ICP-MS vs GF-AAS for Trace Element Quantification
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Trace Element Analysis Evolution and Objectives
Trace element analysis has evolved significantly over the past century, transforming from rudimentary colorimetric methods to sophisticated instrumental techniques capable of detecting elements at parts per trillion levels. The journey began in the early 20th century with spectrophotometric methods that offered limited sensitivity and specificity. By the 1950s, flame atomic absorption spectrometry (FAAS) emerged as a revolutionary technique, providing improved detection capabilities for many elements.
The 1970s marked a significant milestone with the development of Graphite Furnace Atomic Absorption Spectrometry (GF-AAS), which dramatically enhanced sensitivity by utilizing a graphite tube furnace to atomize samples at high temperatures. This advancement enabled detection limits in the parts per billion range, opening new possibilities for environmental and biological analyses.
The 1980s witnessed the commercialization of Inductively Coupled Plasma Mass Spectrometry (ICP-MS), representing a quantum leap in analytical capabilities. By combining the high-temperature ionization efficiency of an argon plasma with the sensitivity and selectivity of mass spectrometry, ICP-MS pushed detection limits to parts per trillion levels while offering multi-element analysis capabilities.
Recent decades have seen continuous refinements in both technologies. GF-AAS has benefited from improved background correction methods, transverse heating, and platform atomization techniques. Meanwhile, ICP-MS has evolved with collision/reaction cell technology to overcome polyatomic interferences, triple quadrupole systems for enhanced selectivity, and single-particle analysis capabilities for nanomaterial characterization.
The primary objective of modern trace element quantification is to achieve accurate, precise, and reliable measurements at increasingly lower concentrations across diverse sample matrices. This is driven by stringent regulatory requirements in environmental monitoring, food safety, pharmaceutical quality control, and clinical diagnostics. Additionally, there is growing demand for techniques that can handle complex matrices with minimal sample preparation while maintaining high throughput.
Current research aims to address remaining challenges in both techniques, including reducing matrix effects, minimizing interferences, improving sample introduction efficiency, and developing more environmentally friendly methodologies with reduced reagent consumption. The ultimate goal is to develop analytical platforms that combine the strengths of both ICP-MS and GF-AAS while mitigating their respective limitations, potentially through hybrid systems or novel ionization and detection approaches.
The 1970s marked a significant milestone with the development of Graphite Furnace Atomic Absorption Spectrometry (GF-AAS), which dramatically enhanced sensitivity by utilizing a graphite tube furnace to atomize samples at high temperatures. This advancement enabled detection limits in the parts per billion range, opening new possibilities for environmental and biological analyses.
The 1980s witnessed the commercialization of Inductively Coupled Plasma Mass Spectrometry (ICP-MS), representing a quantum leap in analytical capabilities. By combining the high-temperature ionization efficiency of an argon plasma with the sensitivity and selectivity of mass spectrometry, ICP-MS pushed detection limits to parts per trillion levels while offering multi-element analysis capabilities.
Recent decades have seen continuous refinements in both technologies. GF-AAS has benefited from improved background correction methods, transverse heating, and platform atomization techniques. Meanwhile, ICP-MS has evolved with collision/reaction cell technology to overcome polyatomic interferences, triple quadrupole systems for enhanced selectivity, and single-particle analysis capabilities for nanomaterial characterization.
The primary objective of modern trace element quantification is to achieve accurate, precise, and reliable measurements at increasingly lower concentrations across diverse sample matrices. This is driven by stringent regulatory requirements in environmental monitoring, food safety, pharmaceutical quality control, and clinical diagnostics. Additionally, there is growing demand for techniques that can handle complex matrices with minimal sample preparation while maintaining high throughput.
Current research aims to address remaining challenges in both techniques, including reducing matrix effects, minimizing interferences, improving sample introduction efficiency, and developing more environmentally friendly methodologies with reduced reagent consumption. The ultimate goal is to develop analytical platforms that combine the strengths of both ICP-MS and GF-AAS while mitigating their respective limitations, potentially through hybrid systems or novel ionization and detection approaches.
