Implementing High Throughput Screening in ICP-MS
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS HTS Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, establishing itself as a cornerstone analytical technique for elemental analysis. This technology combines the high-temperature ionization capabilities of inductively coupled plasma with the precise detection mechanisms of mass spectrometry, enabling researchers to detect and quantify elements at concentrations as low as parts per trillion. The evolution of ICP-MS has been marked by continuous improvements in sensitivity, precision, and analytical throughput, making it an indispensable tool across various scientific disciplines.
Traditional ICP-MS workflows, however, have been characterized by relatively low sample throughput, with typical processing rates of 1-2 minutes per sample. This limitation has become increasingly problematic as modern research and industrial applications demand higher efficiency and greater sample volumes. High Throughput Screening (HTS) methodologies, which have transformed drug discovery and other fields, represent a promising approach to overcome these constraints when applied to ICP-MS.
The integration of HTS principles with ICP-MS technology aims to dramatically increase analytical capacity while maintaining the exceptional sensitivity and accuracy that define ICP-MS. This convergence addresses growing demands in pharmaceutical development, environmental monitoring, food safety, and materials science, where large-scale elemental analysis has become essential for comprehensive assessment and regulatory compliance.
The primary objective of implementing HTS in ICP-MS is to develop systems capable of processing hundreds to thousands of samples daily without compromising analytical performance. This involves optimizing sample introduction systems, enhancing plasma stability during rapid sample changes, and developing sophisticated automation protocols that minimize human intervention while maximizing instrument utilization.
Secondary objectives include reducing per-sample analysis costs, minimizing reagent consumption, and decreasing the environmental footprint of analytical processes. These goals align with broader industry trends toward more sustainable and economically viable laboratory practices.
From a technological perspective, successful implementation requires innovations in sample handling robotics, microfluidic systems for sample introduction, and advanced data processing algorithms capable of handling the substantial increase in generated data. The development of specialized software interfaces that streamline workflow management and facilitate rapid data interpretation represents another critical component of this technological evolution.
The anticipated impact of high-throughput ICP-MS extends beyond mere efficiency gains, potentially enabling entirely new applications in metabolomics, proteomics, and high-volume clinical diagnostics. As research continues to push the boundaries of elemental analysis, HTS-enabled ICP-MS stands poised to transform how scientists approach large-scale elemental screening and quantification challenges.
Traditional ICP-MS workflows, however, have been characterized by relatively low sample throughput, with typical processing rates of 1-2 minutes per sample. This limitation has become increasingly problematic as modern research and industrial applications demand higher efficiency and greater sample volumes. High Throughput Screening (HTS) methodologies, which have transformed drug discovery and other fields, represent a promising approach to overcome these constraints when applied to ICP-MS.
The integration of HTS principles with ICP-MS technology aims to dramatically increase analytical capacity while maintaining the exceptional sensitivity and accuracy that define ICP-MS. This convergence addresses growing demands in pharmaceutical development, environmental monitoring, food safety, and materials science, where large-scale elemental analysis has become essential for comprehensive assessment and regulatory compliance.
The primary objective of implementing HTS in ICP-MS is to develop systems capable of processing hundreds to thousands of samples daily without compromising analytical performance. This involves optimizing sample introduction systems, enhancing plasma stability during rapid sample changes, and developing sophisticated automation protocols that minimize human intervention while maximizing instrument utilization.
Secondary objectives include reducing per-sample analysis costs, minimizing reagent consumption, and decreasing the environmental footprint of analytical processes. These goals align with broader industry trends toward more sustainable and economically viable laboratory practices.
From a technological perspective, successful implementation requires innovations in sample handling robotics, microfluidic systems for sample introduction, and advanced data processing algorithms capable of handling the substantial increase in generated data. The development of specialized software interfaces that streamline workflow management and facilitate rapid data interpretation represents another critical component of this technological evolution.
The anticipated impact of high-throughput ICP-MS extends beyond mere efficiency gains, potentially enabling entirely new applications in metabolomics, proteomics, and high-volume clinical diagnostics. As research continues to push the boundaries of elemental analysis, HTS-enabled ICP-MS stands poised to transform how scientists approach large-scale elemental screening and quantification challenges.
Market Demand Analysis for High-Throughput ICP-MS
The global market for high-throughput ICP-MS technology has experienced significant growth in recent years, driven by increasing demand for rapid, accurate, and efficient elemental analysis across multiple industries. The pharmaceutical sector represents one of the largest market segments, where high-throughput screening capabilities are essential for drug discovery and development processes, enabling researchers to analyze large compound libraries for potential therapeutic candidates.
Environmental monitoring agencies and regulatory bodies constitute another substantial market segment, requiring advanced analytical tools to process numerous samples for contaminant detection and compliance verification. The ability to screen multiple samples quickly while maintaining analytical precision has become a critical requirement for these organizations facing growing workloads and stricter regulatory standards.
The food and beverage industry has emerged as a rapidly expanding market for high-throughput ICP-MS technology, with increasing concerns about food safety and authenticity driving demand for comprehensive elemental analysis. Manufacturers require efficient screening methods to ensure product quality and regulatory compliance across global supply chains.
