Hydrosulfuric Acid Vs Sulfuric Acid: Corrosiveness on Steels
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Corrosion Background and Research Objectives
Acid corrosion represents one of the most significant challenges in industrial applications involving steel components. The interaction between acids and steel surfaces has been extensively studied since the early 20th century, with particular attention to the comparative effects of different acid types. Hydrosulfuric acid (H₂S in aqueous solution) and sulfuric acid (H₂SO₄) exhibit distinct corrosion mechanisms despite their sulfur-based chemistry, making their comparative analysis crucial for material selection in industrial settings.
The evolution of corrosion science has progressed from empirical observations to sophisticated electrochemical understanding. Early research focused primarily on visual documentation of corrosion effects, while modern approaches incorporate atomic-level analysis and real-time monitoring technologies. This progression has enabled more precise characterization of how these acids interact with various steel compositions under different environmental conditions.
Hydrosulfuric acid corrosion gained particular attention following several catastrophic failures in oil and gas infrastructure during the 1970s, leading to the development of specialized testing protocols such as NACE MR0175/ISO 15156. Sulfuric acid corrosion research intensified with the expansion of chemical processing industries, where its concentration-dependent behavior presented unique engineering challenges.
Recent technological advancements in materials science have introduced new steel alloys specifically designed to withstand acidic environments. However, the fundamental understanding of corrosion mechanisms at the microstructural level remains incomplete, particularly regarding the synergistic effects when multiple environmental factors are present alongside acid exposure.
The primary objective of this research is to establish a comprehensive comparative analysis of how hydrosulfuric acid and sulfuric acid affect different steel grades across varying concentrations, temperatures, and exposure durations. This includes quantifying corrosion rates, characterizing corrosion morphologies, and identifying critical thresholds where corrosion behavior significantly changes.
Secondary objectives include developing predictive models for estimating service life of steel components exposed to these acids, evaluating the effectiveness of current corrosion mitigation strategies, and exploring novel approaches to enhance corrosion resistance. The research also aims to address knowledge gaps regarding the influence of trace elements in steel composition on corrosion susceptibility to these specific acids.
The findings from this research will directly inform material selection guidelines for industries where exposure to these acids is unavoidable, including oil and gas, chemical processing, mining, and wastewater treatment. Additionally, the research seeks to establish standardized testing methodologies that more accurately reflect real-world exposure conditions, addressing limitations in current laboratory testing approaches.
The evolution of corrosion science has progressed from empirical observations to sophisticated electrochemical understanding. Early research focused primarily on visual documentation of corrosion effects, while modern approaches incorporate atomic-level analysis and real-time monitoring technologies. This progression has enabled more precise characterization of how these acids interact with various steel compositions under different environmental conditions.
Hydrosulfuric acid corrosion gained particular attention following several catastrophic failures in oil and gas infrastructure during the 1970s, leading to the development of specialized testing protocols such as NACE MR0175/ISO 15156. Sulfuric acid corrosion research intensified with the expansion of chemical processing industries, where its concentration-dependent behavior presented unique engineering challenges.
Recent technological advancements in materials science have introduced new steel alloys specifically designed to withstand acidic environments. However, the fundamental understanding of corrosion mechanisms at the microstructural level remains incomplete, particularly regarding the synergistic effects when multiple environmental factors are present alongside acid exposure.
The primary objective of this research is to establish a comprehensive comparative analysis of how hydrosulfuric acid and sulfuric acid affect different steel grades across varying concentrations, temperatures, and exposure durations. This includes quantifying corrosion rates, characterizing corrosion morphologies, and identifying critical thresholds where corrosion behavior significantly changes.
Secondary objectives include developing predictive models for estimating service life of steel components exposed to these acids, evaluating the effectiveness of current corrosion mitigation strategies, and exploring novel approaches to enhance corrosion resistance. The research also aims to address knowledge gaps regarding the influence of trace elements in steel composition on corrosion susceptibility to these specific acids.
