Hydrofluoric Acid vs Nitric Acid: Chemical Reactivity Comparison
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HF and HNO3 Background and Research Objectives
Hydrofluoric acid (HF) and nitric acid (HNO3) represent two of the most significant inorganic acids in industrial applications, each with distinctive chemical properties and reactivity profiles. The historical development of these acids traces back to the 17th and 18th centuries, with HF first isolated by Carl Wilhelm Scheele in 1771 and HNO3 known since the medieval alchemists, later standardized by Johann Rudolf Glauber in the 17th century. Their evolution from laboratory curiosities to industrial staples marks a critical progression in chemical manufacturing capabilities.
The technological trajectory of both acids has been characterized by continuous refinement in production methods, purification techniques, and application diversification. HF production evolved from the thermal decomposition of fluorite with sulfuric acid to modern electrochemical processes, while HNO3 production advanced from the decomposition of nitrate salts to the current Ostwald process utilizing ammonia oxidation. These developments have significantly enhanced production efficiency and product purity.
Recent technological trends indicate growing interest in safer handling protocols for HF due to its exceptional hazard profile, alongside exploration of alternative etching agents in semiconductor manufacturing. For HNO3, advancements focus on emission reduction during production and development of more environmentally sustainable applications, particularly in metal processing and organic synthesis.
The primary objective of this comparative analysis is to establish a comprehensive understanding of the fundamental chemical reactivity differences between HF and HNO3 across various substrate interactions. This includes examining their behavior with metals, glass, ceramics, polymers, and biological materials, with particular attention to reaction mechanisms, kinetics, and resulting products.
Secondary research goals encompass the identification of application-specific performance differentiators, safety profile comparisons, and environmental impact assessments. We aim to develop predictive models for reactivity patterns that could inform material selection and process design in industries where these acids are utilized, including semiconductor manufacturing, metal processing, and chemical synthesis.
The strategic importance of this research extends to addressing emerging challenges in advanced materials processing, where precise control of surface chemistry is increasingly critical. By elucidating the molecular-level interactions of these acids with various substrates, we anticipate developing more efficient, safer, and environmentally sustainable chemical processes that leverage the unique properties of each acid while mitigating their respective limitations.
The technological trajectory of both acids has been characterized by continuous refinement in production methods, purification techniques, and application diversification. HF production evolved from the thermal decomposition of fluorite with sulfuric acid to modern electrochemical processes, while HNO3 production advanced from the decomposition of nitrate salts to the current Ostwald process utilizing ammonia oxidation. These developments have significantly enhanced production efficiency and product purity.
Recent technological trends indicate growing interest in safer handling protocols for HF due to its exceptional hazard profile, alongside exploration of alternative etching agents in semiconductor manufacturing. For HNO3, advancements focus on emission reduction during production and development of more environmentally sustainable applications, particularly in metal processing and organic synthesis.
The primary objective of this comparative analysis is to establish a comprehensive understanding of the fundamental chemical reactivity differences between HF and HNO3 across various substrate interactions. This includes examining their behavior with metals, glass, ceramics, polymers, and biological materials, with particular attention to reaction mechanisms, kinetics, and resulting products.
Secondary research goals encompass the identification of application-specific performance differentiators, safety profile comparisons, and environmental impact assessments. We aim to develop predictive models for reactivity patterns that could inform material selection and process design in industries where these acids are utilized, including semiconductor manufacturing, metal processing, and chemical synthesis.
The strategic importance of this research extends to addressing emerging challenges in advanced materials processing, where precise control of surface chemistry is increasingly critical. By elucidating the molecular-level interactions of these acids with various substrates, we anticipate developing more efficient, safer, and environmentally sustainable chemical processes that leverage the unique properties of each acid while mitigating their respective limitations.
Market Applications and Demand Analysis
The global market for both hydrofluoric acid (HF) and nitric acid (HNO3) continues to expand, driven by diverse industrial applications that leverage their distinct chemical reactivity profiles. The semiconductor industry represents a primary demand driver for high-purity hydrofluoric acid, with the global semiconductor market projected to reach $1 trillion by 2030. HF's unique ability to etch silicon dioxide makes it irreplaceable in chip manufacturing processes, particularly as device miniaturization advances toward 3nm and beyond technology nodes.
