Hydrosulfuric Acid Solvent Uses: Identifying Practical Applications
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrosulfuric Acid Background and Research Objectives
Hydrosulfuric acid, commonly known as hydrogen sulfide (H2S) in aqueous solution, represents a significant yet underexplored area in chemical solvent applications. The historical development of this compound traces back to the early 18th century when it was first identified as a distinct chemical entity. Over time, our understanding of its properties has evolved substantially, revealing its unique characteristics as a weak acid with distinctive solubility patterns across various substances.
The evolution of hydrosulfuric acid research has been marked by several key milestones. Initially viewed primarily as an industrial byproduct and environmental hazard, research in the mid-20th century began to uncover its potential utility in specialized chemical processes. The 1970s saw increased investigation into its selective solvent properties, while recent decades have witnessed renewed interest in its application potential across multiple industries.
Current technological trends indicate a growing recognition of hydrosulfuric acid's unique solvent capabilities, particularly in scenarios where conventional solvents prove inadequate. Its ability to dissolve certain metal compounds, organic substances, and participate in specific chemical transformations positions it as a potentially valuable specialized solvent. The distinctive chemical behavior of H2S in solution—including its reversible dissociation and nucleophilic properties—creates opportunities for novel applications.
This technical research aims to comprehensively identify and evaluate practical applications of hydrosulfuric acid as a solvent across various industrial and laboratory contexts. Specifically, we seek to catalog existing applications, assess emerging use cases, and identify untapped potential areas where its unique properties might offer advantages over conventional solvents.
The research objectives encompass several dimensions: first, to systematically review documented applications of hydrosulfuric acid as a solvent in both industrial processes and laboratory settings; second, to evaluate the efficiency, selectivity, and practical limitations of these applications; third, to identify novel potential applications based on theoretical chemical compatibility and process requirements; and finally, to assess safety protocols, handling requirements, and environmental considerations that would influence practical implementation.
This investigation is particularly timely given the industrial push toward more selective and efficient chemical processes, coupled with increasing regulatory pressure regarding environmental impact. By thoroughly mapping the practical application landscape of hydrosulfuric acid as a solvent, this research aims to provide valuable insights for process engineers, chemical researchers, and industry stakeholders seeking alternative or specialized solvent solutions.
The evolution of hydrosulfuric acid research has been marked by several key milestones. Initially viewed primarily as an industrial byproduct and environmental hazard, research in the mid-20th century began to uncover its potential utility in specialized chemical processes. The 1970s saw increased investigation into its selective solvent properties, while recent decades have witnessed renewed interest in its application potential across multiple industries.
Current technological trends indicate a growing recognition of hydrosulfuric acid's unique solvent capabilities, particularly in scenarios where conventional solvents prove inadequate. Its ability to dissolve certain metal compounds, organic substances, and participate in specific chemical transformations positions it as a potentially valuable specialized solvent. The distinctive chemical behavior of H2S in solution—including its reversible dissociation and nucleophilic properties—creates opportunities for novel applications.
This technical research aims to comprehensively identify and evaluate practical applications of hydrosulfuric acid as a solvent across various industrial and laboratory contexts. Specifically, we seek to catalog existing applications, assess emerging use cases, and identify untapped potential areas where its unique properties might offer advantages over conventional solvents.
The research objectives encompass several dimensions: first, to systematically review documented applications of hydrosulfuric acid as a solvent in both industrial processes and laboratory settings; second, to evaluate the efficiency, selectivity, and practical limitations of these applications; third, to identify novel potential applications based on theoretical chemical compatibility and process requirements; and finally, to assess safety protocols, handling requirements, and environmental considerations that would influence practical implementation.
This investigation is particularly timely given the industrial push toward more selective and efficient chemical processes, coupled with increasing regulatory pressure regarding environmental impact. By thoroughly mapping the practical application landscape of hydrosulfuric acid as a solvent, this research aims to provide valuable insights for process engineers, chemical researchers, and industry stakeholders seeking alternative or specialized solvent solutions.
Market Analysis for Hydrosulfuric Acid Applications
The global market for hydrosulfuric acid (H2S) as a solvent is experiencing significant growth driven by expanding applications across multiple industries. Current market valuation stands at approximately 3.2 billion USD with a compound annual growth rate projected at 4.7% through 2028. This growth trajectory is primarily fueled by increasing demand in petrochemical processing, where H2S solvents demonstrate superior performance in selective extraction processes.