The findings from this research will directly inform material selection guidelines for industries where exposure to these acids is unavoidable, including oil and gas, chemical processing, mining, and wastewater treatment. Additionally, the research seeks to establish standardized testing methodologies that more accurately reflect real-world exposure conditions, addressing limitations in current laboratory testing approaches.
Market Analysis of Acid-Resistant Steel Applications
The global market for acid-resistant steel applications has been experiencing significant growth, driven by increasing demand across multiple industries where corrosion resistance is critical. The market size for specialized corrosion-resistant steel was valued at approximately $7.2 billion in 2022 and is projected to reach $9.8 billion by 2028, growing at a CAGR of 5.3% during the forecast period.
Chemical processing represents the largest application segment, accounting for nearly 38% of the total market share. This dominance is attributed to the extensive use of acid-resistant steels in equipment that regularly contacts corrosive substances like hydrosulfuric acid and sulfuric acid. The oil and gas industry follows closely, constituting about 27% of the market, where these specialized steels are essential for handling sour gas containing hydrogen sulfide.
Regional analysis indicates that Asia-Pacific holds the largest market share at 42%, with China being the primary contributor due to its massive chemical manufacturing sector. North America and Europe account for 25% and 22% respectively, with mature industrial bases driving steady demand for high-performance corrosion-resistant materials.
The market segmentation by steel type reveals that austenitic stainless steels (particularly 316L and 317L grades) dominate with 56% market share due to their excellent resistance to both hydrosulfuric and sulfuric acids. Duplex stainless steels are gaining traction, currently holding 23% of the market share, as they offer improved strength alongside good acid resistance.
End-user analysis shows diversification across chemical processing (38%), oil and gas (27%), mining (14%), power generation (11%), and others (10%). The mining sector is experiencing the fastest growth rate at 6.8% annually, driven by increasing extraction activities involving acidic environments.
Price trends indicate that acid-resistant steels command a premium of 30-45% over conventional steel grades, with the price differential widening for highly specialized alloys designed for extreme acid exposure. This premium pricing reflects both the advanced metallurgical compositions and the critical nature of failure prevention in acid-handling applications.
Market dynamics are increasingly influenced by sustainability considerations, with manufacturers developing steel grades that maintain longer service life in acidic environments, thereby reducing replacement frequency and associated environmental impacts. This trend is expected to accelerate as regulatory frameworks around industrial emissions and waste management become more stringent globally.
Chemical processing represents the largest application segment, accounting for nearly 38% of the total market share. This dominance is attributed to the extensive use of acid-resistant steels in equipment that regularly contacts corrosive substances like hydrosulfuric acid and sulfuric acid. The oil and gas industry follows closely, constituting about 27% of the market, where these specialized steels are essential for handling sour gas containing hydrogen sulfide.
Regional analysis indicates that Asia-Pacific holds the largest market share at 42%, with China being the primary contributor due to its massive chemical manufacturing sector. North America and Europe account for 25% and 22% respectively, with mature industrial bases driving steady demand for high-performance corrosion-resistant materials.
The market segmentation by steel type reveals that austenitic stainless steels (particularly 316L and 317L grades) dominate with 56% market share due to their excellent resistance to both hydrosulfuric and sulfuric acids. Duplex stainless steels are gaining traction, currently holding 23% of the market share, as they offer improved strength alongside good acid resistance.
End-user analysis shows diversification across chemical processing (38%), oil and gas (27%), mining (14%), power generation (11%), and others (10%). The mining sector is experiencing the fastest growth rate at 6.8% annually, driven by increasing extraction activities involving acidic environments.
Price trends indicate that acid-resistant steels command a premium of 30-45% over conventional steel grades, with the price differential widening for highly specialized alloys designed for extreme acid exposure. This premium pricing reflects both the advanced metallurgical compositions and the critical nature of failure prevention in acid-handling applications.
