ICP-MS Performance Benchmarks: From Sample Introduction to Detection
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Evolution and Performance Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the early 1980s. This analytical technique combines the high-temperature ICP source with a mass spectrometer to provide elemental analysis with exceptional sensitivity, capable of detecting metals and several non-metals at concentrations as low as one part per trillion. The evolution of ICP-MS technology has been driven by the increasing demands for lower detection limits, higher sample throughput, and improved interference management across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and clinical research.
The historical development of ICP-MS can be traced through several distinct generations of instrumentation. First-generation systems established the fundamental technology but suffered from significant limitations in terms of interference handling and stability. Second-generation instruments introduced collision/reaction cell technology, dramatically improving performance by reducing polyatomic interferences. The third generation brought enhanced sensitivity through improved ion optics and detector technology, while current fourth-generation systems feature advanced collision/reaction cells, triple quadrupole configurations, and high-resolution capabilities.
Performance objectives for modern ICP-MS systems focus on several critical parameters. Sensitivity remains paramount, with current high-end systems achieving detection limits in the parts-per-quadrillion range for many elements. Precision and accuracy targets have become increasingly stringent, with expectations of relative standard deviations below 2% for most applications and often below 0.5% for isotope ratio measurements.
Speed and sample throughput have emerged as essential performance metrics, with modern systems capable of analyzing dozens of elements in hundreds of samples per day. This has been facilitated by improvements in sample introduction systems, including high-efficiency nebulizers and rapid washout technologies. Interference management capabilities have also advanced significantly, with sophisticated collision/reaction cell technologies and high-resolution mass analyzers effectively eliminating most polyatomic and isobaric interferences.
Robustness and stability represent another crucial performance objective, particularly for routine analytical laboratories processing large sample batches. Modern systems aim to maintain calibration stability over extended analytical runs, with drift typically below 5% over 8-12 hours of continuous operation. This stability is achieved through improved plasma generation technology, temperature-controlled spray chambers, and advanced electronic systems.
The ongoing technological trajectory suggests future performance objectives will focus on further miniaturization, reduced operating costs, simplified maintenance requirements, and enhanced software capabilities for automated method development and data interpretation. Integration with artificial intelligence for predictive maintenance and automated optimization represents an emerging frontier in ICP-MS development, potentially revolutionizing laboratory workflows and accessibility.
The historical development of ICP-MS can be traced through several distinct generations of instrumentation. First-generation systems established the fundamental technology but suffered from significant limitations in terms of interference handling and stability. Second-generation instruments introduced collision/reaction cell technology, dramatically improving performance by reducing polyatomic interferences. The third generation brought enhanced sensitivity through improved ion optics and detector technology, while current fourth-generation systems feature advanced collision/reaction cells, triple quadrupole configurations, and high-resolution capabilities.
Performance objectives for modern ICP-MS systems focus on several critical parameters. Sensitivity remains paramount, with current high-end systems achieving detection limits in the parts-per-quadrillion range for many elements. Precision and accuracy targets have become increasingly stringent, with expectations of relative standard deviations below 2% for most applications and often below 0.5% for isotope ratio measurements.
Speed and sample throughput have emerged as essential performance metrics, with modern systems capable of analyzing dozens of elements in hundreds of samples per day. This has been facilitated by improvements in sample introduction systems, including high-efficiency nebulizers and rapid washout technologies. Interference management capabilities have also advanced significantly, with sophisticated collision/reaction cell technologies and high-resolution mass analyzers effectively eliminating most polyatomic and isobaric interferences.
Robustness and stability represent another crucial performance objective, particularly for routine analytical laboratories processing large sample batches. Modern systems aim to maintain calibration stability over extended analytical runs, with drift typically below 5% over 8-12 hours of continuous operation. This stability is achieved through improved plasma generation technology, temperature-controlled spray chambers, and advanced electronic systems.
The ongoing technological trajectory suggests future performance objectives will focus on further miniaturization, reduced operating costs, simplified maintenance requirements, and enhanced software capabilities for automated method development and data interpretation. Integration with artificial intelligence for predictive maintenance and automated optimization represents an emerging frontier in ICP-MS development, potentially revolutionizing laboratory workflows and accessibility.
