systems for HF waste streams, standardization of treatment protocols across industries, and the creation of predictive models for assessing environmental impact. These objectives are increasingly driven by stringent regulatory frameworks, including the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) in the United States, as well as comparable legislation internationally.
The technical trajectory for HF waste management is expected to increasingly incorporate advanced materials science, nanotechnology, and artificial intelligence to develop more selective, efficient, and sustainable treatment methods. Emerging research in areas such as selective membrane technologies, advanced oxidation processes, and bioremediation shows particular promise for addressing the persistent challenges of HF waste management while meeting increasingly stringent environmental standards.
The technical landscape surrounding HF waste management has evolved considerably over the past three decades. Initial approaches focused primarily on neutralization techniques, which, while effective at reducing immediate hazards, often resulted in secondary waste streams requiring further treatment. The 1990s saw a shift toward recovery and recycling methodologies, though these were limited by technological constraints and economic feasibility concerns.
Current technical trends indicate a movement toward integrated waste minimization strategies that combine process optimization, substitution technologies, and advanced treatment methods. This holistic approach represents a significant departure from earlier end-of-pipe solutions and aligns with broader sustainability objectives across industries.
The primary technical objective of HF waste management innovation is to develop cost-effective, environmentally sound methods that minimize the volume and toxicity of HF-containing waste while maximizing resource recovery. Specific goals include reducing HF waste generation at source by at least 30%, developing treatment technologies that achieve 99.9% removal efficiency, and creating closed-loop systems that enable the recovery and reuse of fluoride compounds.
Secondary objectives encompass the development of real-time monitoring systems for HF waste streams, standardization of treatment protocols across industries, and the creation of predictive models for assessing environmental impact. These objectives are increasingly driven by stringent regulatory frameworks, including the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) in the United States, as well as comparable legislation internationally.
The technical trajectory for HF waste management is expected to increasingly incorporate advanced materials science, nanotechnology, and artificial intelligence to develop more selective, efficient, and sustainable treatment methods. Emerging research in areas such as selective membrane technologies, advanced oxidation processes, and bioremediation shows particular promise for addressing the persistent challenges of HF waste management while meeting increasingly stringent environmental standards.
Market Analysis of HF Acid Waste Treatment Solutions
The global market for hydrofluoric acid (HF) waste treatment solutions has experienced significant growth in recent years, driven by stringent environmental regulations and increasing awareness of the hazardous nature of HF acid waste. The market size was valued at approximately $1.2 billion in 2022 and is projected to reach $1.8 billion by 2028, representing a compound annual growth rate (CAGR) of 6.7%.
The semiconductor industry remains the largest consumer segment for HF acid waste treatment solutions, accounting for nearly 40% of the market share. This dominance is attributed to the extensive use of HF in silicon wafer cleaning and etching processes. The chemical manufacturing sector follows closely, representing about 25% of the market, while metal processing and glass etching industries collectively account for another 20%.
Geographically, Asia-Pacific leads the market with approximately 45% share, primarily due to the high concentration of semiconductor manufacturing facilities in countries like Taiwan, South Korea, and China. North America and Europe follow with 25% and 20% market shares respectively, driven by strict regulatory frameworks governing hazardous waste management.
The market is witnessing a notable shift toward sustainable and cost-effective treatment solutions. Neutralization technologies currently dominate with about 35% market share, followed by precipitation methods at 25%. However, advanced technologies such as ion exchange, membrane filtration, and electrochemical treatment are gaining traction, collectively growing at a CAGR of 8.5%, outpacing traditional methods.
Key market drivers include increasingly stringent environmental regulations, particularly in developed economies, which mandate comprehensive treatment of HF-containing waste. The REACH regulations in Europe and the EPA guidelines in the United States have significantly influenced market dynamics, pushing companies toward adoption of more effective treatment solutions.
Cost considerations remain a significant factor influencing market adoption. The average implementation cost for comprehensive HF waste treatment systems ranges from $500,000 to $3 million, depending on capacity and technology sophistication. However, the long-term operational savings and avoided environmental compliance penalties are creating a favorable return on investment scenario for many industrial users.