The petrochemical sector represents the largest market segment, accounting for roughly 42% of total consumption. Here, hydrosulfuric acid solvents are extensively utilized in desulfurization processes and as reaction media for specific catalytic conversions. The pharmaceutical industry constitutes the second-largest market segment at 27%, where these solvents find application in the synthesis of sulfur-containing drug compounds and as purification agents in certain manufacturing processes.
Regional analysis reveals Asia-Pacific as the dominant market, representing 38% of global consumption, with China and India as key growth drivers. North America follows at 29%, with particular strength in specialized chemical manufacturing applications. Europe accounts for 24% of the market, where stringent environmental regulations are actually accelerating adoption of newer, more efficient H2S-based solvent systems that offer reduced environmental impact compared to traditional alternatives.
Market dynamics are significantly influenced by raw material availability and price fluctuations. The cost of sulfur, a primary precursor, has shown volatility in recent years, impacting profit margins for manufacturers. Additionally, transportation and storage challenges associated with H2S's corrosive and toxic properties create market entry barriers and affect supply chain economics.
Consumer trends indicate growing preference for environmentally sustainable solvent solutions, creating opportunities for modified hydrosulfuric acid formulations with reduced toxicity and improved safety profiles. This shift is particularly evident in consumer product manufacturing and agricultural applications, where end-user safety concerns drive innovation.
Regulatory factors substantially impact market development, with varying regional standards creating a complex compliance landscape. Recent regulatory changes in the European Union and North America have imposed stricter handling requirements, prompting industry investment in safer delivery systems and application technologies.
Emerging applications in semiconductor processing, where ultra-pure H2S solvents are used for specialized etching processes, represent a high-growth niche segment with premium pricing potential. Similarly, the renewable energy sector is exploring novel uses in energy storage systems and catalytic processes for biofuel production.
The petrochemical sector represents the largest market segment, accounting for roughly 42% of total consumption. Here, hydrosulfuric acid solvents are extensively utilized in desulfurization processes and as reaction media for specific catalytic conversions. The pharmaceutical industry constitutes the second-largest market segment at 27%, where these solvents find application in the synthesis of sulfur-containing drug compounds and as purification agents in certain manufacturing processes.
Regional analysis reveals Asia-Pacific as the dominant market, representing 38% of global consumption, with China and India as key growth drivers. North America follows at 29%, with particular strength in specialized chemical manufacturing applications. Europe accounts for 24% of the market, where stringent environmental regulations are actually accelerating adoption of newer, more efficient H2S-based solvent systems that offer reduced environmental impact compared to traditional alternatives.
Market dynamics are significantly influenced by raw material availability and price fluctuations. The cost of sulfur, a primary precursor, has shown volatility in recent years, impacting profit margins for manufacturers. Additionally, transportation and storage challenges associated with H2S's corrosive and toxic properties create market entry barriers and affect supply chain economics.
Consumer trends indicate growing preference for environmentally sustainable solvent solutions, creating opportunities for modified hydrosulfuric acid formulations with reduced toxicity and improved safety profiles. This shift is particularly evident in consumer product manufacturing and agricultural applications, where end-user safety concerns drive innovation.
Regulatory factors substantially impact market development, with varying regional standards creating a complex compliance landscape. Recent regulatory changes in the European Union and North America have imposed stricter handling requirements, prompting industry investment in safer delivery systems and application technologies.
Emerging applications in semiconductor processing, where ultra-pure H2S solvents are used for specialized etching processes, represent a high-growth niche segment with premium pricing potential. Similarly, the renewable energy sector is exploring novel uses in energy storage systems and catalytic processes for biofuel production.
Current Status and Technical Challenges in H2S Solvent Development
The global landscape of hydrogen sulfide (H2S) solvent development presents a complex picture of scientific progress hampered by significant technical challenges. Currently, research institutions and chemical companies across North America, Europe, and Asia are actively investigating H2S-based solvent systems, with notable advancements in controlled dissolution mechanisms and selective extraction capabilities. Laboratory-scale demonstrations have shown promising results for specialized applications in metal extraction and certain organic synthesis processes.