Market Analysis for High-Precision Elemental Analysis
The global market for high-precision elemental analysis continues to expand, driven by increasing demands across multiple sectors including environmental monitoring, pharmaceuticals, food safety, and advanced materials research. The ICP-MS (Inductively Coupled Plasma Mass Spectrometry) technology segment represents a significant portion of this market, valued at approximately $4.3 billion in 2022 with projections to reach $6.2 billion by 2027, growing at a CAGR of 7.6%.
Environmental testing remains the largest application segment, accounting for nearly 32% of the total market share. This dominance stems from increasingly stringent regulations worldwide regarding water quality, soil contamination, and air pollution monitoring. The pharmaceutical and biotechnology sectors follow closely, representing 28% of market demand, where ultra-trace elemental analysis is critical for ensuring product safety and quality control.
Geographically, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 9.2% annually, primarily driven by China's expanding industrial base and increasing environmental regulations. Emerging economies in Latin America and Africa are showing promising growth potential as they strengthen their environmental monitoring infrastructure.
The competitive landscape features both established analytical instrument manufacturers and specialized providers. Key market players include Thermo Fisher Scientific (market share: 26%), Agilent Technologies (22%), PerkinElmer (18%), and Shimadzu Corporation (12%). These companies compete primarily on instrument sensitivity, detection limits, throughput capabilities, and software integration.
Customer segmentation reveals distinct needs across different sectors. Research institutions prioritize detection limits and analytical flexibility, while industrial users emphasize throughput, reliability, and ease of use. Government regulatory agencies focus on standardization, data integrity, and compliance features.
Price sensitivity varies significantly by segment. High-end research instruments command premium prices ($300,000-500,000), while more standardized industrial systems typically range from $150,000-250,000. The total cost of ownership remains a critical factor, with consumables and maintenance representing approximately 15-20% of annual operational costs.
Market trends indicate growing demand for portable and field-deployable systems, which currently represent only 8% of the market but are growing at 12% annually. Additionally, integration with automation systems and data analytics platforms is becoming increasingly important as laboratories seek to improve efficiency and reduce human error in analytical workflows.
Environmental testing remains the largest application segment, accounting for nearly 32% of the total market share. This dominance stems from increasingly stringent regulations worldwide regarding water quality, soil contamination, and air pollution monitoring. The pharmaceutical and biotechnology sectors follow closely, representing 28% of market demand, where ultra-trace elemental analysis is critical for ensuring product safety and quality control.
Geographically, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 9.2% annually, primarily driven by China's expanding industrial base and increasing environmental regulations. Emerging economies in Latin America and Africa are showing promising growth potential as they strengthen their environmental monitoring infrastructure.
The competitive landscape features both established analytical instrument manufacturers and specialized providers. Key market players include Thermo Fisher Scientific (market share: 26%), Agilent Technologies (22%), PerkinElmer (18%), and Shimadzu Corporation (12%). These companies compete primarily on instrument sensitivity, detection limits, throughput capabilities, and software integration.
Customer segmentation reveals distinct needs across different sectors. Research institutions prioritize detection limits and analytical flexibility, while industrial users emphasize throughput, reliability, and ease of use. Government regulatory agencies focus on standardization, data integrity, and compliance features.
Price sensitivity varies significantly by segment. High-end research instruments command premium prices ($300,000-500,000), while more standardized industrial systems typically range from $150,000-250,000. The total cost of ownership remains a critical factor, with consumables and maintenance representing approximately 15-20% of annual operational costs.
Market trends indicate growing demand for portable and field-deployable systems, which currently represent only 8% of the market but are growing at 12% annually. Additionally, integration with automation systems and data analytics platforms is becoming increasingly important as laboratories seek to improve efficiency and reduce human error in analytical workflows.
Current Challenges in ICP-MS Technology
Despite significant advancements in ICP-MS technology over recent decades, several persistent challenges continue to limit the analytical capabilities and reliability of these systems. Sample introduction remains a critical bottleneck, with conventional nebulizers typically achieving only 1-3% transport efficiency, resulting in substantial sample waste and reduced sensitivity. The formation of polyatomic interferences, particularly in complex matrices, continues to compromise detection limits and accuracy for certain elements. These interferences, such as 40Ar16O+ affecting 56Fe determination, require sophisticated correction strategies that add complexity to analytical workflows.