Market Applications and Demand for Trace Element Quantification
Trace element quantification technologies have witnessed significant market growth across multiple sectors due to increasing regulatory requirements and quality control standards. The global market for trace element analysis equipment was valued at approximately 5.3 billion USD in 2022 and is projected to reach 7.8 billion USD by 2028, representing a compound annual growth rate of 6.7%. This growth is primarily driven by expanding applications in environmental monitoring, food safety, pharmaceutical quality control, and clinical diagnostics.
The environmental monitoring sector constitutes the largest market segment, accounting for nearly 32% of the total market share. Stringent regulations regarding water quality, soil contamination, and air pollution have necessitated precise quantification of heavy metals and other trace elements. Government agencies and environmental consulting firms represent the primary customers in this segment, with particular demand for portable and field-deployable solutions.
The food and beverage industry represents the fastest-growing market segment with an annual growth rate of 8.3%. Consumer awareness regarding food safety and nutritional content has prompted manufacturers to implement comprehensive testing protocols. Trace element analysis is critical for detecting contaminants such as lead, mercury, and arsenic, as well as for verifying nutritional claims regarding mineral content.
Healthcare applications, including clinical diagnostics and pharmaceutical quality control, account for approximately 24% of the market. The growing recognition of trace elements' role in human health has increased demand for analytical technologies capable of detecting biomarkers and monitoring therapeutic drug levels. Hospitals, clinical laboratories, and pharmaceutical companies are investing in advanced analytical capabilities to support personalized medicine initiatives.
The mining and metallurgy sector represents another significant market, with particular emphasis on ore characterization and process optimization. Precise quantification of valuable elements in complex matrices directly impacts extraction efficiency and profitability. This sector shows preference for robust, high-throughput systems capable of analyzing large sample volumes.
Geographically, North America and Europe currently dominate the market with combined share of 58%, though Asia-Pacific represents the fastest-growing region with 9.2% annual growth rate. China, India, and South Korea are experiencing rapid market expansion due to strengthening environmental regulations, industrial growth, and increasing research activities.
Market research indicates a clear trend toward multi-element analysis capabilities, improved detection limits, and enhanced sample throughput. End-users increasingly demand integrated solutions that combine sample preparation, analysis, and data management functionalities, driving manufacturers to develop more comprehensive analytical platforms.
The environmental monitoring sector constitutes the largest market segment, accounting for nearly 32% of the total market share. Stringent regulations regarding water quality, soil contamination, and air pollution have necessitated precise quantification of heavy metals and other trace elements. Government agencies and environmental consulting firms represent the primary customers in this segment, with particular demand for portable and field-deployable solutions.
The food and beverage industry represents the fastest-growing market segment with an annual growth rate of 8.3%. Consumer awareness regarding food safety and nutritional content has prompted manufacturers to implement comprehensive testing protocols. Trace element analysis is critical for detecting contaminants such as lead, mercury, and arsenic, as well as for verifying nutritional claims regarding mineral content.
Healthcare applications, including clinical diagnostics and pharmaceutical quality control, account for approximately 24% of the market. The growing recognition of trace elements' role in human health has increased demand for analytical technologies capable of detecting biomarkers and monitoring therapeutic drug levels. Hospitals, clinical laboratories, and pharmaceutical companies are investing in advanced analytical capabilities to support personalized medicine initiatives.
The mining and metallurgy sector represents another significant market, with particular emphasis on ore characterization and process optimization. Precise quantification of valuable elements in complex matrices directly impacts extraction efficiency and profitability. This sector shows preference for robust, high-throughput systems capable of analyzing large sample volumes.
Geographically, North America and Europe currently dominate the market with combined share of 58%, though Asia-Pacific represents the fastest-growing region with 9.2% annual growth rate. China, India, and South Korea are experiencing rapid market expansion due to strengthening environmental regulations, industrial growth, and increasing research activities.
Market research indicates a clear trend toward multi-element analysis capabilities, improved detection limits, and enhanced sample throughput. End-users increasingly demand integrated solutions that combine sample preparation, analysis, and data management functionalities, driving manufacturers to develop more comprehensive analytical platforms.
ICP-MS and GF-AAS Technologies: Current Status and Limitations
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Graphite Furnace Atomic Absorption Spectroscopy (GF-AAS) represent two of the most advanced analytical techniques for trace element quantification. Currently, ICP-MS dominates the market with approximately 65% share in trace element analysis applications, while GF-AAS holds about 25%, with the remainder distributed among other techniques.