In contrast, nitric acid finds its largest market in fertilizer production, accounting for approximately 80% of global nitric acid consumption. The agricultural sector's growing demand for increased crop yields, particularly in developing regions, sustains a steady 3-4% annual growth rate in this segment. The remaining 20% of nitric acid production serves diverse industrial applications including explosives manufacturing, metal processing, and specialty chemicals.
The electronics cleaning segment represents a competitive battleground where both acids serve different purposes. Hydrofluoric acid's selective etching capabilities make it valuable for precision cleaning of electronic components, while nitric acid's strong oxidizing properties are preferred for removing metallic contaminants. This market segment is experiencing rapid growth due to increasing electronic device production and miniaturization trends.
Metal processing industries utilize both acids differently based on their reactivity profiles. Nitric acid dominates in stainless steel pickling operations due to its passivation capabilities, while hydrofluoric acid finds application in aluminum processing and specialized metal surface treatments. The automotive and aerospace sectors drive significant demand in this segment, with lightweight metal components requiring precise surface preparation.
Regional demand patterns reveal interesting contrasts. Asia-Pacific dominates hydrofluoric acid consumption due to concentrated semiconductor manufacturing, while nitric acid demand is more globally distributed with significant consumption in agricultural regions. North America and Europe maintain steady demand for both acids in specialty applications, particularly in pharmaceutical manufacturing and advanced materials processing.
Environmental regulations increasingly influence market dynamics for both acids. Hydrofluoric acid faces stricter handling requirements due to its extreme toxicity, while nitric acid production facilities must address NOx emissions concerns. This regulatory landscape is driving investment in safer handling technologies and alternative processes, particularly in developed markets where environmental compliance costs significantly impact operational economics.
In contrast, nitric acid finds its largest market in fertilizer production, accounting for approximately 80% of global nitric acid consumption. The agricultural sector's growing demand for increased crop yields, particularly in developing regions, sustains a steady 3-4% annual growth rate in this segment. The remaining 20% of nitric acid production serves diverse industrial applications including explosives manufacturing, metal processing, and specialty chemicals.
The electronics cleaning segment represents a competitive battleground where both acids serve different purposes. Hydrofluoric acid's selective etching capabilities make it valuable for precision cleaning of electronic components, while nitric acid's strong oxidizing properties are preferred for removing metallic contaminants. This market segment is experiencing rapid growth due to increasing electronic device production and miniaturization trends.
Metal processing industries utilize both acids differently based on their reactivity profiles. Nitric acid dominates in stainless steel pickling operations due to its passivation capabilities, while hydrofluoric acid finds application in aluminum processing and specialized metal surface treatments. The automotive and aerospace sectors drive significant demand in this segment, with lightweight metal components requiring precise surface preparation.
Regional demand patterns reveal interesting contrasts. Asia-Pacific dominates hydrofluoric acid consumption due to concentrated semiconductor manufacturing, while nitric acid demand is more globally distributed with significant consumption in agricultural regions. North America and Europe maintain steady demand for both acids in specialty applications, particularly in pharmaceutical manufacturing and advanced materials processing.
Environmental regulations increasingly influence market dynamics for both acids. Hydrofluoric acid faces stricter handling requirements due to its extreme toxicity, while nitric acid production facilities must address NOx emissions concerns. This regulatory landscape is driving investment in safer handling technologies and alternative processes, particularly in developed markets where environmental compliance costs significantly impact operational economics.
Current Technical Challenges in Acid Reactivity
The comparative reactivity of hydrofluoric acid (HF) and nitric acid (HNO₃) presents significant challenges for industrial applications, particularly in semiconductor manufacturing, metal processing, and chemical synthesis. Despite their widespread use, several technical hurdles remain unresolved when implementing these acids in production environments.
One primary challenge involves the unique corrosion mechanisms of HF. Unlike most acids that attack metals through hydrogen ion activity, HF's corrosivity stems from fluoride ions forming metal fluoride complexes. This distinctive behavior makes conventional corrosion-resistant materials ineffective against HF, necessitating specialized containment solutions that significantly increase implementation costs and complexity.