Market challenges include high initial capital requirements, technical complexity of advanced treatment systems, and varying regulatory standards across different regions. These factors have created entry barriers for smaller players and have led to market consolidation, with the top five solution providers controlling approximately 40% of the global market share.
The semiconductor industry remains the largest consumer segment for HF acid waste treatment solutions, accounting for nearly 40% of the market share. This dominance is attributed to the extensive use of HF in silicon wafer cleaning and etching processes. The chemical manufacturing sector follows closely, representing about 25% of the market, while metal processing and glass etching industries collectively account for another 20%.
Geographically, Asia-Pacific leads the market with approximately 45% share, primarily due to the high concentration of semiconductor manufacturing facilities in countries like Taiwan, South Korea, and China. North America and Europe follow with 25% and 20% market shares respectively, driven by strict regulatory frameworks governing hazardous waste management.
The market is witnessing a notable shift toward sustainable and cost-effective treatment solutions. Neutralization technologies currently dominate with about 35% market share, followed by precipitation methods at 25%. However, advanced technologies such as ion exchange, membrane filtration, and electrochemical treatment are gaining traction, collectively growing at a CAGR of 8.5%, outpacing traditional methods.
Key market drivers include increasingly stringent environmental regulations, particularly in developed economies, which mandate comprehensive treatment of HF-containing waste. The REACH regulations in Europe and the EPA guidelines in the United States have significantly influenced market dynamics, pushing companies toward adoption of more effective treatment solutions.
Cost considerations remain a significant factor influencing market adoption. The average implementation cost for comprehensive HF waste treatment systems ranges from $500,000 to $3 million, depending on capacity and technology sophistication. However, the long-term operational savings and avoided environmental compliance penalties are creating a favorable return on investment scenario for many industrial users.
Market challenges include high initial capital requirements, technical complexity of advanced treatment systems, and varying regulatory standards across different regions. These factors have created entry barriers for smaller players and have led to market consolidation, with the top five solution providers controlling approximately 40% of the global market share.
Current Challenges in HF Acid Waste Management
Hydrofluoric acid (HF) waste management presents significant challenges across multiple industries, particularly in semiconductor manufacturing, chemical processing, and metal treatment. The corrosive and highly toxic nature of HF creates complex handling requirements that conventional waste treatment systems often struggle to address adequately. Current disposal methods frequently fail to completely neutralize or safely contain HF, resulting in potential environmental contamination and health hazards.
One primary challenge is the inadequate neutralization efficiency in existing treatment systems. Traditional lime-based neutralization methods often achieve incomplete reactions with HF, leaving residual acid that can later reactivate under certain environmental conditions. This "rebound effect" poses significant long-term monitoring challenges and increases the risk of delayed environmental impacts, particularly in groundwater systems where detection may be delayed.
The transportation of HF waste presents another critical challenge, as specialized containment vessels are required due to HF's ability to penetrate standard materials. Current transportation protocols often lack sufficient safeguards against accidental releases during transit, creating potential exposure risks for workers and communities along transportation routes. The high cost of specialized containment systems further complicates proper management, particularly for smaller industrial operations.
Regulatory frameworks governing HF waste management remain inconsistent across different regions, creating compliance challenges for multinational corporations and allowing for potential regulatory arbitrage. The absence of standardized treatment protocols results in varying levels of treatment effectiveness and safety measures. This regulatory fragmentation impedes the development and implementation of best practices across the industry.
Monitoring technologies for HF waste streams show significant limitations in accuracy and reliability, particularly for dilute concentrations that still pose environmental risks. Current detection systems often fail to provide real-time data necessary for rapid response to potential releases or treatment failures. The inability to effectively track HF throughout the waste management lifecycle creates blind spots in environmental protection efforts.
The economic burden of proper HF waste management drives some facilities toward minimally compliant solutions rather than optimal treatment approaches. The high costs associated with advanced treatment technologies, specialized handling equipment, and trained personnel create significant barriers to implementation, particularly in developing economies where regulatory enforcement may be less stringent.