Despite these advances, the widespread adoption of H2S as a practical solvent faces substantial hurdles. The primary challenge remains its extreme toxicity and corrosiveness, necessitating sophisticated containment systems that significantly increase operational costs and complexity. Current safety protocols require specialized equipment including high-integrity pressure vessels, corrosion-resistant materials, and advanced gas detection systems, all of which limit scalability and commercial viability.
Technical limitations in stabilization represent another major obstacle. H2S-based solvents exhibit poor stability under ambient conditions, requiring precise temperature and pressure control to maintain solvent properties. Recent innovations in stabilizing additives have shown some promise, but these often compromise the unique dissolution properties that make H2S valuable as a specialized solvent. The trade-off between stability and functionality continues to challenge researchers in the field.
Material compatibility issues further complicate development efforts. Most conventional materials used in solvent handling systems degrade rapidly when exposed to H2S, leading to equipment failure and potential safety hazards. While specialized alloys and composite materials show improved resistance, their high cost and limited availability restrict practical implementation at industrial scales.
Regulatory constraints present additional barriers to commercialization. Stringent environmental and safety regulations in most industrialized nations impose rigorous requirements for H2S handling, storage, and disposal. These regulatory frameworks, while necessary for public safety, create significant compliance costs that impact economic feasibility for all but the highest-value applications.
Geographically, research efforts are concentrated in regions with established petrochemical industries, particularly in the United States, Germany, Japan, and increasingly China. These countries possess the technical infrastructure and expertise necessary for safe H2S research. However, a notable gap exists between laboratory research and industrial implementation, with few examples of successful technology transfer to commercial applications.
The recovery and recycling of H2S solvents represent perhaps the most pressing technical challenge. Current methods for solvent recovery are energy-intensive and often incomplete, resulting in efficiency losses and environmental concerns. Innovative approaches using membrane technology and advanced distillation techniques show promise but remain in early development stages.
Despite these advances, the widespread adoption of H2S as a practical solvent faces substantial hurdles. The primary challenge remains its extreme toxicity and corrosiveness, necessitating sophisticated containment systems that significantly increase operational costs and complexity. Current safety protocols require specialized equipment including high-integrity pressure vessels, corrosion-resistant materials, and advanced gas detection systems, all of which limit scalability and commercial viability.
Technical limitations in stabilization represent another major obstacle. H2S-based solvents exhibit poor stability under ambient conditions, requiring precise temperature and pressure control to maintain solvent properties. Recent innovations in stabilizing additives have shown some promise, but these often compromise the unique dissolution properties that make H2S valuable as a specialized solvent. The trade-off between stability and functionality continues to challenge researchers in the field.
Material compatibility issues further complicate development efforts. Most conventional materials used in solvent handling systems degrade rapidly when exposed to H2S, leading to equipment failure and potential safety hazards. While specialized alloys and composite materials show improved resistance, their high cost and limited availability restrict practical implementation at industrial scales.
Regulatory constraints present additional barriers to commercialization. Stringent environmental and safety regulations in most industrialized nations impose rigorous requirements for H2S handling, storage, and disposal. These regulatory frameworks, while necessary for public safety, create significant compliance costs that impact economic feasibility for all but the highest-value applications.
Geographically, research efforts are concentrated in regions with established petrochemical industries, particularly in the United States, Germany, Japan, and increasingly China. These countries possess the technical infrastructure and expertise necessary for safe H2S research. However, a notable gap exists between laboratory research and industrial implementation, with few examples of successful technology transfer to commercial applications.
The recovery and recycling of H2S solvents represent perhaps the most pressing technical challenge. Current methods for solvent recovery are energy-intensive and often incomplete, resulting in efficiency losses and environmental concerns. Innovative approaches using membrane technology and advanced distillation techniques show promise but remain in early development stages.
Existing Hydrosulfuric Acid Solvent Applications
01 Methods for hydrogen sulfide removal and treatment
Various processes and systems have been developed for the removal and treatment of hydrogen sulfide (hydrosulfuric acid) from gas streams and industrial effluents. These methods include absorption, adsorption, and chemical conversion techniques to eliminate this toxic and corrosive compound. The technologies aim to reduce environmental pollution and protect equipment from corrosion damage while meeting regulatory emission standards.- Methods for hydrogen sulfide removal and treatment: Various methods and systems for removing hydrogen sulfide (hydrosulfuric acid) from gas streams or liquid media. These processes typically involve chemical reactions, absorption, or adsorption techniques to convert the toxic hydrogen sulfide into less harmful compounds or to capture it for further processing. Such methods are crucial in industrial settings like natural gas processing, petroleum refining, and wastewater treatment to prevent environmental pollution and equipment corrosion.