Matrix effects represent another significant challenge, where high concentrations of dissolved solids can suppress or enhance analyte signals, leading to inaccurate quantification. Current systems struggle to maintain linearity across the wide concentration ranges often encountered in environmental and biological samples, necessitating multiple dilutions and reruns that reduce laboratory throughput.
Instrument drift remains problematic, with sensitivity changes occurring during analytical runs due to salt deposition on interface cones and ion lenses. This necessitates frequent recalibration and potentially compromises data quality for long sequence runs. The trade-off between sensitivity and precision continues to challenge manufacturers, as higher sensitivity often comes at the cost of signal stability, particularly at ultra-trace levels.
Memory effects persist as a significant issue, especially for elements like boron, mercury, and iodine, which can adsorb to system components and cause cross-contamination between samples. Current washout procedures extend analysis time and consume substantial volumes of reagents, reducing overall laboratory efficiency.
From a detection perspective, abundance sensitivity limitations affect the measurement of low-abundance isotopes adjacent to high-abundance ones, particularly important in isotope ratio measurements. The dynamic range constraints of detector systems (typically 109) still fall short of the concentration ranges encountered in many real-world samples, from ultra-trace to major components.
Emerging applications in single-cell and nanoparticle analysis reveal timing limitations in current detection systems, where the microsecond-scale events require faster data acquisition than many commercial systems can provide. Additionally, the integration of ICP-MS with separation techniques like chromatography and field-flow fractionation introduces synchronization challenges that affect quantitative accuracy in speciation analysis.
Matrix effects represent another significant challenge, where high concentrations of dissolved solids can suppress or enhance analyte signals, leading to inaccurate quantification. Current systems struggle to maintain linearity across the wide concentration ranges often encountered in environmental and biological samples, necessitating multiple dilutions and reruns that reduce laboratory throughput.
Instrument drift remains problematic, with sensitivity changes occurring during analytical runs due to salt deposition on interface cones and ion lenses. This necessitates frequent recalibration and potentially compromises data quality for long sequence runs. The trade-off between sensitivity and precision continues to challenge manufacturers, as higher sensitivity often comes at the cost of signal stability, particularly at ultra-trace levels.
Memory effects persist as a significant issue, especially for elements like boron, mercury, and iodine, which can adsorb to system components and cause cross-contamination between samples. Current washout procedures extend analysis time and consume substantial volumes of reagents, reducing overall laboratory efficiency.
From a detection perspective, abundance sensitivity limitations affect the measurement of low-abundance isotopes adjacent to high-abundance ones, particularly important in isotope ratio measurements. The dynamic range constraints of detector systems (typically 109) still fall short of the concentration ranges encountered in many real-world samples, from ultra-trace to major components.
Emerging applications in single-cell and nanoparticle analysis reveal timing limitations in current detection systems, where the microsecond-scale events require faster data acquisition than many commercial systems can provide. Additionally, the integration of ICP-MS with separation techniques like chromatography and field-flow fractionation introduces synchronization challenges that affect quantitative accuracy in speciation analysis.
Contemporary ICP-MS Analytical Solutions
01 Sensitivity and detection limit improvements in ICP-MS
Various techniques and modifications have been developed to enhance the sensitivity and lower detection limits of ICP-MS systems. These improvements include optimized ion optics, enhanced sample introduction systems, and specialized interface designs that increase ion transmission efficiency. Advanced signal processing algorithms and detector technologies also contribute to better performance by reducing background noise and improving signal-to-noise ratios, allowing for detection of trace elements at increasingly lower concentrations.- Sensitivity and detection limit improvements in ICP-MS: Various techniques and modifications have been developed to enhance the sensitivity and lower detection limits of ICP-MS systems. These improvements include optimized ion optics, enhanced sample introduction systems, and specialized interface designs that increase ion transmission efficiency. Advanced signal processing algorithms and detector technologies also contribute to better sensitivity, allowing for trace element analysis at parts-per-trillion levels or below.
- Interference reduction and resolution enhancement: Methods for reducing spectral interferences and improving mass resolution in ICP-MS systems are critical for accurate analysis. These include collision/reaction cell technologies that remove polyatomic interferences, high-resolution mass analyzers that can separate ions with very small mass differences, and mathematical correction models that compensate for known interferences. These approaches significantly improve the accuracy of measurements, especially for complex sample matrices.