ICP-MS technology has reached a high level of maturity, with detection limits typically in the parts-per-trillion (ppt) range for most elements. Modern systems feature multi-quadrupole configurations that effectively minimize polyatomic interferences, a historical limitation of the technology. The latest ICP-MS instruments incorporate collision/reaction cell technology, which has dramatically improved performance for traditionally problematic elements such as iron, potassium, and calcium in complex matrices.
Despite these advances, ICP-MS still faces challenges including high initial investment costs ($150,000-$500,000), significant operational expenses, and complex sample preparation requirements. The technology also demands specialized laboratory infrastructure including high-purity argon supply, specialized ventilation, and temperature-controlled environments.
GF-AAS, while older technology, maintains relevance due to its lower cost ($50,000-$120,000), simpler operation, and reduced infrastructure requirements. Modern GF-AAS systems feature enhanced background correction capabilities, improved graphite tube designs, and automated sample introduction systems. These improvements have extended instrument lifetimes and improved analytical precision.
However, GF-AAS is limited by its sequential analysis nature, analyzing only one element at a time, which significantly reduces sample throughput compared to ICP-MS. It also generally exhibits higher detection limits (parts-per-billion range) and narrower linear dynamic ranges, requiring more frequent sample dilutions or concentrations.
Geographically, ICP-MS technology development is concentrated in the United States, Japan, and Germany, with companies like Agilent, Thermo Fisher, and PerkinElmer leading innovation. GF-AAS development remains strong in Germany and Japan, with Analytik Jena and Shimadzu maintaining significant market presence.
Recent technological trends include the miniaturization of both technologies, with benchtop and even portable systems emerging. ICP-MS has seen significant advances in time-of-flight mass analyzers, improving multi-element capabilities. For GF-AAS, developments in high-resolution continuum source technology have expanded multi-element capabilities, though still not matching ICP-MS throughput.
The primary technical constraint limiting wider adoption of both technologies remains the expertise required for method development, validation, and data interpretation. This has created a growing market for automated software solutions that incorporate machine learning algorithms to optimize analytical parameters and interpret complex spectral data.
ICP-MS technology has reached a high level of maturity, with detection limits typically in the parts-per-trillion (ppt) range for most elements. Modern systems feature multi-quadrupole configurations that effectively minimize polyatomic interferences, a historical limitation of the technology. The latest ICP-MS instruments incorporate collision/reaction cell technology, which has dramatically improved performance for traditionally problematic elements such as iron, potassium, and calcium in complex matrices.
Despite these advances, ICP-MS still faces challenges including high initial investment costs ($150,000-$500,000), significant operational expenses, and complex sample preparation requirements. The technology also demands specialized laboratory infrastructure including high-purity argon supply, specialized ventilation, and temperature-controlled environments.
GF-AAS, while older technology, maintains relevance due to its lower cost ($50,000-$120,000), simpler operation, and reduced infrastructure requirements. Modern GF-AAS systems feature enhanced background correction capabilities, improved graphite tube designs, and automated sample introduction systems. These improvements have extended instrument lifetimes and improved analytical precision.
However, GF-AAS is limited by its sequential analysis nature, analyzing only one element at a time, which significantly reduces sample throughput compared to ICP-MS. It also generally exhibits higher detection limits (parts-per-billion range) and narrower linear dynamic ranges, requiring more frequent sample dilutions or concentrations.
Geographically, ICP-MS technology development is concentrated in the United States, Japan, and Germany, with companies like Agilent, Thermo Fisher, and PerkinElmer leading innovation. GF-AAS development remains strong in Germany and Japan, with Analytik Jena and Shimadzu maintaining significant market presence.
Recent technological trends include the miniaturization of both technologies, with benchtop and even portable systems emerging. ICP-MS has seen significant advances in time-of-flight mass analyzers, improving multi-element capabilities. For GF-AAS, developments in high-resolution continuum source technology have expanded multi-element capabilities, though still not matching ICP-MS throughput.
The primary technical constraint limiting wider adoption of both technologies remains the expertise required for method development, validation, and data interpretation. This has created a growing market for automated software solutions that incorporate machine learning algorithms to optimize analytical parameters and interpret complex spectral data.