Worker safety remains a persistent challenge, with current personal protective equipment offering inadequate protection against HF exposure in many waste management scenarios. Training programs often fail to fully prepare personnel for the unique hazards associated with HF waste handling, increasing occupational risk factors throughout the waste management chain.
One primary challenge is the inadequate neutralization efficiency in existing treatment systems. Traditional lime-based neutralization methods often achieve incomplete reactions with HF, leaving residual acid that can later reactivate under certain environmental conditions. This "rebound effect" poses significant long-term monitoring challenges and increases the risk of delayed environmental impacts, particularly in groundwater systems where detection may be delayed.
The transportation of HF waste presents another critical challenge, as specialized containment vessels are required due to HF's ability to penetrate standard materials. Current transportation protocols often lack sufficient safeguards against accidental releases during transit, creating potential exposure risks for workers and communities along transportation routes. The high cost of specialized containment systems further complicates proper management, particularly for smaller industrial operations.
Regulatory frameworks governing HF waste management remain inconsistent across different regions, creating compliance challenges for multinational corporations and allowing for potential regulatory arbitrage. The absence of standardized treatment protocols results in varying levels of treatment effectiveness and safety measures. This regulatory fragmentation impedes the development and implementation of best practices across the industry.
Monitoring technologies for HF waste streams show significant limitations in accuracy and reliability, particularly for dilute concentrations that still pose environmental risks. Current detection systems often fail to provide real-time data necessary for rapid response to potential releases or treatment failures. The inability to effectively track HF throughout the waste management lifecycle creates blind spots in environmental protection efforts.
The economic burden of proper HF waste management drives some facilities toward minimally compliant solutions rather than optimal treatment approaches. The high costs associated with advanced treatment technologies, specialized handling equipment, and trained personnel create significant barriers to implementation, particularly in developing economies where regulatory enforcement may be less stringent.
Worker safety remains a persistent challenge, with current personal protective equipment offering inadequate protection against HF exposure in many waste management scenarios. Training programs often fail to fully prepare personnel for the unique hazards associated with HF waste handling, increasing occupational risk factors throughout the waste management chain.
Existing HF Acid Neutralization and Containment Methods
01 Neutralization and chemical treatment methods
Various chemical treatments can be employed to neutralize hydrofluoric acid and reduce its harmful impacts. These methods include using alkaline substances like calcium hydroxide, sodium hydroxide, or magnesium compounds to neutralize the acid. Chemical treatments can convert the highly corrosive hydrofluoric acid into less harmful compounds, thereby reducing its impact on both human health and the environment. These neutralization processes are critical in industrial settings where hydrofluoric acid is used or produced.- Neutralization and chemical treatment methods: Various chemical treatments can be employed to neutralize hydrofluoric acid and reduce its harmful impacts. These include using alkaline substances like calcium hydroxide, sodium hydroxide, or magnesium compounds to neutralize the acid. Chemical treatments can convert the highly corrosive hydrofluoric acid into less harmful compounds, thereby reducing its impact on both human health and the environment. These methods are particularly important in industrial settings where hydrofluoric acid is commonly used.
- Protective equipment and safety measures: Implementing proper protective equipment and safety measures is crucial for reducing the impact of hydrofluoric acid exposure. This includes specialized acid-resistant clothing, gloves, face shields, and respiratory protection. Additionally, emergency response protocols, such as immediate washing procedures and the availability of calcium gluconate gel for treatment of exposure, can significantly reduce the severity of hydrofluoric acid injuries. Training personnel on proper handling techniques and emergency procedures is also essential for minimizing risks.
- Waste treatment and environmental protection systems: Systems designed for treating hydrofluoric acid waste and protecting the environment from its harmful effects are essential for impact reduction. These include specialized wastewater treatment processes that can remove fluoride ions, scrubbing systems for air emissions containing hydrofluoric acid, and containment strategies to prevent environmental contamination. Such systems often incorporate multiple stages of treatment to ensure that hydrofluoric acid is adequately neutralized before discharge into the environment.