Temperature dependency variations between these acids create substantial process control difficulties. HF exhibits relatively consistent reactivity across temperature ranges, while nitric acid's oxidizing power increases dramatically with temperature. This disparity complicates processes requiring precise reaction rates, especially in etching applications where uniform material removal is critical.
The volatility characteristics of both acids present serious safety and handling challenges. HF produces dangerous vapors even at room temperature, while concentrated nitric acid releases nitrogen dioxide fumes. Current vapor suppression technologies remain inadequate for large-scale industrial applications, limiting deployment in facilities without extensive ventilation systems.
Reaction selectivity represents another significant technical obstacle. While nitric acid demonstrates predictable oxidation behavior with most metals, HF shows highly variable selectivity depending on substrate composition. This variability complicates process development for multi-material components, particularly in advanced electronics manufacturing where precise material interfaces are crucial.
Waste treatment and neutralization present ongoing environmental challenges. Fluoride-containing waste streams require specialized treatment protocols that exceed conventional acid waste management approaches. Similarly, nitric acid waste contains nitrogen compounds that can contribute to water eutrophication if improperly handled. Current treatment technologies struggle to address these issues cost-effectively at industrial scales.
Analytical monitoring capabilities for real-time process control remain underdeveloped. While nitric acid concentration can be reliably monitored through conductivity or spectroscopic methods, accurate real-time HF concentration measurement in industrial settings continues to challenge existing sensor technologies. This limitation hampers precise process control and optimization efforts.
The synergistic effects when these acids are combined with other chemicals create additional complexity. For instance, HF mixed with nitric acid (forming aqua regia variants) exhibits dramatically different reactivity profiles than either acid alone. Current predictive models inadequately capture these interaction effects, necessitating extensive empirical testing for new applications.
One primary challenge involves the unique corrosion mechanisms of HF. Unlike most acids that attack metals through hydrogen ion activity, HF's corrosivity stems from fluoride ions forming metal fluoride complexes. This distinctive behavior makes conventional corrosion-resistant materials ineffective against HF, necessitating specialized containment solutions that significantly increase implementation costs and complexity.
Temperature dependency variations between these acids create substantial process control difficulties. HF exhibits relatively consistent reactivity across temperature ranges, while nitric acid's oxidizing power increases dramatically with temperature. This disparity complicates processes requiring precise reaction rates, especially in etching applications where uniform material removal is critical.
The volatility characteristics of both acids present serious safety and handling challenges. HF produces dangerous vapors even at room temperature, while concentrated nitric acid releases nitrogen dioxide fumes. Current vapor suppression technologies remain inadequate for large-scale industrial applications, limiting deployment in facilities without extensive ventilation systems.
Reaction selectivity represents another significant technical obstacle. While nitric acid demonstrates predictable oxidation behavior with most metals, HF shows highly variable selectivity depending on substrate composition. This variability complicates process development for multi-material components, particularly in advanced electronics manufacturing where precise material interfaces are crucial.
Waste treatment and neutralization present ongoing environmental challenges. Fluoride-containing waste streams require specialized treatment protocols that exceed conventional acid waste management approaches. Similarly, nitric acid waste contains nitrogen compounds that can contribute to water eutrophication if improperly handled. Current treatment technologies struggle to address these issues cost-effectively at industrial scales.
Analytical monitoring capabilities for real-time process control remain underdeveloped. While nitric acid concentration can be reliably monitored through conductivity or spectroscopic methods, accurate real-time HF concentration measurement in industrial settings continues to challenge existing sensor technologies. This limitation hampers precise process control and optimization efforts.
The synergistic effects when these acids are combined with other chemicals create additional complexity. For instance, HF mixed with nitric acid (forming aqua regia variants) exhibits dramatically different reactivity profiles than either acid alone. Current predictive models inadequately capture these interaction effects, necessitating extensive empirical testing for new applications.