- Detection and monitoring systems for hydrogen sulfide: Technologies and devices designed to detect, measure, and monitor hydrogen sulfide concentrations in various environments. These systems utilize different sensing mechanisms such as electrochemical sensors, optical sensors, or colorimetric indicators to provide real-time or periodic measurements of hydrogen sulfide levels. Such monitoring is essential for safety in industrial facilities, confined spaces, and environmental applications to prevent exposure to this toxic gas.
- Biological processes involving hydrogen sulfide: Biological methods that utilize microorganisms or enzymatic processes to either produce or consume hydrogen sulfide. These processes include sulfate-reducing bacteria that generate hydrogen sulfide under anaerobic conditions, as well as sulfide-oxidizing bacteria that convert hydrogen sulfide to less harmful compounds. Biological approaches are employed in wastewater treatment, bioremediation, and certain industrial applications as environmentally friendly alternatives to chemical methods.
- Industrial applications of hydrogen sulfide: Utilization of hydrogen sulfide or its derivatives in various industrial processes and applications. Despite its toxicity, hydrogen sulfide serves as a valuable reagent in chemical synthesis, metallurgy, and manufacturing. It is used in the production of sulfur compounds, metal sulfides, and certain pharmaceuticals. These applications require careful handling and specialized equipment due to the hazardous nature of the compound.
- Equipment and materials for hydrogen sulfide handling: Specialized equipment, materials, and containment systems designed for the safe handling, storage, and transport of hydrogen sulfide or materials that may generate it. These include corrosion-resistant containers, specialized valves, sealing materials, and protective coatings that can withstand the highly corrosive nature of hydrogen sulfide. Such equipment is essential in industries where hydrogen sulfide is present to prevent leaks, equipment failure, and potential safety hazards.
02 Analytical detection and measurement of hydrosulfuric acid
Techniques and devices for detecting and measuring hydrosulfuric acid concentrations in various environments. These include sensors, analytical instruments, and testing methods that can accurately quantify hydrogen sulfide levels for safety monitoring, quality control, and research purposes. The technologies enable rapid detection of this hazardous gas to prevent exposure and ensure workplace safety.Expand Specific Solutions03 Biological processing of hydrogen sulfide
Biological methods for the treatment and conversion of hydrogen sulfide using microorganisms and enzymatic processes. These approaches leverage natural biological systems to transform toxic hydrogen sulfide into less harmful compounds or useful byproducts. Bioreactors and specialized bacterial cultures are employed to facilitate these transformations in an environmentally friendly manner.Expand Specific Solutions04 Industrial applications of hydrosulfuric acid
Despite its hazardous nature, hydrosulfuric acid has various industrial applications in chemical manufacturing, metallurgy, and other processes. These applications utilize the chemical properties of hydrogen sulfide for specific reactions, treatments, or as a reagent in production processes. Controlled use of this compound enables certain chemical transformations that are valuable in industrial settings.Expand Specific Solutions05 Safety systems and protective equipment for hydrogen sulfide exposure
Specialized safety systems, protective equipment, and protocols designed to prevent and mitigate the risks associated with hydrogen sulfide exposure. These include gas detection alarms, personal protective equipment, emergency response systems, and containment technologies. The focus is on protecting workers and communities from the potentially lethal effects of this highly toxic gas.Expand Specific Solutions
Key Industry Players in Hydrosulfuric Acid Research and Production
The hydrosulfuric acid solvent market is currently in a growth phase, with increasing applications across petrochemical, pharmaceutical, and agricultural sectors. The global market size is estimated to reach approximately $3.5 billion by 2025, driven by demand in chemical processing and environmental remediation. Leading players include established petrochemical giants like China Petroleum & Chemical Corp. (Sinopec) and JFE Steel, alongside specialized chemical manufacturers such as Arkema, Fluid Energy Group, and Esseco Srl. Pharmaceutical applications are being advanced by Fidia Farmaceutici, Mitsubishi Tanabe Pharma, and Pfizer. Technical innovation is primarily led by research institutions like Sinopec Research Institute of Petroleum Processing and specialized companies like Ecolab and Haldor Topsøe, focusing on improving safety profiles and environmental sustainability of hydrosulfuric acid applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced hydrosulfuric acid solvent applications for petroleum refining processes. Their technology focuses on using hydrosulfuric acid as a catalyst in desulfurization processes, particularly in their VRDS (Vacuum Residue Desulfurization) systems. Sinopec has engineered specialized reactor designs that optimize the interaction between hydrosulfuric acid and petroleum compounds, achieving sulfur removal efficiencies of up to 98% in heavy crude oil processing. Their proprietary acid-resistant alloy materials for equipment construction extend operational lifespans by approximately 40% compared to conventional materials when exposed to hydrosulfuric acid environments. Additionally, Sinopec has pioneered recovery systems that capture and recycle over 85% of the hydrosulfuric acid used in their processes, significantly reducing waste and environmental impact.