Market dynamics are increasingly influenced by sustainability considerations, with manufacturers developing steel grades that maintain longer service life in acidic environments, thereby reducing replacement frequency and associated environmental impacts. This trend is expected to accelerate as regulatory frameworks around industrial emissions and waste management become more stringent globally.
Current Challenges in Acid Corrosion Resistance
The corrosion of steel by acids represents one of the most significant challenges in various industrial applications, particularly when comparing hydrosulfuric acid (H2S in aqueous solution) and sulfuric acid (H2SO4). These two acids exhibit distinct corrosion mechanisms and severity levels when interacting with different steel grades, creating persistent challenges for engineers and materials scientists.
Sulfuric acid's corrosiveness varies dramatically with concentration. At concentrations below 80%, it aggressively attacks carbon steels through hydrogen evolution and metal dissolution. However, at very high concentrations (>98%), carbon steels may develop a protective iron sulfate film, creating a counterintuitive situation where dilution actually increases corrosion rates. This concentration-dependent behavior complicates material selection and protection strategies.
Hydrosulfuric acid presents different challenges, primarily through hydrogen sulfide-induced damage. When dissolved in water, H2S dissociates to form a weak acid that initiates corrosion, but its most damaging effect comes from hydrogen embrittlement and sulfide stress cracking (SSC). The atomic hydrogen produced during corrosion can penetrate steel microstructures, causing catastrophic mechanical failures even at relatively low stress levels.
Temperature effects further complicate acid corrosion resistance. While corrosion rates typically increase with temperature, the relationship is not always linear. For sulfuric acid, temperature increases can accelerate corrosion exponentially in certain concentration ranges, while potentially reducing attack at very high concentrations by promoting protective film formation. For hydrosulfuric acid solutions, elevated temperatures often accelerate both general corrosion and hydrogen permeation rates.
The presence of contaminants and oxidizing agents creates additional challenges. Chlorides, dissolved oxygen, and ferric ions can disrupt protective films and initiate localized corrosion mechanisms like pitting and crevice corrosion. In industrial settings, these contaminants are nearly impossible to eliminate completely, making laboratory corrosion data often unreliable for real-world applications.
Current testing methodologies also present limitations. Standard immersion tests may not accurately represent flow conditions in industrial equipment, while electrochemical techniques might not capture long-term corrosion behaviors. The industry lacks standardized methods specifically designed for comparing hydrosulfuric and sulfuric acid corrosion under identical conditions, making direct comparisons difficult.
Perhaps most challenging is the development of cost-effective corrosion-resistant alloys. While highly alloyed stainless steels and nickel-based alloys offer excellent resistance to both acids, their high cost prohibits widespread use. Finding the optimal balance between corrosion resistance and economic feasibility remains an ongoing challenge, particularly for applications requiring exposure to both acid types or varying concentrations.
Sulfuric acid's corrosiveness varies dramatically with concentration. At concentrations below 80%, it aggressively attacks carbon steels through hydrogen evolution and metal dissolution. However, at very high concentrations (>98%), carbon steels may develop a protective iron sulfate film, creating a counterintuitive situation where dilution actually increases corrosion rates. This concentration-dependent behavior complicates material selection and protection strategies.
Hydrosulfuric acid presents different challenges, primarily through hydrogen sulfide-induced damage. When dissolved in water, H2S dissociates to form a weak acid that initiates corrosion, but its most damaging effect comes from hydrogen embrittlement and sulfide stress cracking (SSC). The atomic hydrogen produced during corrosion can penetrate steel microstructures, causing catastrophic mechanical failures even at relatively low stress levels.
Temperature effects further complicate acid corrosion resistance. While corrosion rates typically increase with temperature, the relationship is not always linear. For sulfuric acid, temperature increases can accelerate corrosion exponentially in certain concentration ranges, while potentially reducing attack at very high concentrations by promoting protective film formation. For hydrosulfuric acid solutions, elevated temperatures often accelerate both general corrosion and hydrogen permeation rates.