- Alternative processes and acid substitution: Developing and implementing alternative processes that reduce or eliminate the need for hydrofluoric acid can significantly decrease its impact. This includes using less hazardous acids or chemicals as substitutes in industrial processes, modifying manufacturing techniques to avoid hydrofluoric acid usage, and developing new technologies that achieve the same results without the associated risks. These approaches not only reduce direct exposure risks but also minimize the generation of hazardous waste requiring special handling.
- Monitoring and detection systems: Advanced monitoring and detection systems play a crucial role in reducing hydrofluoric acid impacts by providing early warning of leaks or dangerous concentration levels. These systems include specialized sensors that can detect hydrofluoric acid vapors, automated monitoring equipment for workplace environments, and integrated alarm systems that alert personnel to potential hazards. Real-time monitoring allows for immediate response to leaks or spills, significantly reducing exposure time and potential damage to both personnel and equipment.
02 Protective equipment and safety measures
Implementing appropriate protective equipment and safety measures is essential for reducing the impact of hydrofluoric acid exposure. This includes specialized acid-resistant clothing, gloves, face shields, and respiratory protection. Safety protocols such as emergency shower stations, eyewash facilities, and proper ventilation systems are also crucial. Training personnel on proper handling procedures and emergency response can significantly reduce the risk of injury from hydrofluoric acid exposure.Expand Specific Solutions03 Waste treatment and environmental protection
Methods for treating hydrofluoric acid waste to minimize environmental impact include specialized waste treatment processes, recycling techniques, and containment strategies. These approaches focus on preventing the release of hydrofluoric acid into the environment, treating contaminated water, and managing acid waste in environmentally responsible ways. Advanced filtration systems, chemical precipitation methods, and ion exchange technologies can be employed to remove fluoride ions from wastewater before discharge.Expand Specific Solutions04 Medical treatments for hydrofluoric acid exposure
Specific medical interventions have been developed to treat hydrofluoric acid exposure and reduce its impact on human tissue. These include calcium gluconate gel applications, calcium gluconate injections for deeper burns, and specialized washing protocols. Quick medical response is critical as hydrofluoric acid can penetrate skin and cause deep tissue damage. Treatment protocols focus on neutralizing the acid and preventing the calcium depletion that makes hydrofluoric acid particularly dangerous to living tissue.Expand Specific Solutions05 Alternative technologies and substitution methods
Development of alternative technologies and substances to replace hydrofluoric acid in industrial processes can significantly reduce its impact. These alternatives include modified etching compounds, non-fluoride based cleaning agents, and redesigned manufacturing processes. By substituting hydrofluoric acid with less hazardous materials or implementing processes that require smaller quantities of the acid, industries can minimize the risks associated with its use while maintaining production efficiency.Expand Specific Solutions
Leading Companies in HF Waste Management Industry
The hydrofluoric acid waste management technology landscape is currently in a growth phase, with an estimated market size of $1.2-1.5 billion annually and expanding at 5-7% CAGR. The competitive environment features established players with diverse technological approaches. Companies like Kurita Water Industries, DuPont, and Organo Corp lead with advanced neutralization and recovery systems, while Stella Chemifa and Mitsubishi Chemical Engineering focus on specialized fluoride recycling technologies. Emerging players such as Daiseki and Anhui Chaoyue Environmental Technology are developing innovative biological treatment methods. The technology maturity varies significantly across different treatment approaches, with chemical neutralization being most established while membrane filtration and electrochemical recovery systems (championed by Samsung Display and Sharp Corp) represent cutting-edge solutions still gaining commercial adoption.
Kurita Water Industries Ltd.
Technical Solution: Kurita Water Industries has developed advanced hydrofluoric acid (HF) waste treatment systems utilizing calcium precipitation technology. Their process involves a two-stage neutralization approach where HF waste is first treated with calcium hydroxide to form calcium fluoride precipitates, followed by a polishing stage using proprietary adsorbent media to capture residual fluoride ions. The company has engineered specialized reactor designs that optimize mixing efficiency and precipitation kinetics, reducing reaction time by approximately 40% compared to conventional systems. Their automated control systems continuously monitor pH, fluoride concentration, and calcium dosage to maintain optimal treatment conditions. Kurita's technology includes a closed-loop water recycling component that reduces freshwater consumption by up to 70% in the treatment process. The resulting calcium fluoride sludge undergoes dewatering using their proprietary filter press technology, achieving solid content exceeding 40% by weight, significantly reducing disposal volume and associated costs[1][3].