Comparative Analysis of ICP-MS and GF-AAS Methodologies
01 ICP-MS techniques for trace element analysis
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is widely used for trace element quantification due to its high sensitivity and multi-element detection capabilities. This technique ionizes samples using an argon plasma and separates ions based on their mass-to-charge ratio, allowing for detection of elements at parts per trillion levels. ICP-MS is particularly valuable for analyzing complex matrices and can be coupled with various sample introduction systems to enhance performance for specific applications.- ICP-MS techniques for trace element analysis: Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is widely used for trace element quantification due to its high sensitivity and multi-element detection capabilities. This technique ionizes samples using an inductively coupled plasma and then separates and quantifies ions based on their mass-to-charge ratio. ICP-MS allows for detection of elements at parts per trillion levels, making it ideal for ultra-trace analysis in various matrices including environmental, biological, and industrial samples.
- GF-AAS methods for elemental determination: Graphite Furnace Atomic Absorption Spectroscopy (GF-AAS) provides highly sensitive detection of trace elements in complex matrices. This technique uses a graphite tube furnace to atomize samples and measure the absorption of specific wavelengths of light by ground-state atoms. GF-AAS offers advantages including small sample volume requirements, minimal sample preparation, and high sensitivity for certain elements, particularly metals like lead, cadmium, and arsenic at parts per billion levels.
- Sample preparation techniques for trace analysis: Effective sample preparation is crucial for accurate trace element quantification using ICP-MS and GF-AAS. Methods include acid digestion, microwave-assisted extraction, ultrasonic extraction, and solid-phase extraction to isolate target analytes from complex matrices. These preparation techniques aim to minimize matrix interferences, reduce contamination risks, and ensure complete recovery of target elements. Proper sample preparation significantly improves detection limits and analytical precision for both ICP-MS and GF-AAS methodologies.
- Calibration and quality control for trace element analysis: Accurate calibration and robust quality control procedures are essential for reliable trace element quantification. Techniques include internal standardization, isotope dilution, standard addition methods, and matrix-matched calibration. Quality control measures involve analyzing certified reference materials, method blanks, and duplicate samples. These approaches help compensate for matrix effects, instrument drift, and potential contamination, ensuring the validity and reproducibility of analytical results from both ICP-MS and GF-AAS techniques.
- Specialized applications and method development: Advanced applications of ICP-MS and GF-AAS include speciation analysis, isotope ratio measurements, and ultra-trace detection in challenging matrices. Method development focuses on optimizing parameters such as plasma conditions, atomization temperatures, and interference reduction strategies. Specialized techniques like laser ablation ICP-MS enable direct solid sample analysis, while hyphenated methods combining chromatographic separation with these detection techniques allow for enhanced selectivity and sensitivity in complex samples.
02 GF-AAS methods for elemental quantification
Graphite Furnace Atomic Absorption Spectroscopy (GF-AAS) provides highly sensitive detection of trace elements in various sample types. This technique uses a graphite tube furnace to atomize samples through controlled heating cycles, allowing for analysis of small sample volumes with minimal preparation. GF-AAS is particularly effective for determining elements like lead, cadmium, and arsenic at ultra-trace levels, offering detection limits in the parts per billion range with high selectivity.Expand Specific Solutions03 Sample preparation techniques for trace analysis
Effective sample preparation is crucial for accurate trace element quantification using ICP-MS and GF-AAS. Methods include acid digestion, microwave-assisted extraction, and chelation techniques to isolate target elements from complex matrices. Proper preparation minimizes matrix interferences and contamination while maximizing analyte recovery. Specialized protocols have been developed for different sample types including biological tissues, environmental samples, and industrial materials to ensure reliable quantification at ultra-trace levels.Expand Specific Solutions04 Calibration and quality control for analytical accuracy
Achieving accurate trace element quantification requires robust calibration strategies and quality control measures. Techniques include internal standardization, isotope dilution, standard addition methods, and matrix-matched calibration to compensate for matrix effects and instrument drift. Certified reference materials are essential for method validation and ongoing quality assurance. Statistical approaches for data processing help minimize measurement uncertainty and ensure reliable results across different analytical sessions.Expand Specific Solutions05 Hyphenated and complementary analytical techniques