The presence of contaminants and oxidizing agents creates additional challenges. Chlorides, dissolved oxygen, and ferric ions can disrupt protective films and initiate localized corrosion mechanisms like pitting and crevice corrosion. In industrial settings, these contaminants are nearly impossible to eliminate completely, making laboratory corrosion data often unreliable for real-world applications.
Current testing methodologies also present limitations. Standard immersion tests may not accurately represent flow conditions in industrial equipment, while electrochemical techniques might not capture long-term corrosion behaviors. The industry lacks standardized methods specifically designed for comparing hydrosulfuric and sulfuric acid corrosion under identical conditions, making direct comparisons difficult.
Perhaps most challenging is the development of cost-effective corrosion-resistant alloys. While highly alloyed stainless steels and nickel-based alloys offer excellent resistance to both acids, their high cost prohibits widespread use. Finding the optimal balance between corrosion resistance and economic feasibility remains an ongoing challenge, particularly for applications requiring exposure to both acid types or varying concentrations.
Comparative Analysis of H2S vs H2SO4 Corrosion Mechanisms
01 Corrosion mechanisms of hydrosulfuric and sulfuric acids
Hydrosulfuric acid (H2S) and sulfuric acid (H2SO4) exhibit different corrosion mechanisms. Sulfuric acid is highly corrosive due to its strong oxidizing properties and ability to donate protons, causing rapid deterioration of many metals. Hydrosulfuric acid, while less aggressive than sulfuric acid, causes corrosion through sulfide stress cracking and hydrogen embrittlement. The corrosiveness of both acids varies with concentration, temperature, and the presence of other compounds.- Corrosion mechanisms of hydrosulfuric and sulfuric acids: Hydrosulfuric acid (H2S) and sulfuric acid (H2SO4) exhibit different corrosion mechanisms. Sulfuric acid is highly corrosive due to its strong oxidizing properties and ability to donate protons, causing rapid deterioration of many metals. Hydrosulfuric acid, while less aggressive than sulfuric acid, causes corrosion through sulfide stress cracking and hydrogen embrittlement. The combination of both acids can create synergistic corrosion effects that accelerate material degradation, particularly in industrial environments where both acids may be present.
- Corrosion-resistant materials and coatings: Various materials and coatings have been developed to resist corrosion from hydrosulfuric and sulfuric acids. These include specialized alloys containing chromium, nickel, and molybdenum that form protective oxide layers. Polymer-based coatings such as fluoropolymers, epoxy resins, and phenolic linings provide effective barriers against acid attack. Ceramic materials and glass linings are also utilized in extremely corrosive environments. The selection of appropriate corrosion-resistant materials depends on factors such as acid concentration, temperature, and pressure conditions.
- Corrosion monitoring and prevention techniques: Effective monitoring and prevention techniques are essential for managing corrosion caused by hydrosulfuric and sulfuric acids. These include electrochemical monitoring systems that detect early signs of corrosion, inhibitor chemicals that form protective films on metal surfaces, and cathodic protection systems. Regular inspection protocols using ultrasonic testing, radiography, and visual examination help identify corrosion before critical failure occurs. Proper material selection, design considerations such as avoiding crevices, and controlling environmental factors like temperature and humidity also play crucial roles in corrosion prevention.
- Industrial applications and corrosion challenges: Hydrosulfuric and sulfuric acids present significant corrosion challenges across various industries. In oil and gas processing, these acids corrode pipelines, storage tanks, and processing equipment. Chemical manufacturing facilities face corrosion in reactors, heat exchangers, and transfer lines. Wastewater treatment plants encounter corrosion in pumps, valves, and containment structures. Mining operations deal with acid-induced deterioration of extraction and processing equipment. Each industry requires specialized corrosion management strategies tailored to their specific operating conditions and exposure scenarios.