Strengths: Highly efficient fluoride removal (>99.5% removal efficiency); closed-loop water recycling reduces environmental impact; automated process control ensures consistent treatment quality. Weaknesses: Higher initial capital investment compared to basic neutralization systems; requires specialized maintenance expertise; calcium fluoride sludge still requires proper disposal management.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering has developed an innovative biological treatment system for hydrofluoric acid waste management. Their approach utilizes specially adapted microbial consortia that can tolerate and metabolize fluoride compounds at concentrations that would typically inhibit biological activity. The process employs a sequential bioreactor configuration with gradually increasing fluoride concentrations to acclimate the microbial populations. Their system incorporates specialized immobilization techniques using porous ceramic carriers that protect microorganisms from toxic shock while maximizing surface area for biofilm development. The institute has engineered the process to operate under carefully controlled pH and nutrient conditions that optimize biological fluoride transformation. Their technology includes a hybrid approach combining biological treatment with subsequent adsorption using modified natural materials such as bone char and modified clay minerals. Research has demonstrated fluoride removal efficiencies of up to 95% from dilute waste streams with significantly reduced chemical inputs compared to conventional chemical precipitation methods[8][10].
Strengths: Lower chemical consumption and operating costs compared to purely chemical methods; reduced sludge production; environmentally sustainable approach with minimal secondary pollution. Weaknesses: Limited effectiveness for high-concentration HF waste; requires careful control of operating conditions; longer treatment time compared to chemical precipitation methods.
Key Innovations in HF Waste Detoxification Processes
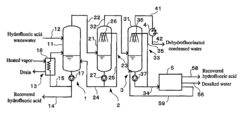
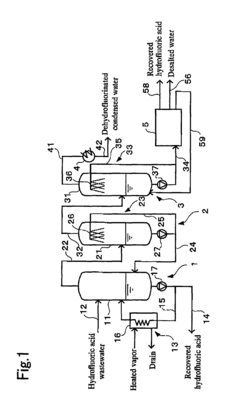
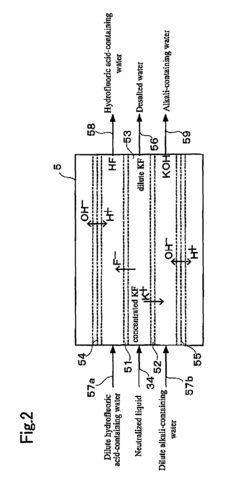
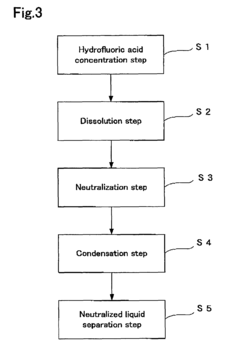
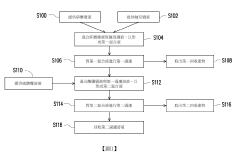
Hydrofluoric acid wastewater treatment method and device
PatentInactiveUS7311799B2
Innovation
- A method involving concentration by evaporation, followed by dissolution with water, neutralization with an alkali, and condensation, utilizing ion exchange membranes to separate hydrofluoric acid-containing water, alkali-containing water, and desalted water, effectively recovering hydrofluoric acid and reducing its concentration in the treated water.
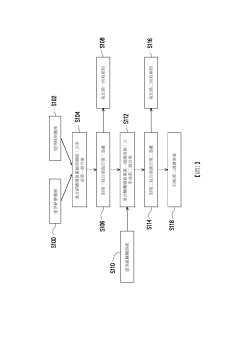
Method for processing waste polishing solution and waste solution containing hydrofluoric acid
PatentActiveTW202202452A
Innovation
- A method involving the reaction of grinding waste liquid with hydrofluoric acid to form water-insoluble fluorides, followed by reacting fluorosilicic acid with metal salts to produce water-insoluble fluorosilicates, utilizing cerium oxide, alumina, and sodium sulfate to reduce toxicity and recover valuable products.