Comparative Analysis of HF and HNO3 Properties
01 Etching applications in semiconductor manufacturing
Hydrofluoric acid and nitric acid mixtures are widely used in semiconductor manufacturing for etching silicon and silicon compounds. The combination provides controlled etching rates and surface quality, with nitric acid acting as an oxidizing agent and hydrofluoric acid dissolving the oxidized silicon. These mixtures can be formulated with specific ratios to achieve desired etching characteristics for different semiconductor materials and processing steps.- Etching applications in semiconductor manufacturing: Mixtures of hydrofluoric acid and nitric acid are widely used in semiconductor manufacturing for etching silicon and other materials. The combination creates a powerful etching solution where nitric acid oxidizes the silicon surface while hydrofluoric acid dissolves the resulting silicon dioxide. This reaction is controlled by varying the concentration ratio of the acids and adding other components to achieve specific etch rates and surface profiles. The reactivity can be modulated for different applications including wafer cleaning, pattern formation, and surface preparation.
- Safety and handling considerations: The combination of hydrofluoric acid and nitric acid presents significant safety hazards due to their high reactivity. These mixtures can generate toxic gases including nitrogen oxides and hydrogen fluoride vapor. Special containment systems, neutralization procedures, and personal protective equipment are required when handling these acids together. Safety protocols include specialized storage containers, ventilation systems, and emergency response procedures to mitigate risks associated with accidental mixing or spills. Temperature control is critical as the reaction between these acids can be exothermic under certain conditions.
- Metal surface treatment and cleaning: Hydrofluoric acid and nitric acid mixtures are effective for metal surface treatment, particularly for stainless steel, titanium, and other corrosion-resistant alloys. The combination removes oxide layers, contaminants, and passivates metal surfaces. The nitric acid component oxidizes the metal surface while hydrofluoric acid dissolves the oxides, resulting in a clean, uniform surface. These treatments improve adhesion properties for subsequent coating processes, enhance corrosion resistance, and prepare surfaces for welding or other joining techniques.
- Waste treatment and recycling processes: The chemical reactivity between hydrofluoric acid and nitric acid is relevant in waste treatment and recycling processes for spent etchants from industrial applications. Methods have been developed to neutralize these acid mixtures, recover valuable components, and reduce environmental impact. Techniques include precipitation reactions, membrane filtration, distillation, and chemical conversion to less hazardous compounds. These processes aim to minimize waste volume and recover acids or their derivatives for reuse in manufacturing processes.
- Chemical synthesis applications: The reactive properties of hydrofluoric acid and nitric acid mixtures are utilized in various chemical synthesis applications. These include the production of fluorinated compounds, nitration reactions, and specialized oxidation processes. The combination can facilitate unique reaction pathways not achievable with either acid alone. By controlling reaction conditions such as temperature, concentration, and catalysts, these acid mixtures can be used to synthesize intermediates for pharmaceuticals, agrochemicals, and specialty materials. The synergistic effects of the acids enable selective functionalization of organic and inorganic substrates.
02 Metal surface treatment and cleaning
The combination of hydrofluoric acid and nitric acid is effective for metal surface treatment, particularly for stainless steel, titanium, and other corrosion-resistant alloys. This acid mixture removes oxide layers, contaminants, and provides passivation of metal surfaces. The chemical reactivity between these acids creates a powerful cleaning solution that can remove stubborn deposits and prepare surfaces for subsequent processing or coating applications.Expand Specific Solutions03 Safety measures and handling protocols
Due to the highly corrosive and reactive nature of hydrofluoric acid and nitric acid mixtures, specialized safety measures and handling protocols are essential. This includes the use of specific containment materials resistant to these acids, ventilation systems to remove toxic fumes, neutralization procedures for spills, and personal protective equipment designed for extreme chemical hazards. The reaction between these acids can generate toxic gases and heat, requiring careful mixing procedures and storage conditions.Expand Specific Solutions04 Waste treatment and environmental considerations
The treatment of waste containing hydrofluoric and nitric acid mixtures requires specialized processes to neutralize their reactivity and toxicity before disposal. Methods include chemical neutralization with alkaline compounds, precipitation of fluoride ions, and treatment systems designed to handle multiple hazardous components. Environmental regulations govern the disposal of these acid mixtures due to their potential impact on aquatic ecosystems and groundwater.Expand Specific Solutions05 Analytical and laboratory applications
Hydrofluoric acid and nitric acid mixtures are used in analytical chemistry and laboratory applications for sample digestion and preparation. The strong oxidizing properties of nitric acid combined with the ability of hydrofluoric acid to dissolve silicates make this mixture particularly effective for breaking down complex matrices in geological, metallurgical, and environmental samples. Specific ratios and reaction conditions are employed depending on the analytical target and sample composition.Expand Specific Solutions
Major Industry Players and Research Institutions
The hydrofluoric acid versus nitric acid chemical reactivity comparison market is in a mature development stage with established applications across multiple industries. The global market size for these acids is substantial, estimated at several billion dollars, driven by their critical roles in semiconductor manufacturing, metal processing, and chemical synthesis. Technologically, both acids have well-understood reactivity profiles, with companies like Solvay SA, DAIKIN INDUSTRIES Ltd., and Do-Fluoride New Materials Co. leading hydrofluoric acid innovations, while Stella Chemifa Corp. and CSIR focus on high-purity applications. In the nitric acid segment, LANXESS Deutschland GmbH and Curia Global have developed advanced handling and application technologies. Research institutions like MIT and Sun Yat-Sen University continue to explore novel applications, particularly in semiconductor etching processes where these acids' distinct reactivity properties are leveraged for precision manufacturing.