- Neutralization and treatment methods: Various neutralization and treatment methods are employed to mitigate the corrosive effects of hydrosulfuric and sulfuric acids. These include chemical neutralization using alkaline substances like sodium hydroxide, calcium carbonate, or magnesium hydroxide. Scrubbing systems remove acidic gases from process streams before they can condense and cause corrosion. Acid dilution techniques reduce corrosivity in certain applications. Specialized treatment processes convert acids into less corrosive compounds or recover them for reuse, minimizing both corrosion damage and environmental impact while improving operational efficiency.
02 Corrosion-resistant materials and coatings
Various materials and coatings have been developed to resist corrosion from hydrosulfuric and sulfuric acids. These include specialized alloys containing chromium, nickel, and molybdenum, as well as polymer-based linings and coatings. Fluoropolymers, epoxy resins, and certain ceramic materials demonstrate excellent resistance to both acids. The selection of appropriate materials depends on factors such as acid concentration, operating temperature, and pressure conditions.Expand Specific Solutions03 Corrosion inhibitors and treatment methods
Chemical inhibitors can be added to reduce the corrosive effects of hydrosulfuric and sulfuric acids. These inhibitors work by forming protective films on metal surfaces or neutralizing acid components. Treatment methods include passivation techniques, pH adjustment, and oxygen scavenging. Organic compounds containing nitrogen, sulfur, or oxygen atoms are particularly effective as inhibitors against acid corrosion, creating a protective barrier between the acid and the metal surface.Expand Specific Solutions04 Monitoring and detection systems for acid corrosion
Advanced monitoring systems have been developed to detect and measure corrosion caused by hydrosulfuric and sulfuric acids. These include electrochemical sensors, ultrasonic thickness measurement devices, and real-time corrosion rate monitors. Early detection allows for timely intervention and maintenance, preventing catastrophic failures in industrial equipment. Some systems incorporate predictive analytics to forecast potential corrosion issues before they become critical.Expand Specific Solutions05 Industrial applications and corrosion management
In industrial settings where hydrosulfuric and sulfuric acids are present, comprehensive corrosion management strategies are essential. These include regular inspection protocols, material selection guidelines, and process control measures. Industries such as oil and gas, chemical processing, and wastewater treatment have developed specialized approaches to manage acid corrosion. Techniques include controlling process parameters, implementing proper drainage systems, and establishing maintenance schedules based on corrosion risk assessment.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The corrosion resistance of steels against hydrosulfuric acid versus sulfuric acid represents a critical technical challenge in the mature steel industry, currently valued at approximately $2.5 trillion globally. The competitive landscape is dominated by established players like POSCO Holdings, Nippon Steel, and JFE Steel, who have developed specialized corrosion-resistant steel grades. Major Chinese producers including Baoshan Iron & Steel and Shougang Group are rapidly advancing their technical capabilities in this field. The technology has reached moderate maturity with most major steel manufacturers offering corrosion-resistant product lines, though innovation continues in highly specialized applications. Companies like Outokumpu and Mitsubishi Heavy Industries have established leadership in developing proprietary steel compositions specifically engineered for extreme acid environments in petrochemical and energy sectors.
POSCO Holdings, Inc.
Technical Solution: POSCO has developed advanced corrosion-resistant steel grades specifically engineered to withstand both hydrosulfuric acid and sulfuric acid environments. Their proprietary PosMAC (POSCO Magnesium Alloy Coated) steel utilizes a zinc-magnesium-aluminum coating system that provides superior protection against acid corrosion compared to conventional galvanized steels. For sulfuric acid environments, POSCO employs high-molybdenum austenitic stainless steels (6% Mo grades) that demonstrate exceptional resistance to sulfuric acid at various concentrations and temperatures. Their research has shown that while hydrosulfuric acid (H2S) causes hydrogen-induced cracking and sulfide stress corrosion in carbon steels, their specialized high-strength low-alloy (HSLA) steels with carefully controlled microstructures exhibit up to 40% better resistance to H2S environments than standard carbon steels.