- Sample introduction and preparation innovations: Advancements in sample introduction systems for ICP-MS include specialized nebulizers, spray chambers, and desolvation systems that improve sample transport efficiency and stability. Automated sample preparation techniques, including microfluidic platforms and integrated digestion systems, enhance throughput and reproducibility. These innovations reduce matrix effects, minimize sample consumption, and improve overall analytical performance.
- Calibration and standardization methods: Novel calibration approaches for ICP-MS performance benchmarking include internal standardization techniques, isotope dilution methods, and matrix-matched calibration strategies. Standard reference materials and certified reference materials are used to validate instrument performance and ensure accuracy across different laboratories. These methods establish traceability and comparability of analytical results, which is essential for quality control and regulatory compliance.
- Hyphenated techniques and specialized applications: Integration of ICP-MS with separation techniques such as chromatography, electrophoresis, and laser ablation creates powerful hyphenated systems with enhanced analytical capabilities. These combined approaches enable speciation analysis, nanoparticle characterization, and spatial mapping applications. Performance benchmarks for these specialized systems focus on parameters such as species conversion, recovery rates, and spatial resolution in addition to traditional sensitivity and precision metrics.
02 Interference reduction technologies for ICP-MS
Innovations in ICP-MS focus on reducing spectral and non-spectral interferences that can affect measurement accuracy. These include collision/reaction cell technologies that use specific gases to eliminate polyatomic interferences, high-resolution mass analyzers that can separate interfering species, and mathematical correction models. Advanced sample preparation methods and specialized sample introduction systems also help minimize matrix effects and other interference sources, improving overall analytical performance.Expand Specific Solutions03 Sample introduction and plasma stability enhancements
Improvements in sample introduction systems and plasma stability contribute significantly to ICP-MS performance benchmarks. Innovations include advanced nebulizers, spray chambers, and torch designs that improve sample transport efficiency and plasma robustness. Temperature-controlled spray chambers, desolvation systems, and specialized sample introduction accessories help maintain consistent plasma conditions and reduce matrix effects, leading to more reliable and reproducible analytical results across diverse sample types.Expand Specific Solutions04 Calibration and quantification methods for ICP-MS
Advanced calibration and quantification strategies have been developed to improve the accuracy and reliability of ICP-MS measurements. These include internal standardization techniques, isotope dilution methods, and matrix-matched calibration approaches. Automated calibration systems, quality control protocols, and reference material development help establish performance benchmarks and ensure consistent results across different instruments and laboratories. Software algorithms for drift correction and signal processing further enhance quantitative performance.Expand Specific Solutions05 Automation and high-throughput capabilities in ICP-MS
Modern ICP-MS systems incorporate automation and high-throughput features to improve laboratory efficiency and throughput. These include automated sample changers, integrated sample preparation systems, and rapid washout technologies that reduce analysis time. Software improvements enable unattended operation, automated quality control, and intelligent scheduling of samples. Multi-element analysis capabilities and fast scanning technologies allow for processing large sample batches while maintaining performance benchmarks for sensitivity, precision, and accuracy.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS performance benchmarking market is currently in a growth phase, with an expanding global market driven by increasing demand for trace element analysis across pharmaceutical, environmental, and semiconductor industries. The technology has reached moderate maturity, with established players like Agilent Technologies, Thermo Fisher Scientific, and PerkinElmer (Revvity) dominating with comprehensive solutions. Emerging companies such as Elemental Scientific and Kimia Analytics are driving innovation in sample introduction systems, while academic institutions like ETH Zurich and EPFL contribute to fundamental research. Regional players including Shimadzu and Jiangsu Skyray are expanding market reach in Asia. The competitive landscape features differentiation through automation capabilities, detection limits, and specialized applications for nanoparticle analysis and single-cell measurements.
Revvity Health Sciences, Inc.