Solvay SA
Technical Solution: Solvay has developed advanced processes for handling and utilizing both hydrofluoric acid (HF) and nitric acid (HNO3) in industrial applications. Their comparative reactivity research shows that HF (pKa = 3.17) is a weak acid in aqueous solution but exhibits strong reactivity due to the strength of the H-F bond and the small size of fluoride ions, allowing for penetration of tissues and materials. Their proprietary fluorination technologies leverage HF's unique ability to form hydrogen bonds and attack silica-based materials. For nitric acid applications, Solvay has engineered specialized containment systems that account for HNO3's strong oxidizing properties (reduction potential of +0.96V), particularly in concentrated forms where it can react violently with organic compounds. Their research demonstrates that while HF attacks glass and silicates specifically, HNO3 is more broadly reactive with metals, producing nitrogen dioxide gas during metal dissolution processes. Solvay's material compatibility charts indicate distinct corrosion profiles: HF requiring specialized polymers like PTFE for safe handling, while HNO3 is compatible with a wider range of materials but requires specific grade stainless steels.
Strengths: Solvay's extensive experience in industrial chemical processing has resulted in highly optimized handling protocols for both acids. Their fluoropolymer technologies specifically designed for HF containment represent industry-leading safety standards. Weaknesses: Their solutions tend to be more costly than competitors, and some of their specialized containment systems require significant capital investment and maintenance.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has pioneered comprehensive research comparing hydrofluoric and nitric acid reactivity, particularly in fluoropolymer manufacturing contexts. Their studies demonstrate that HF's reactivity is characterized by its strong hydrogen bonding capability and nucleophilic properties of the fluoride ion, making it particularly aggressive toward silica-based materials. DAIKIN's proprietary etching processes utilize HF's ability to dissolve glass (SiO2 + 6HF → H2SiF6 + 2H2O) at rates approximately 4-5 times faster than comparable concentrations of nitric acid. Their research shows that while HF is a weaker acid (pKa 3.17) than nitric acid (pKa -1.4), it demonstrates higher reactivity in certain applications due to the small size and high charge density of the fluoride ion. DAIKIN's materials compatibility database indicates that HF rapidly attacks alkali metals, forming hydrogen gas, while nitric acid's primary reaction mechanism involves oxidation rather than direct hydrogen replacement. For metal processing applications, they've documented that nitric acid's passivation capabilities on stainless steel create protective oxide layers, whereas HF actively removes such layers. DAIKIN's specialized containment systems account for HF's unique permeation properties through many polymers that would otherwise contain nitric acid effectively.
Strengths: DAIKIN's fluoropolymer expertise gives them unparalleled insight into HF handling and containment, with their PTFE and related materials being industry standards for HF-resistant applications. Their comparative reactivity data is among the most comprehensive available. Weaknesses: Their research focuses heavily on fluoropolymer applications, potentially limiting broader applicability, and their specialized HF handling equipment tends to command premium pricing in the market.