Strengths: Superior desulfurization efficiency in petroleum processing; advanced materials science for acid-resistant equipment; excellent acid recovery systems reducing operational costs and environmental impact. Weaknesses: High initial capital investment required; technology primarily optimized for petroleum applications rather than broader industrial uses; requires specialized handling protocols due to the corrosive nature of hydrosulfuric acid.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group has developed a revolutionary approach to hydrosulfuric acid applications through their HydroSolv™ technology platform. Their innovation centers on modified hydrosulfuric acid formulations that maintain effectiveness while reducing corrosivity by up to 70% compared to conventional acid solutions. The company has engineered proprietary surfactant packages that enhance the penetration capabilities of hydrosulfuric acid in oil and gas well stimulation, achieving up to 35% greater permeability improvement in carbonate formations. Their technology incorporates specialized inhibitor compounds that selectively protect metal infrastructure while allowing the acid to react with targeted mineral deposits. Fluid Energy Group has also pioneered environmentally enhanced formulations that reduce the ecological footprint of hydrosulfuric acid applications, with biodegradability rates approximately 3-4 times faster than traditional acid systems. Their solutions have been successfully deployed in over 5,000 well treatments across North America with documented production improvements averaging 40-60% in previously underperforming wells.
Strengths: Significantly reduced corrosivity while maintaining acid effectiveness; enhanced environmental profile with faster biodegradation; proven field performance in oil and gas applications with substantial production improvements. Weaknesses: Higher cost per volume compared to conventional acid systems; requires specialized handling equipment despite reduced corrosivity; limited applications outside the energy sector.
Critical Patents and Technical Literature on H2S Solvent Properties
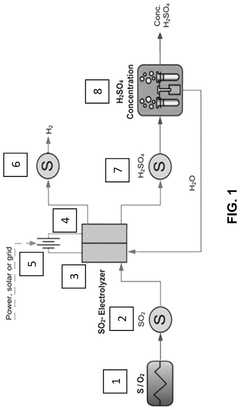
Process to convert reduced sulfur species and water into hydrogen and sulfuric acid
PatentActiveUS12110601B2
Innovation
- Electrochemical methods that thermally convert sulfur-containing species into sulfur dioxide, which is then electrochemically oxidized to produce sulfuric acid and hydrogen gas, offering a less energy-intensive, on-demand, and more versatile approach with reduced CO2 emissions, suitable for both small-scale and large-scale applications.
Free-flowing sulfur transport, storage and use to produce energy, fertilizer or hydrogen without carbon dioxide
PatentInactiveUS20060239785A1
Innovation
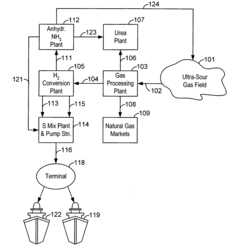
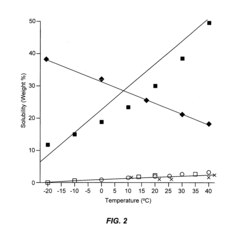
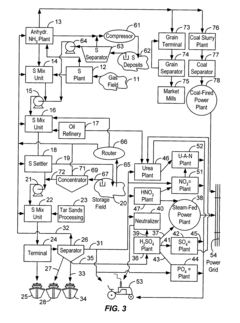
- The use of anhydrous ammonia and sulfur dioxide as fluid vehicles for elemental sulfur, which exhibit unique temperature-solubility relationships and reduced corrosiveness, allowing for the formation of stable solutions and slurries that prevent precipitation and reduce pipeline clogging, and the recovery of hydrogen sulfide to produce elemental sulfur and sulfur dioxide, facilitating efficient transportation and storage.