Technical Solution: Revvity (formerly part of PerkinElmer) has developed specialized ICP-MS solutions focused on bioanalytical applications. Their systems feature a high-efficiency sample introduction system optimized for limited sample volumes, achieving reliable results with as little as 50 μL of biological samples. The company's proprietary Dynamic Reaction Cell (DRC) technology uses controlled ion-molecule chemistry to eliminate spectral interferences, particularly critical for challenging bioanalytical matrices. Their instruments incorporate a specialized interface designed to handle high salt content and organic matrices common in biological samples without compromising performance. Revvity's ICP-MS systems achieve detection limits in the sub-ppt range for most elements with a linear dynamic range spanning eight orders of magnitude. Their integrated software platform provides specialized workflows for bioanalytical applications, including FDA 21 CFR Part 11 compliance features and automated data processing for large sample batches. The systems include specialized sample introduction accessories for direct coupling with LC, GC, and CE separation techniques for speciation analysis.
Strengths: Specialized optimization for bioanalytical applications; excellent performance with limited sample volumes; comprehensive integration with separation techniques; robust compliance features for regulated environments. Weaknesses: Less versatile for general environmental or industrial applications; higher per-sample analysis costs; more specialized training required for optimal operation.
Agilent Technologies, Inc.
Technical Solution: Agilent's ICP-MS technology features the Ultra High Matrix Introduction (UHMI) system that enables direct measurement of samples containing up to 25% total dissolved solids without manual dilution. Their 8900 Triple Quadrupole ICP-MS provides MS/MS capability with helium and reactive cell gases to eliminate polyatomic and isobaric interferences. The system incorporates a high-efficiency sample introduction system with a temperature-controlled spray chamber and optimized nebulizer design that achieves >2% sample transport efficiency. Agilent's proprietary ion lens technology delivers superior sensitivity while minimizing background noise, achieving detection limits in the sub-ppt range for most elements. Their instruments feature fast time-resolved analysis with 1 ms integration times and include automated performance optimization through their ICP-MS MassHunter software that provides real-time monitoring and adjustment of plasma conditions.
Strengths: Superior interference removal using triple quadrupole technology; excellent matrix tolerance with UHMI; high sensitivity across the mass range; comprehensive software integration. Weaknesses: Higher acquisition costs compared to single quadrupole systems; more complex operation requiring additional training; increased helium and cell gas consumption.
Critical Patents and Breakthroughs in ICP-MS
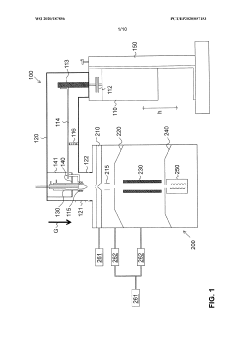
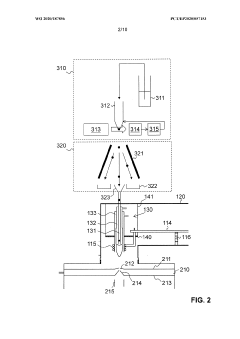
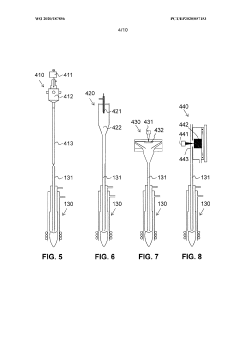
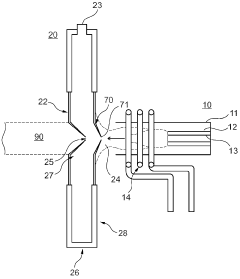
Ion source for inductively coupled plasma mass spectrometry
PatentWO2020187856A1
Innovation
- An ICP source with a vertically oriented plasma torch and injector tube allows sample introduction along a downwards-pointing vertical direction, reducing dependence on carrier gas flow and enabling 100% transport efficiency by utilizing gravity, and includes a metallic cooling plate and electromagnetic coupling element for efficient plasma generation.
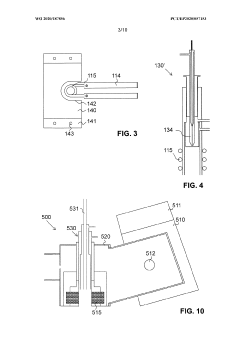
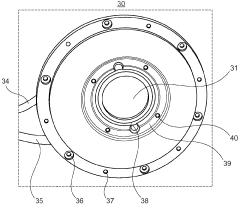
Cooling plate for icp-ms
PatentActiveGB2585327A
Innovation
- A cooling plate made from bronze is used, which provides sufficient thermal conductivity and enhanced chemical resistance, reducing the risk of corrosion and degradation, and eliminating the need for a corrosion-resistant coating.