Combining ICP-MS or GF-AAS with separation techniques creates powerful hyphenated methods for complex sample analysis. Approaches include coupling with chromatography (HPLC, GC, IC) or field-flow fractionation for speciation analysis and characterization of element distribution. Complementary techniques like X-ray fluorescence and neutron activation analysis provide additional information when used alongside ICP-MS and GF-AAS. These integrated analytical strategies enable comprehensive trace element profiling with enhanced selectivity and information content.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Analytical Instrumentation
The trace element quantification market is in a mature growth phase, with ICP-MS (Inductively Coupled Plasma Mass Spectrometry) gradually gaining dominance over GF-AAS (Graphite Furnace Atomic Absorption Spectroscopy) due to superior sensitivity, multi-element capabilities, and higher throughput. The global market is estimated at $2.5-3 billion, growing at 5-7% annually, driven by environmental monitoring, pharmaceutical analysis, and food safety applications. Leading players include established instrumentation companies like Hitachi, SPECTRO Analytical, and Elemental Scientific, alongside specialized firms such as Bioyong Technology and XOS. Research institutions like China Institute of Metrology and Swiss Federal Institute of Technology continue advancing analytical methodologies, while companies like Kimia Analytics are developing next-generation ICP-MS technologies with enhanced sensitivity and matrix tolerance.
Hitachi Ltd.
Technical Solution: Hitachi has developed advanced ICP-MS systems featuring their proprietary collision/reaction cell technology that effectively removes polyatomic interferences. Their latest ICP-MS instruments incorporate a unique octopole collision system that operates at higher cell gas pressures, enabling superior interference removal while maintaining sensitivity. Hitachi's systems also feature an improved ion optics design with off-axis lens technology that enhances ion transmission efficiency while reducing background noise. For trace element quantification, they've implemented a dual-mode detector system that provides a linear dynamic range spanning nine orders of magnitude, allowing simultaneous measurement of major and trace elements without dilution. Their software platform includes advanced calibration algorithms specifically designed for complex matrices encountered in environmental and biological samples.
Strengths: Superior interference management through advanced collision cell technology; exceptional detection limits (sub-ppt level for most elements); robust performance with complex matrices. Weaknesses: Higher acquisition and operational costs compared to GF-AAS systems; requires specialized training and infrastructure; more complex maintenance requirements.
China Institute of Metrology
Technical Solution: The China Institute of Metrology has developed comprehensive reference methodologies for trace element quantification that leverage the complementary strengths of ICP-MS and GF-AAS. Their approach centers on creating certified reference materials specifically designed for method validation and quality control in both techniques. For ICP-MS applications, they've established isotope dilution mass spectrometry (IDMS) protocols that achieve exceptional accuracy for priority elements in environmental and food safety monitoring. Their methodology incorporates advanced interference correction algorithms based on mathematical modeling of polyatomic species formation in various matrices. For GF-AAS, the Institute has developed matrix-matched calibration strategies with optimized modifier combinations that enhance sensitivity while minimizing background interference. Their integrated quality assurance framework includes uncertainty estimation models that account for all significant error sources in both analytical techniques, enabling reliable comparison of results between methods.
Strengths: Exceptional measurement accuracy through metrologically sound approaches; comprehensive uncertainty quantification; established traceability to SI units. Weaknesses: Methods prioritize accuracy over throughput; higher complexity in implementation; requires significant analytical expertise and specialized reference materials.
Key Technical Innovations in Trace Element Detection
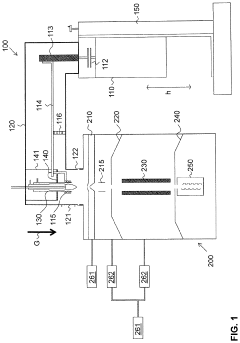
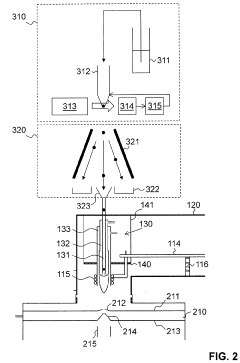
Ion source for inductively coupled plasma mass spectrometry
PatentActiveUS20220181135A1
Innovation
- An ICP source with a vertically oriented plasma torch and injector tube allows sample introduction along a downwards-pointing vertical direction, reducing reliance on carrier gas flow and enabling 100% transport efficiency by utilizing gravity, accommodating various sample sizes and types, and integrating with mass spectrometers for efficient ion extraction.