Key Reaction Mechanisms and Scientific Literature
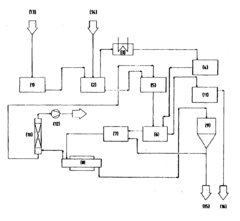
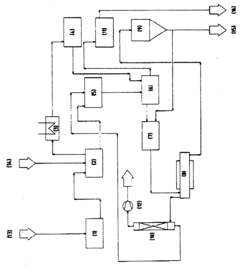
Process for producing and/or recovering hydrofluoric acid and nitric acid
PatentInactiveEP0984078A1
Innovation
- Adding metal oxides to the precipitate before or during pyrohydrolysis to absorb the metal fluoride melt, preventing deposit formation and enhancing the process's economic viability by recycling the used oxides.
Reverse osmosis for purifying mixtures of hydrofluoric acid and nitric acid
PatentWO2014173788A1
Innovation
- A reverse osmosis (RO) membrane process is used to selectively remove silicon impurities from solutions containing hydrofluoric acid and nitric acid, employing thin film composite membranes made from materials like polyamides and polysulfones, operating at controlled pressures and temperatures to maintain acid concentrations while rejecting impurities effectively.
Safety Protocols and Handling Requirements
The handling of hydrofluoric acid (HF) and nitric acid (HNO₃) requires stringent safety protocols due to their hazardous properties. For hydrofluoric acid, specialized personal protective equipment (PPE) is mandatory, including chemical-resistant suits, face shields, and specific HF-resistant gloves made from materials like neoprene or butyl rubber. Standard nitrile gloves provide insufficient protection against HF penetration. Additionally, calcium gluconate gel must be immediately accessible as a first-aid measure for HF exposure.
Nitric acid handling requires different but equally rigorous precautions. Chemical splash goggles, acid-resistant gloves, and laboratory coats are essential. When working with concentrated nitric acid (>68%), additional respiratory protection may be necessary due to nitrogen oxide fumes. Unlike HF, nitric acid does not require specialized antidotes, but immediate water flushing remains critical for exposure incidents.
Storage requirements differ significantly between these acids. HF must be stored in polyethylene or fluorocarbon plastic containers, as it attacks glass and most metals. Nitric acid should be kept in glass or specific plastic containers approved for oxidizing acids, separated from organic materials and reducing agents. Both acids require secondary containment systems, but HF demands additional ventilation considerations due to its volatile nature.
Laboratory facilities working with these acids must implement specific engineering controls. HF operations necessitate dedicated fume hoods with acid-resistant liners and specialized ventilation systems. Emergency eyewash stations and safety showers must be located within 10 seconds' reach of workstations. For nitric acid work, standard chemical fume hoods are generally sufficient, though they must be regularly inspected for acid damage.
Emergency response protocols also differ between these acids. HF exposure requires immediate application of calcium gluconate gel to affected areas, followed by urgent medical attention. Nitric acid spills can be neutralized with sodium bicarbonate or commercial acid neutralizers, while HF spills require specialized HF neutralizers containing calcium compounds. Facilities must maintain detailed incident response plans specific to each acid's unique hazards.
Personnel training represents a critical safety component when working with these acids. Workers handling HF require specialized training on its unique penetrating properties and delayed symptoms, while nitric acid handlers need specific instruction on managing oxidation hazards and nitrogen oxide exposure. Regular emergency drills and certification renewal ensure preparedness for potential incidents with either acid.
Nitric acid handling requires different but equally rigorous precautions. Chemical splash goggles, acid-resistant gloves, and laboratory coats are essential. When working with concentrated nitric acid (>68%), additional respiratory protection may be necessary due to nitrogen oxide fumes. Unlike HF, nitric acid does not require specialized antidotes, but immediate water flushing remains critical for exposure incidents.
Storage requirements differ significantly between these acids. HF must be stored in polyethylene or fluorocarbon plastic containers, as it attacks glass and most metals. Nitric acid should be kept in glass or specific plastic containers approved for oxidizing acids, separated from organic materials and reducing agents. Both acids require secondary containment systems, but HF demands additional ventilation considerations due to its volatile nature.
Laboratory facilities working with these acids must implement specific engineering controls. HF operations necessitate dedicated fume hoods with acid-resistant liners and specialized ventilation systems. Emergency eyewash stations and safety showers must be located within 10 seconds' reach of workstations. For nitric acid work, standard chemical fume hoods are generally sufficient, though they must be regularly inspected for acid damage.