Standardization and Quality Control Protocols
Standardization and quality control protocols are essential components of ICP-MS performance benchmarking, ensuring reliable and comparable analytical results across different laboratories and instruments. These protocols establish systematic approaches to validate instrument performance, monitor analytical quality, and maintain consistency in measurement processes.
The development of standardized operating procedures (SOPs) represents a cornerstone of quality control in ICP-MS analysis. These procedures encompass detailed guidelines for instrument calibration, sample preparation, analysis execution, and data processing. Leading organizations such as ASTM International, ISO, and national metrology institutes have contributed significantly to establishing these protocols, providing frameworks that laboratories can adopt and customize according to their specific requirements.
Quality control measures for ICP-MS typically include regular performance checks using certified reference materials (CRMs). These materials, with well-characterized elemental compositions, serve as benchmarks for assessing accuracy and precision. Daily quality control routines often involve analyzing blank solutions, calibration verification standards, and internal standard recovery checks to monitor instrument stability and detect potential drift or contamination issues.
Statistical process control techniques play a vital role in maintaining analytical quality. Control charts tracking key performance indicators such as sensitivity, background levels, oxide formation rates, and doubly charged ion ratios provide visual representations of instrument performance over time. These charts enable analysts to identify trends, detect anomalies, and implement corrective actions before measurement quality deteriorates significantly.
Interlaboratory comparison programs further strengthen quality assurance by evaluating analytical performance across multiple facilities. Participation in these programs allows laboratories to benchmark their capabilities against peers and identify areas for improvement. Organizations such as NIST, IAEA, and commercial proficiency testing providers coordinate these comparison exercises, distributing identical samples to participating laboratories and statistically evaluating the reported results.
Method validation protocols constitute another critical aspect of standardization in ICP-MS analysis. These protocols establish procedures for determining method performance characteristics including detection limits, quantification limits, linear dynamic range, selectivity, and measurement uncertainty. Validation studies typically involve analyzing samples with known concentrations under various conditions to assess method robustness and reliability.
Documentation and traceability requirements complete the quality control framework, ensuring that analytical processes are transparent and reproducible. Comprehensive records of instrument maintenance, calibration history, standard preparation, and analytical conditions provide an audit trail that supports data integrity and facilitates troubleshooting when performance issues arise.
The development of standardized operating procedures (SOPs) represents a cornerstone of quality control in ICP-MS analysis. These procedures encompass detailed guidelines for instrument calibration, sample preparation, analysis execution, and data processing. Leading organizations such as ASTM International, ISO, and national metrology institutes have contributed significantly to establishing these protocols, providing frameworks that laboratories can adopt and customize according to their specific requirements.
Quality control measures for ICP-MS typically include regular performance checks using certified reference materials (CRMs). These materials, with well-characterized elemental compositions, serve as benchmarks for assessing accuracy and precision. Daily quality control routines often involve analyzing blank solutions, calibration verification standards, and internal standard recovery checks to monitor instrument stability and detect potential drift or contamination issues.
Statistical process control techniques play a vital role in maintaining analytical quality. Control charts tracking key performance indicators such as sensitivity, background levels, oxide formation rates, and doubly charged ion ratios provide visual representations of instrument performance over time. These charts enable analysts to identify trends, detect anomalies, and implement corrective actions before measurement quality deteriorates significantly.
Interlaboratory comparison programs further strengthen quality assurance by evaluating analytical performance across multiple facilities. Participation in these programs allows laboratories to benchmark their capabilities against peers and identify areas for improvement. Organizations such as NIST, IAEA, and commercial proficiency testing providers coordinate these comparison exercises, distributing identical samples to participating laboratories and statistically evaluating the reported results.
Method validation protocols constitute another critical aspect of standardization in ICP-MS analysis. These protocols establish procedures for determining method performance characteristics including detection limits, quantification limits, linear dynamic range, selectivity, and measurement uncertainty. Validation studies typically involve analyzing samples with known concentrations under various conditions to assess method robustness and reliability.