Environmental and Regulatory Considerations for Trace Analysis
Environmental regulations worldwide are increasingly focusing on trace element monitoring in various matrices, driving the need for more sensitive and accurate analytical methods. Both ICP-MS and GF-AAS technologies must comply with stringent regulatory frameworks established by organizations such as the US Environmental Protection Agency (EPA), European Environmental Agency (EEA), and the World Health Organization (WHO). These frameworks set specific detection limits for toxic elements like lead, mercury, arsenic, and cadmium in drinking water, soil, and food products.
ICP-MS generally offers advantages in meeting these regulatory requirements due to its lower detection limits, which often fall well below the maximum contaminant levels established by regulatory bodies. For instance, the EPA's limit for lead in drinking water is 15 ppb, while ICP-MS can routinely detect lead at sub-ppb levels. GF-AAS, while also capable of meeting many regulatory standards, may require additional sample preparation or concentration steps for ultra-trace analysis.
Environmental considerations also extend to the operational aspects of these technologies. ICP-MS systems consume significant amounts of argon gas and require more extensive laboratory infrastructure, resulting in a larger carbon footprint. GF-AAS, conversely, has lower gas consumption requirements but may use graphite tubes that require regular replacement, generating waste materials.
Sample preparation procedures for both techniques may involve environmentally concerning chemicals, including strong acids and organic solvents. However, modern green analytical chemistry approaches are addressing these concerns through the development of environmentally friendly extraction methods and reduced reagent consumption protocols.
The waste management aspects differ significantly between the two techniques. ICP-MS generates more liquid waste due to its continuous sample introduction system, while GF-AAS produces less waste volume but may contain higher concentrations of analytes. Both require proper disposal protocols in accordance with local environmental regulations.
Regulatory compliance documentation is another critical consideration. ICP-MS offers advantages in multi-element analysis capabilities, allowing laboratories to efficiently generate comprehensive compliance reports. GF-AAS, while excellent for targeted analysis, may require multiple analytical runs to cover the same regulatory scope, potentially increasing documentation complexity.
As environmental regulations continue to evolve toward lower detection limits and broader element coverage, analytical laboratories must carefully evaluate both technologies' capabilities against current and anticipated regulatory requirements, ensuring sustainable analytical practices while maintaining compliance with environmental protection standards.
ICP-MS generally offers advantages in meeting these regulatory requirements due to its lower detection limits, which often fall well below the maximum contaminant levels established by regulatory bodies. For instance, the EPA's limit for lead in drinking water is 15 ppb, while ICP-MS can routinely detect lead at sub-ppb levels. GF-AAS, while also capable of meeting many regulatory standards, may require additional sample preparation or concentration steps for ultra-trace analysis.
Environmental considerations also extend to the operational aspects of these technologies. ICP-MS systems consume significant amounts of argon gas and require more extensive laboratory infrastructure, resulting in a larger carbon footprint. GF-AAS, conversely, has lower gas consumption requirements but may use graphite tubes that require regular replacement, generating waste materials.
Sample preparation procedures for both techniques may involve environmentally concerning chemicals, including strong acids and organic solvents. However, modern green analytical chemistry approaches are addressing these concerns through the development of environmentally friendly extraction methods and reduced reagent consumption protocols.
The waste management aspects differ significantly between the two techniques. ICP-MS generates more liquid waste due to its continuous sample introduction system, while GF-AAS produces less waste volume but may contain higher concentrations of analytes. Both require proper disposal protocols in accordance with local environmental regulations.
Regulatory compliance documentation is another critical consideration. ICP-MS offers advantages in multi-element analysis capabilities, allowing laboratories to efficiently generate comprehensive compliance reports. GF-AAS, while excellent for targeted analysis, may require multiple analytical runs to cover the same regulatory scope, potentially increasing documentation complexity.
As environmental regulations continue to evolve toward lower detection limits and broader element coverage, analytical laboratories must carefully evaluate both technologies' capabilities against current and anticipated regulatory requirements, ensuring sustainable analytical practices while maintaining compliance with environmental protection standards.