Safety and Handling Protocols for Hydrosulfuric Acid
The handling of hydrosulfuric acid (H2S in solution) requires stringent safety protocols due to its highly toxic and corrosive nature. Personnel working with this chemical must undergo comprehensive training that covers exposure risks, emergency procedures, and proper handling techniques. Appropriate personal protective equipment (PPE) is mandatory, including chemical-resistant gloves, face shields, splash goggles, and respiratory protection with appropriate filters for H2S gas.
Storage facilities for hydrosulfuric acid must be designed with specialized ventilation systems to prevent accumulation of potentially lethal H2S gas. Containers should be kept in cool, dry areas away from direct sunlight and incompatible materials such as strong oxidizers, metals, and certain catalysts that may trigger violent reactions. All storage vessels must be clearly labeled with hazard information and equipped with secondary containment systems to manage potential spills.
Transportation of hydrosulfuric acid falls under strict regulatory frameworks including DOT regulations in the United States and ADR in Europe. Specialized transport containers with pressure relief mechanisms are required, and vehicles must display appropriate hazard placards. Chain of custody documentation must accompany all shipments to ensure traceability and proper handling throughout the transportation process.
Emergency response protocols for hydrosulfuric acid incidents must be established prior to any handling operations. These include evacuation procedures, spill containment strategies, and decontamination methods. Facilities should be equipped with emergency eyewash stations, safety showers, and H2S gas detectors with audible alarms set at appropriate threshold levels. Regular drills should be conducted to ensure all personnel can execute emergency procedures efficiently.
Waste disposal of hydrosulfuric acid and contaminated materials must follow environmental regulations, typically requiring neutralization before disposal. The neutralization process generally involves careful addition of basic solutions such as sodium hydroxide under controlled conditions to convert the acid to less hazardous sulfide salts. All neutralization processes must be performed in well-ventilated areas with appropriate engineering controls.
Health monitoring programs should be implemented for workers regularly exposed to hydrosulfuric acid. These programs should include baseline and periodic medical examinations focusing on respiratory function, as chronic low-level exposure can cause cumulative health effects. Exposure monitoring using personal sampling devices helps ensure that workplace concentrations remain below established occupational exposure limits.
Documentation and record-keeping systems must track all aspects of hydrosulfuric acid handling, including training records, exposure monitoring data, maintenance of safety equipment, and incident reports. These records are essential for regulatory compliance and continuous improvement of safety protocols through regular review and analysis of near-miss incidents and actual exposures.
Storage facilities for hydrosulfuric acid must be designed with specialized ventilation systems to prevent accumulation of potentially lethal H2S gas. Containers should be kept in cool, dry areas away from direct sunlight and incompatible materials such as strong oxidizers, metals, and certain catalysts that may trigger violent reactions. All storage vessels must be clearly labeled with hazard information and equipped with secondary containment systems to manage potential spills.
Transportation of hydrosulfuric acid falls under strict regulatory frameworks including DOT regulations in the United States and ADR in Europe. Specialized transport containers with pressure relief mechanisms are required, and vehicles must display appropriate hazard placards. Chain of custody documentation must accompany all shipments to ensure traceability and proper handling throughout the transportation process.
Emergency response protocols for hydrosulfuric acid incidents must be established prior to any handling operations. These include evacuation procedures, spill containment strategies, and decontamination methods. Facilities should be equipped with emergency eyewash stations, safety showers, and H2S gas detectors with audible alarms set at appropriate threshold levels. Regular drills should be conducted to ensure all personnel can execute emergency procedures efficiently.
Waste disposal of hydrosulfuric acid and contaminated materials must follow environmental regulations, typically requiring neutralization before disposal. The neutralization process generally involves careful addition of basic solutions such as sodium hydroxide under controlled conditions to convert the acid to less hazardous sulfide salts. All neutralization processes must be performed in well-ventilated areas with appropriate engineering controls.
Health monitoring programs should be implemented for workers regularly exposed to hydrosulfuric acid. These programs should include baseline and periodic medical examinations focusing on respiratory function, as chronic low-level exposure can cause cumulative health effects. Exposure monitoring using personal sampling devices helps ensure that workplace concentrations remain below established occupational exposure limits.