Strengths: Superior corrosion resistance in both acid environments through specialized alloy compositions and coating technologies. Their integrated materials science approach allows customization for specific industrial applications. Weaknesses: Higher production costs compared to standard steels, and potential limitations in extremely high concentration acid environments where even their advanced alloys may experience accelerated corrosion rates.
NIPPON STEEL CORP.
Technical Solution: Nippon Steel has pioneered the development of specialized stainless steel grades for acid resistance, particularly their NAR series (Nippon Acid Resistant) designed specifically for sulfuric and hydrosulfuric acid environments. Their research demonstrates that hydrosulfuric acid (H2S) primarily causes hydrogen embrittlement and sulfide stress cracking in steels, while sulfuric acid causes general corrosion through direct chemical attack. To address these different corrosion mechanisms, Nippon Steel developed their Super304H austenitic stainless steel with enhanced copper content (2.5-3.5%) that forms protective precipitates, improving resistance to both acids. For severe hydrosulfuric acid environments, they've engineered their NSSC FW series with controlled nitrogen content and optimized microstructure that reduces susceptibility to hydrogen penetration by up to 65% compared to conventional steels. Their corrosion testing protocols have established that while sulfuric acid corrosion rates increase dramatically with temperature and concentration, hydrosulfuric acid corrosion is more influenced by pH and partial pressure of H2S gas.
Strengths: Comprehensive understanding of different corrosion mechanisms allows for targeted material solutions. Their proprietary alloy compositions demonstrate exceptional resistance to both acid types across various industrial conditions. Weaknesses: Higher material costs and potential manufacturing complexity for their most advanced grades. Some specialized compositions may have limited weldability or formability compared to standard steels.
Key Patents and Research on Steel Corrosion Protection
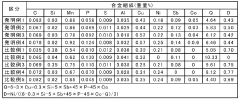
Steel having excellent resistance to corrosion by hydrochloric acid and sulfuric acid and method for manufacturing the same
PatentActiveIN5248DELNP2010A
Innovation
- A steel composition with specific weight percentages of elements such as C, Si, Mn, P, Al, Cu, Co, Sb, Sn, and W, along with a manufacturing process involving reheating, hot-rolling, and coiling at controlled temperatures to enhance corrosion resistance, is developed to address the corrosion issues.
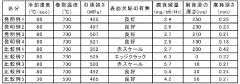
Sulfuric acid and hydrochloric acid composite corrosion-resistant steel plate with excellent wear resistance and surface quality, and its manufacturing method
PatentInactiveJP2016535171A
Innovation
- A steel composition with controlled elements (C, Si, Mn, P, S, Al, Cu, Ni, Co, Sb) and specific processing conditions (reheating, hot-rolling, cooling, and coiling) to form a corrosion-resistant layer with Cu, Co, Ni, and Sb oxides, ensuring excellent wear resistance and surface quality.
Material Testing Standards and Protocols
The evaluation of corrosion effects from hydrosulfuric acid versus sulfuric acid on steel materials requires adherence to established testing standards and protocols to ensure reliable, reproducible results. The American Society for Testing and Materials (ASTM) provides several key standards specifically designed for acid corrosion testing, including ASTM G31 for laboratory immersion testing and ASTM G1 for preparing, cleaning, and evaluating corrosion test specimens.
For comprehensive corrosion assessment, the NACE International (National Association of Corrosion Engineers) TM0169/G31 standard outlines procedures for laboratory immersion corrosion testing of metals, particularly applicable when comparing the corrosive effects of different acid solutions on steel substrates. These standards specify precise parameters including temperature control (±1°C), solution volume to specimen surface area ratios, and exposure duration requirements.