Sample Preparation Techniques and Their Impact on Results
Sample preparation represents a critical pre-analytical phase that significantly influences the accuracy, precision, and reliability of trace element quantification using both ICP-MS and GF-AAS techniques. The preparation methodology must be carefully selected based on sample matrix, target analytes, and the analytical technique employed.
For ICP-MS analysis, sample preparation typically involves acid digestion procedures using combinations of nitric acid, hydrochloric acid, hydrogen peroxide, or hydrofluoric acid in closed-vessel microwave systems. This approach ensures complete matrix decomposition while minimizing contamination risks and analyte loss. However, the aggressive nature of these reagents may introduce spectral interferences that must be addressed during subsequent analysis.
GF-AAS sample preparation often employs less aggressive methods, including dilution, acid extraction, or matrix modification. The technique's tolerance for certain matrix components allows for simpler preparation protocols in many cases. Matrix modifiers such as palladium nitrate or magnesium nitrate are frequently added to stabilize volatile elements during the thermal program, enhancing measurement reliability.
The choice between aqueous calibration and matrix-matched calibration standards represents another critical consideration. ICP-MS typically requires matrix-matched standards to compensate for matrix effects, while GF-AAS may utilize standard addition methods to overcome matrix interferences when analyzing complex samples.
Sample homogeneity presents unique challenges for both techniques. Solid samples require pulverization, grinding, and sieving to ensure representative sampling. Biological tissues often necessitate cryogenic homogenization followed by lyophilization to preserve analyte integrity while achieving sample uniformity.
Contamination control during sample preparation directly impacts detection limits for both techniques. Ultra-clean laboratory environments, high-purity reagents, and acid-cleaned labware are essential, particularly for ICP-MS which offers superior detection capabilities but greater susceptibility to contamination effects.
Recovery studies using certified reference materials reveal that sample preparation efficiency varies significantly between techniques. ICP-MS generally achieves 95-99% recovery rates for most elements when using optimized digestion protocols, while GF-AAS typically demonstrates 90-95% recovery, with greater variability for volatile elements like mercury and arsenic.
Recent innovations in sample preparation include automated microextraction techniques, which reduce reagent consumption and minimize analyst exposure to hazardous chemicals while improving throughput for both analytical platforms.
For ICP-MS analysis, sample preparation typically involves acid digestion procedures using combinations of nitric acid, hydrochloric acid, hydrogen peroxide, or hydrofluoric acid in closed-vessel microwave systems. This approach ensures complete matrix decomposition while minimizing contamination risks and analyte loss. However, the aggressive nature of these reagents may introduce spectral interferences that must be addressed during subsequent analysis.
GF-AAS sample preparation often employs less aggressive methods, including dilution, acid extraction, or matrix modification. The technique's tolerance for certain matrix components allows for simpler preparation protocols in many cases. Matrix modifiers such as palladium nitrate or magnesium nitrate are frequently added to stabilize volatile elements during the thermal program, enhancing measurement reliability.
The choice between aqueous calibration and matrix-matched calibration standards represents another critical consideration. ICP-MS typically requires matrix-matched standards to compensate for matrix effects, while GF-AAS may utilize standard addition methods to overcome matrix interferences when analyzing complex samples.
Sample homogeneity presents unique challenges for both techniques. Solid samples require pulverization, grinding, and sieving to ensure representative sampling. Biological tissues often necessitate cryogenic homogenization followed by lyophilization to preserve analyte integrity while achieving sample uniformity.
Contamination control during sample preparation directly impacts detection limits for both techniques. Ultra-clean laboratory environments, high-purity reagents, and acid-cleaned labware are essential, particularly for ICP-MS which offers superior detection capabilities but greater susceptibility to contamination effects.
Recovery studies using certified reference materials reveal that sample preparation efficiency varies significantly between techniques. ICP-MS generally achieves 95-99% recovery rates for most elements when using optimized digestion protocols, while GF-AAS typically demonstrates 90-95% recovery, with greater variability for volatile elements like mercury and arsenic.
Recent innovations in sample preparation include automated microextraction techniques, which reduce reagent consumption and minimize analyst exposure to hazardous chemicals while improving throughput for both analytical platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!