Environmental Compliance and Regulatory Framework
The regulatory landscape governing hydrofluoric acid (HF) waste management is complex and multifaceted, with frameworks established at international, national, and local levels. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal specifically categorizes HF waste as hazardous, mandating strict protocols for its cross-border transportation and disposal. This international framework provides the foundation upon which many national regulations are built.
In the United States, the Environmental Protection Agency (EPA) regulates HF waste under multiple statutes, including the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). The RCRA classifies HF waste as F001, requiring cradle-to-grave tracking and specialized disposal methods. Additionally, the Clean Air Act and Clean Water Act impose stringent emission and discharge limits for facilities handling HF waste.
European regulations, particularly under the European Union's Waste Framework Directive (2008/98/EC) and REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, implement even more stringent controls. These frameworks emphasize waste prevention, recycling, and recovery over disposal, with specific attention to hazardous substances like HF.
Compliance requirements typically include detailed waste characterization, proper labeling, secure containment, comprehensive record-keeping, regular reporting, and specialized training for personnel handling HF waste. Facilities must obtain specific permits that often mandate implementation of Best Available Techniques (BAT) for waste treatment and disposal.
Recent regulatory trends show increasing stringency in permissible emission levels and growing emphasis on circular economy principles. Several jurisdictions have introduced extended producer responsibility (EPR) schemes, requiring manufacturers using HF to participate in end-of-life management of their products and associated waste streams.
Penalties for non-compliance with HF waste regulations are severe, often including substantial fines, facility closure, and even criminal prosecution for egregious violations. The European Union's Environmental Liability Directive and similar frameworks in other regions establish the "polluter pays" principle, holding organizations financially responsible for environmental remediation costs.
Companies operating globally must navigate this complex regulatory mosaic by implementing comprehensive environmental management systems that can adapt to varying regional requirements while maintaining consistent internal standards for HF waste handling, treatment, and disposal.
In the United States, the Environmental Protection Agency (EPA) regulates HF waste under multiple statutes, including the Resource Conservation and Recovery Act (RCRA) and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). The RCRA classifies HF waste as F001, requiring cradle-to-grave tracking and specialized disposal methods. Additionally, the Clean Air Act and Clean Water Act impose stringent emission and discharge limits for facilities handling HF waste.
European regulations, particularly under the European Union's Waste Framework Directive (2008/98/EC) and REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, implement even more stringent controls. These frameworks emphasize waste prevention, recycling, and recovery over disposal, with specific attention to hazardous substances like HF.
Compliance requirements typically include detailed waste characterization, proper labeling, secure containment, comprehensive record-keeping, regular reporting, and specialized training for personnel handling HF waste. Facilities must obtain specific permits that often mandate implementation of Best Available Techniques (BAT) for waste treatment and disposal.
Recent regulatory trends show increasing stringency in permissible emission levels and growing emphasis on circular economy principles. Several jurisdictions have introduced extended producer responsibility (EPR) schemes, requiring manufacturers using HF to participate in end-of-life management of their products and associated waste streams.
Penalties for non-compliance with HF waste regulations are severe, often including substantial fines, facility closure, and even criminal prosecution for egregious violations. The European Union's Environmental Liability Directive and similar frameworks in other regions establish the "polluter pays" principle, holding organizations financially responsible for environmental remediation costs.
Companies operating globally must navigate this complex regulatory mosaic by implementing comprehensive environmental management systems that can adapt to varying regional requirements while maintaining consistent internal standards for HF waste handling, treatment, and disposal.
Risk Assessment and Safety Protocols for HF Handling
Handling hydrofluoric acid (HF) requires comprehensive risk assessment and stringent safety protocols due to its unique hazard profile. The dual threat of HF stems from its corrosive properties and systemic toxicity, which can cause severe tissue damage and potentially fatal systemic effects through calcium sequestration. Risk assessment for HF handling in waste management facilities must begin with a thorough hazard identification process, documenting all potential exposure routes including inhalation, skin contact, and ingestion scenarios.