Emergency response protocols also differ between these acids. HF exposure requires immediate application of calcium gluconate gel to affected areas, followed by urgent medical attention. Nitric acid spills can be neutralized with sodium bicarbonate or commercial acid neutralizers, while HF spills require specialized HF neutralizers containing calcium compounds. Facilities must maintain detailed incident response plans specific to each acid's unique hazards.
Personnel training represents a critical safety component when working with these acids. Workers handling HF require specialized training on its unique penetrating properties and delayed symptoms, while nitric acid handlers need specific instruction on managing oxidation hazards and nitrogen oxide exposure. Regular emergency drills and certification renewal ensure preparedness for potential incidents with either acid.
Environmental Impact and Regulatory Compliance
The environmental impact of hydrofluoric acid (HF) and nitric acid (HNO₃) presents significant concerns for industrial operations, requiring comprehensive regulatory compliance strategies. Both acids contribute to environmental degradation through different pathways, with HF posing particularly severe threats to aquatic ecosystems even at low concentrations. When released into water bodies, HF can cause substantial pH changes and introduce fluoride ions that disrupt calcium metabolism in aquatic organisms, potentially leading to widespread ecological damage.
Nitric acid, while highly corrosive, presents different environmental challenges primarily related to its role in atmospheric pollution and acid rain formation. When nitric acid or its vapors are released into the atmosphere, they contribute to nitrogen oxide emissions that participate in complex photochemical reactions, exacerbating air quality issues and contributing to respiratory health problems in affected populations.
Regulatory frameworks governing these acids vary globally but generally follow similar principles. In the United States, both acids are regulated under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) and the Emergency Planning and Community Right-to-Know Act (EPCRA), with reportable quantity thresholds of 1,000 pounds for HF and 1,000 pounds for nitric acid. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes strict documentation and risk management requirements for both acids.
Waste management protocols differ significantly between these acids due to their distinct chemical properties. HF waste typically requires specialized neutralization procedures using calcium compounds to form insoluble calcium fluoride before disposal. Nitric acid waste often undergoes neutralization with bases like sodium hydroxide or limestone, with careful attention to the exothermic nature of these reactions.
Emissions control technologies for facilities using these acids include wet scrubbers, which are particularly effective for capturing acid vapors. For HF, specialized scrubbing solutions containing calcium or sodium compounds are employed, while nitric acid emissions may be controlled using selective catalytic reduction systems that convert nitrogen oxides to nitrogen and water.
Recent regulatory trends indicate increasingly stringent requirements for both acids, with particular focus on workplace exposure limits and community notification protocols. The global harmonization of chemical management systems has led to standardized hazard communication requirements, including detailed safety data sheets and labeling that explicitly identify environmental hazards and disposal considerations for both acids.
Nitric acid, while highly corrosive, presents different environmental challenges primarily related to its role in atmospheric pollution and acid rain formation. When nitric acid or its vapors are released into the atmosphere, they contribute to nitrogen oxide emissions that participate in complex photochemical reactions, exacerbating air quality issues and contributing to respiratory health problems in affected populations.
Regulatory frameworks governing these acids vary globally but generally follow similar principles. In the United States, both acids are regulated under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) and the Emergency Planning and Community Right-to-Know Act (EPCRA), with reportable quantity thresholds of 1,000 pounds for HF and 1,000 pounds for nitric acid. The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes strict documentation and risk management requirements for both acids.
Waste management protocols differ significantly between these acids due to their distinct chemical properties. HF waste typically requires specialized neutralization procedures using calcium compounds to form insoluble calcium fluoride before disposal. Nitric acid waste often undergoes neutralization with bases like sodium hydroxide or limestone, with careful attention to the exothermic nature of these reactions.
Emissions control technologies for facilities using these acids include wet scrubbers, which are particularly effective for capturing acid vapors. For HF, specialized scrubbing solutions containing calcium or sodium compounds are employed, while nitric acid emissions may be controlled using selective catalytic reduction systems that convert nitrogen oxides to nitrogen and water.
Recent regulatory trends indicate increasingly stringent requirements for both acids, with particular focus on workplace exposure limits and community notification protocols. The global harmonization of chemical management systems has led to standardized hazard communication requirements, including detailed safety data sheets and labeling that explicitly identify environmental hazards and disposal considerations for both acids.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!