ISO 8407 and ISO 9226 standards provide additional methodologies for removing corrosion products from test specimens and conducting atmospheric corrosion tests, respectively. When specifically examining sulfuric and hydrosulfuric acid effects, ASTM G59 for electrochemical polarization measurements becomes particularly valuable for quantifying corrosion rates and mechanisms.
Sample preparation protocols demand meticulous attention to surface finish consistency, with ASTM E3 specifying metallographic specimen preparation techniques. Test specimens typically require standardized dimensions, with surface areas calculated to three significant figures, and edges properly prepared to prevent preferential corrosion that could skew results.
Data collection methodologies must include weight loss measurements (accurate to ±0.1mg), corrosion rate calculations (expressed in mils per year or millimeters per year), photographic documentation, and microscopic examination of surface morphology. For electrochemical testing, potentiodynamic polarization techniques following ASTM G102 enable determination of corrosion current density and calculation of corrosion rates.
Statistical analysis requirements typically mandate a minimum of three replicate specimens per test condition to ensure result validity. The ISO 8407 standard further details procedures for removing corrosion products without removing base metal, critical for accurate weight loss determination when comparing the differential effects of hydrosulfuric versus sulfuric acid.
Environmental test parameters must be carefully controlled and documented, including solution pH, temperature, aeration conditions, and agitation rates. For industrial relevance, cyclic testing protocols that alternate between immersion and atmospheric exposure may be implemented according to ASTM G44 to simulate real-world service conditions where steel components might experience intermittent acid exposure.
For comprehensive corrosion assessment, the NACE International (National Association of Corrosion Engineers) TM0169/G31 standard outlines procedures for laboratory immersion corrosion testing of metals, particularly applicable when comparing the corrosive effects of different acid solutions on steel substrates. These standards specify precise parameters including temperature control (±1°C), solution volume to specimen surface area ratios, and exposure duration requirements.
ISO 8407 and ISO 9226 standards provide additional methodologies for removing corrosion products from test specimens and conducting atmospheric corrosion tests, respectively. When specifically examining sulfuric and hydrosulfuric acid effects, ASTM G59 for electrochemical polarization measurements becomes particularly valuable for quantifying corrosion rates and mechanisms.
Sample preparation protocols demand meticulous attention to surface finish consistency, with ASTM E3 specifying metallographic specimen preparation techniques. Test specimens typically require standardized dimensions, with surface areas calculated to three significant figures, and edges properly prepared to prevent preferential corrosion that could skew results.
Data collection methodologies must include weight loss measurements (accurate to ±0.1mg), corrosion rate calculations (expressed in mils per year or millimeters per year), photographic documentation, and microscopic examination of surface morphology. For electrochemical testing, potentiodynamic polarization techniques following ASTM G102 enable determination of corrosion current density and calculation of corrosion rates.
Statistical analysis requirements typically mandate a minimum of three replicate specimens per test condition to ensure result validity. The ISO 8407 standard further details procedures for removing corrosion products without removing base metal, critical for accurate weight loss determination when comparing the differential effects of hydrosulfuric versus sulfuric acid.
Environmental test parameters must be carefully controlled and documented, including solution pH, temperature, aeration conditions, and agitation rates. For industrial relevance, cyclic testing protocols that alternate between immersion and atmospheric exposure may be implemented according to ASTM G44 to simulate real-world service conditions where steel components might experience intermittent acid exposure.
Environmental Impact and Safety Considerations
The environmental impact and safety considerations of hydrosulfuric acid (H2S in aqueous solution) and sulfuric acid (H2SO4) present significant concerns when evaluating their corrosiveness on steel materials. Both acids pose distinct environmental hazards that must be carefully managed in industrial applications.