Quantitative risk assessment methodologies should incorporate both the probability and severity of potential HF exposure incidents. This includes modeling various accident scenarios such as spills, container failures, and vapor releases, with particular attention to concentration-dependent effects. The assessment must account for cumulative exposure risks to workers and potential environmental impacts through groundwater or atmospheric contamination pathways.
Facility-specific risk matrices should be developed, categorizing operational activities according to their HF exposure potential. Critical control points must be identified throughout the waste handling process, from receipt and storage to treatment and disposal phases. These assessments should be regularly updated to reflect changes in waste composition, processing technologies, or regulatory requirements.
Safety protocols must establish clear hierarchies of control measures, prioritizing elimination and substitution where feasible. When HF cannot be eliminated, engineering controls become paramount, including closed handling systems, local exhaust ventilation with acid-resistant components, and automated neutralization systems. Continuous monitoring systems with real-time HF vapor detection capabilities should be strategically positioned throughout processing areas.
Administrative controls must include comprehensive training programs covering HF chemistry, exposure symptoms, emergency response procedures, and proper use of personal protective equipment (PPE). Standard operating procedures should detail specific handling requirements, including temperature control parameters to minimize volatilization and precise neutralization protocols.
PPE requirements for HF handling must exceed standard acid protection measures, incorporating HF-specific elements such as neoprene or butyl rubber gloves of appropriate thickness, face shields with chin protection, chemical splash goggles, and acid-resistant full-body protection. Respiratory protection programs should specify appropriate cartridges or supplied air systems based on potential exposure concentrations.
Emergency response protocols require specialized considerations, including immediate access to calcium gluconate gel for skin exposures and shower facilities with tepid water. Medical surveillance programs should be implemented for workers regularly handling HF waste, with baseline and periodic testing for serum calcium and fluoride levels where appropriate.
Documentation and compliance verification systems must track all aspects of the safety program, including training records, exposure monitoring data, equipment maintenance logs, and incident reports to enable continuous improvement of safety protocols.
Quantitative risk assessment methodologies should incorporate both the probability and severity of potential HF exposure incidents. This includes modeling various accident scenarios such as spills, container failures, and vapor releases, with particular attention to concentration-dependent effects. The assessment must account for cumulative exposure risks to workers and potential environmental impacts through groundwater or atmospheric contamination pathways.
Facility-specific risk matrices should be developed, categorizing operational activities according to their HF exposure potential. Critical control points must be identified throughout the waste handling process, from receipt and storage to treatment and disposal phases. These assessments should be regularly updated to reflect changes in waste composition, processing technologies, or regulatory requirements.
Safety protocols must establish clear hierarchies of control measures, prioritizing elimination and substitution where feasible. When HF cannot be eliminated, engineering controls become paramount, including closed handling systems, local exhaust ventilation with acid-resistant components, and automated neutralization systems. Continuous monitoring systems with real-time HF vapor detection capabilities should be strategically positioned throughout processing areas.
Administrative controls must include comprehensive training programs covering HF chemistry, exposure symptoms, emergency response procedures, and proper use of personal protective equipment (PPE). Standard operating procedures should detail specific handling requirements, including temperature control parameters to minimize volatilization and precise neutralization protocols.
PPE requirements for HF handling must exceed standard acid protection measures, incorporating HF-specific elements such as neoprene or butyl rubber gloves of appropriate thickness, face shields with chin protection, chemical splash goggles, and acid-resistant full-body protection. Respiratory protection programs should specify appropriate cartridges or supplied air systems based on potential exposure concentrations.
Emergency response protocols require specialized considerations, including immediate access to calcium gluconate gel for skin exposures and shower facilities with tepid water. Medical surveillance programs should be implemented for workers regularly handling HF waste, with baseline and periodic testing for serum calcium and fluoride levels where appropriate.
Documentation and compliance verification systems must track all aspects of the safety program, including training records, exposure monitoring data, equipment maintenance logs, and incident reports to enable continuous improvement of safety protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!