Documentation and traceability requirements complete the quality control framework, ensuring that analytical processes are transparent and reproducible. Comprehensive records of instrument maintenance, calibration history, standard preparation, and analytical conditions provide an audit trail that supports data integrity and facilitates troubleshooting when performance issues arise.
Environmental and Safety Considerations
The operation of ICP-MS systems presents significant environmental and safety considerations that must be addressed to ensure responsible laboratory practices. Plasma-based analytical techniques inherently involve high temperatures (6,000-10,000K), high voltage components, and potentially hazardous materials that require careful management. Laboratory facilities must implement proper ventilation systems to handle the exhaust gases produced during analysis, particularly argon and trace amounts of sample aerosols that may contain toxic elements.
Waste management represents a critical environmental concern in ICP-MS operations. Sample preparation often involves strong acids and organic solvents that require specialized disposal protocols. The liquid waste generated during analysis may contain heavy metals and other toxic elements that must be collected separately and treated according to local environmental regulations before disposal. Many laboratories have implemented waste reduction strategies, including miniaturization of sample preparation techniques and recycling of certain reagents.
Personnel safety demands comprehensive training and protective measures. Operators must be equipped with appropriate personal protective equipment (PPE) including lab coats, safety glasses, and chemical-resistant gloves when handling samples and reagents. The risk of exposure to high-energy radio frequency fields and UV radiation from the plasma source necessitates proper shielding and safety interlocks on modern instruments. Emergency protocols for handling potential hazards such as gas leaks, electrical failures, or chemical spills must be clearly established and regularly practiced.
Energy consumption represents another environmental consideration for ICP-MS laboratories. These instruments typically require significant electrical power for operation, particularly for plasma generation and vacuum systems. Recent instrument designs have focused on improving energy efficiency through optimized RF generators and more efficient vacuum pumps. Some facilities have implemented standby modes that reduce power consumption during periods of inactivity while maintaining essential system parameters.
Noise pollution, though often overlooked, can be significant in ICP-MS laboratories due to vacuum pumps and cooling systems. Modern instrument designs incorporate sound-dampening features, and proper laboratory design can further mitigate this issue through strategic equipment placement and acoustic treatments. This consideration is particularly important for laboratories located in shared research facilities or academic environments.
Regulatory compliance frameworks vary globally but typically include specific requirements for handling, storing, and disposing of the chemicals and samples used in ICP-MS analysis. Laboratories must maintain comprehensive documentation of their environmental and safety practices, including regular audits and inspections to ensure ongoing compliance with evolving standards and best practices.
Waste management represents a critical environmental concern in ICP-MS operations. Sample preparation often involves strong acids and organic solvents that require specialized disposal protocols. The liquid waste generated during analysis may contain heavy metals and other toxic elements that must be collected separately and treated according to local environmental regulations before disposal. Many laboratories have implemented waste reduction strategies, including miniaturization of sample preparation techniques and recycling of certain reagents.
Personnel safety demands comprehensive training and protective measures. Operators must be equipped with appropriate personal protective equipment (PPE) including lab coats, safety glasses, and chemical-resistant gloves when handling samples and reagents. The risk of exposure to high-energy radio frequency fields and UV radiation from the plasma source necessitates proper shielding and safety interlocks on modern instruments. Emergency protocols for handling potential hazards such as gas leaks, electrical failures, or chemical spills must be clearly established and regularly practiced.
Energy consumption represents another environmental consideration for ICP-MS laboratories. These instruments typically require significant electrical power for operation, particularly for plasma generation and vacuum systems. Recent instrument designs have focused on improving energy efficiency through optimized RF generators and more efficient vacuum pumps. Some facilities have implemented standby modes that reduce power consumption during periods of inactivity while maintaining essential system parameters.
Noise pollution, though often overlooked, can be significant in ICP-MS laboratories due to vacuum pumps and cooling systems. Modern instrument designs incorporate sound-dampening features, and proper laboratory design can further mitigate this issue through strategic equipment placement and acoustic treatments. This consideration is particularly important for laboratories located in shared research facilities or academic environments.
Regulatory compliance frameworks vary globally but typically include specific requirements for handling, storing, and disposing of the chemicals and samples used in ICP-MS analysis. Laboratories must maintain comprehensive documentation of their environmental and safety practices, including regular audits and inspections to ensure ongoing compliance with evolving standards and best practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!