Hydrosulfuric acid releases hydrogen sulfide gas, which is not only highly toxic but also flammable, creating dual environmental and safety risks. Even at concentrations as low as 100 ppm, hydrogen sulfide can cause respiratory paralysis, while prolonged exposure to lower concentrations may lead to chronic health issues including neurological damage. When hydrosulfuric acid corrodes steel infrastructure, the resulting leaks can contaminate soil and water systems with sulfides that disrupt aquatic ecosystems.
Sulfuric acid presents different but equally serious environmental challenges. As one of the most widely used industrial chemicals, its production, transportation, and application create numerous opportunities for environmental exposure. Accidental releases can dramatically lower the pH of water bodies, causing immediate mortality of aquatic organisms and long-term ecosystem damage. The acid rain phenomenon, partially attributed to sulfuric acid formation in the atmosphere, demonstrates its broader environmental impact.
From a safety management perspective, the corrosion of steel by these acids requires distinct protective measures. Sulfuric acid necessitates specialized containment systems typically utilizing high-silicon cast iron, specialized stainless steels, or polymer-lined vessels. Hydrosulfuric acid systems require additional gas detection equipment and emergency ventilation systems due to the potential for hydrogen sulfide release.
Regulatory frameworks worldwide have established strict guidelines for both acids. The transportation of concentrated sulfuric acid is subject to hazardous materials regulations requiring specialized containers and handling procedures. Hydrogen sulfide emissions are regulated under air quality standards, with mandatory monitoring in facilities where hydrosulfuric acid is present.
Waste management presents another critical consideration. Neutralization of sulfuric acid waste generates significant heat and requires careful handling to prevent secondary accidents. Hydrosulfuric acid waste treatment must address both the liquid component and potential gas emissions, often requiring multi-stage treatment processes including oxidation to convert sulfides to less harmful compounds.
Recent technological advances have improved safety systems for both acids, including real-time corrosion monitoring systems, advanced polymer coatings for steel protection, and automated emergency response systems that can detect leaks and initiate containment procedures before significant environmental damage occurs.
Hydrosulfuric acid releases hydrogen sulfide gas, which is not only highly toxic but also flammable, creating dual environmental and safety risks. Even at concentrations as low as 100 ppm, hydrogen sulfide can cause respiratory paralysis, while prolonged exposure to lower concentrations may lead to chronic health issues including neurological damage. When hydrosulfuric acid corrodes steel infrastructure, the resulting leaks can contaminate soil and water systems with sulfides that disrupt aquatic ecosystems.
Sulfuric acid presents different but equally serious environmental challenges. As one of the most widely used industrial chemicals, its production, transportation, and application create numerous opportunities for environmental exposure. Accidental releases can dramatically lower the pH of water bodies, causing immediate mortality of aquatic organisms and long-term ecosystem damage. The acid rain phenomenon, partially attributed to sulfuric acid formation in the atmosphere, demonstrates its broader environmental impact.
From a safety management perspective, the corrosion of steel by these acids requires distinct protective measures. Sulfuric acid necessitates specialized containment systems typically utilizing high-silicon cast iron, specialized stainless steels, or polymer-lined vessels. Hydrosulfuric acid systems require additional gas detection equipment and emergency ventilation systems due to the potential for hydrogen sulfide release.
Regulatory frameworks worldwide have established strict guidelines for both acids. The transportation of concentrated sulfuric acid is subject to hazardous materials regulations requiring specialized containers and handling procedures. Hydrogen sulfide emissions are regulated under air quality standards, with mandatory monitoring in facilities where hydrosulfuric acid is present.
Waste management presents another critical consideration. Neutralization of sulfuric acid waste generates significant heat and requires careful handling to prevent secondary accidents. Hydrosulfuric acid waste treatment must address both the liquid component and potential gas emissions, often requiring multi-stage treatment processes including oxidation to convert sulfides to less harmful compounds.
Recent technological advances have improved safety systems for both acids, including real-time corrosion monitoring systems, advanced polymer coatings for steel protection, and automated emergency response systems that can detect leaks and initiate containment procedures before significant environmental damage occurs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!