Documentation and record-keeping systems must track all aspects of hydrosulfuric acid handling, including training records, exposure monitoring data, maintenance of safety equipment, and incident reports. These records are essential for regulatory compliance and continuous improvement of safety protocols through regular review and analysis of near-miss incidents and actual exposures.
Environmental Impact and Regulatory Compliance
The utilization of hydrosulfuric acid as a solvent presents significant environmental considerations that must be carefully addressed in any practical application. Hydrogen sulfide, the gaseous form of hydrosulfuric acid, is classified as a highly toxic substance with severe environmental implications. When released into the atmosphere, it contributes to acid rain formation and can cause substantial damage to ecosystems, particularly aquatic environments where even low concentrations can be lethal to fish and other organisms.
Regulatory frameworks governing hydrosulfuric acid usage vary globally but consistently emphasize stringent controls. In the United States, the Environmental Protection Agency (EPA) regulates hydrogen sulfide under the Clean Air Act and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), with reportable release thresholds set at 100 pounds. The Occupational Safety and Health Administration (OSHA) has established a permissible exposure limit of 20 ppm ceiling concentration for workplace environments.
European regulations, particularly under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), impose additional requirements for registration and safety assessments when hydrosulfuric acid is used in industrial processes. These regulations necessitate comprehensive documentation of risk management measures and exposure scenarios for all identified applications.
Waste management presents another critical compliance challenge. Hydrosulfuric acid waste streams require specialized treatment processes to neutralize the acid and remove sulfur compounds before disposal. Common treatment methods include oxidation with hydrogen peroxide or sodium hypochlorite, precipitation with metal salts, and biological treatment systems specifically designed for sulfur compounds.
Emissions control technologies must be implemented in facilities utilizing hydrosulfuric acid as a solvent. These typically include scrubber systems, thermal oxidizers, and activated carbon filtration units designed to capture and neutralize hydrogen sulfide before release. Continuous monitoring systems are often mandated to ensure compliance with emission limits and to provide early warning of potential releases.
The transportation of hydrosulfuric acid solutions is subject to hazardous materials regulations that require specialized containers, clear labeling, and detailed shipping documentation. Personnel involved in handling must receive comprehensive training on emergency response procedures and the use of appropriate personal protective equipment.
Companies developing applications for hydrosulfuric acid as a solvent must conduct thorough environmental impact assessments and implement robust environmental management systems to ensure regulatory compliance throughout the product lifecycle. This includes regular auditing, documentation of compliance activities, and continuous improvement of environmental performance metrics.
Regulatory frameworks governing hydrosulfuric acid usage vary globally but consistently emphasize stringent controls. In the United States, the Environmental Protection Agency (EPA) regulates hydrogen sulfide under the Clean Air Act and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), with reportable release thresholds set at 100 pounds. The Occupational Safety and Health Administration (OSHA) has established a permissible exposure limit of 20 ppm ceiling concentration for workplace environments.
European regulations, particularly under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), impose additional requirements for registration and safety assessments when hydrosulfuric acid is used in industrial processes. These regulations necessitate comprehensive documentation of risk management measures and exposure scenarios for all identified applications.
Waste management presents another critical compliance challenge. Hydrosulfuric acid waste streams require specialized treatment processes to neutralize the acid and remove sulfur compounds before disposal. Common treatment methods include oxidation with hydrogen peroxide or sodium hypochlorite, precipitation with metal salts, and biological treatment systems specifically designed for sulfur compounds.
Emissions control technologies must be implemented in facilities utilizing hydrosulfuric acid as a solvent. These typically include scrubber systems, thermal oxidizers, and activated carbon filtration units designed to capture and neutralize hydrogen sulfide before release. Continuous monitoring systems are often mandated to ensure compliance with emission limits and to provide early warning of potential releases.
The transportation of hydrosulfuric acid solutions is subject to hazardous materials regulations that require specialized containers, clear labeling, and detailed shipping documentation. Personnel involved in handling must receive comprehensive training on emergency response procedures and the use of appropriate personal protective equipment.
Companies developing applications for hydrosulfuric acid as a solvent must conduct thorough environmental impact assessments and implement robust environmental management systems to ensure regulatory compliance throughout the product lifecycle. This includes regular auditing, documentation of compliance activities, and continuous improvement of environmental performance metrics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!