Academic and research institutions represent a stable market segment, with ongoing research projects in fields such as geochemistry, materials science, and biomedical research creating sustained demand for advanced analytical capabilities. The ability to process large sample sets efficiently is particularly valuable in these settings where research productivity is paramount.
Market analysis indicates that the global ICP-MS market was valued at approximately $1.2 billion in 2022, with high-throughput systems accounting for roughly 35% of this value. Industry forecasts project a compound annual growth rate of 7.8% through 2028, with high-throughput applications expected to grow at an even faster rate of 9.3% during this period.
Regional analysis reveals that North America currently holds the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding industrial bases in China and India, increasing environmental regulations, and growing investment in research infrastructure.
Customer surveys indicate that key purchasing factors for high-throughput ICP-MS systems include sample throughput capacity, detection limits, operational costs, and integration capabilities with existing laboratory workflows. There is particularly strong demand for systems that can reduce per-sample analysis costs while maintaining or improving analytical performance.
Environmental monitoring agencies and regulatory bodies constitute another substantial market segment, requiring advanced analytical tools to process numerous samples for contaminant detection and compliance verification. The ability to screen multiple samples quickly while maintaining analytical precision has become a critical requirement for these organizations facing growing workloads and stricter regulatory standards.
The food and beverage industry has emerged as a rapidly expanding market for high-throughput ICP-MS technology, with increasing concerns about food safety and authenticity driving demand for comprehensive elemental analysis. Manufacturers require efficient screening methods to ensure product quality and regulatory compliance across global supply chains.
Academic and research institutions represent a stable market segment, with ongoing research projects in fields such as geochemistry, materials science, and biomedical research creating sustained demand for advanced analytical capabilities. The ability to process large sample sets efficiently is particularly valuable in these settings where research productivity is paramount.
Market analysis indicates that the global ICP-MS market was valued at approximately $1.2 billion in 2022, with high-throughput systems accounting for roughly 35% of this value. Industry forecasts project a compound annual growth rate of 7.8% through 2028, with high-throughput applications expected to grow at an even faster rate of 9.3% during this period.
Regional analysis reveals that North America currently holds the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding industrial bases in China and India, increasing environmental regulations, and growing investment in research infrastructure.
Customer surveys indicate that key purchasing factors for high-throughput ICP-MS systems include sample throughput capacity, detection limits, operational costs, and integration capabilities with existing laboratory workflows. There is particularly strong demand for systems that can reduce per-sample analysis costs while maintaining or improving analytical performance.
Current Challenges in ICP-MS Throughput Enhancement
Despite significant advancements in ICP-MS technology, several critical challenges continue to impede high-throughput screening implementation. Sample introduction systems represent a primary bottleneck, with conventional nebulizers and spray chambers requiring 2-3 minutes per sample, significantly limiting throughput capacity. The inherent trade-off between speed and analytical quality remains problematic, as faster sample introduction often leads to increased signal instability, memory effects, and cross-contamination between samples.
Instrument stabilization presents another substantial challenge, with many ICP-MS systems requiring extended warm-up periods and frequent recalibration during high-volume sample processing. This necessity for periodic calibration checks and quality control samples can consume up to 20% of total analytical time in high-throughput environments, creating significant inefficiencies in workflow.
Data processing capabilities struggle to keep pace with accelerated sample introduction rates. Current software platforms often experience processing delays when handling the massive data volumes generated during high-throughput screening, particularly when performing complex interference corrections or isotope ratio calculations in real-time. This computational bottleneck frequently necessitates offline data processing, negating some throughput advantages.
Sample preparation automation remains inadequately integrated with ICP-MS systems. While automated sample preparation platforms exist, their seamless integration with ICP-MS instrumentation is often suboptimal, creating workflow discontinuities that compromise overall throughput efficiency. The interface between sample preparation and analysis frequently requires manual intervention, introducing delays and potential contamination risks.
Matrix effects and spectral interferences become increasingly problematic at higher throughput rates. The reduced dwell times and faster scanning speeds necessary for high-throughput applications limit the effectiveness of collision/reaction cell technologies in resolving complex interferences. This challenge is particularly acute when analyzing diverse sample matrices in rapid succession without adequate washout periods.
Regulatory compliance and quality assurance protocols impose additional constraints on throughput optimization. Validation requirements for pharmaceutical and clinical applications often mandate extensive system suitability testing and quality control measures that are difficult to streamline without compromising regulatory compliance.
Consumable limitations also impact sustained high-throughput operations, with rapid sample introduction accelerating wear on components like nebulizers, torches, and cones. The frequent maintenance requirements and associated downtime significantly affect the practical throughput achievable in routine laboratory settings.
Instrument stabilization presents another substantial challenge, with many ICP-MS systems requiring extended warm-up periods and frequent recalibration during high-volume sample processing. This necessity for periodic calibration checks and quality control samples can consume up to 20% of total analytical time in high-throughput environments, creating significant inefficiencies in workflow.
Data processing capabilities struggle to keep pace with accelerated sample introduction rates. Current software platforms often experience processing delays when handling the massive data volumes generated during high-throughput screening, particularly when performing complex interference corrections or isotope ratio calculations in real-time. This computational bottleneck frequently necessitates offline data processing, negating some throughput advantages.
Sample preparation automation remains inadequately integrated with ICP-MS systems. While automated sample preparation platforms exist, their seamless integration with ICP-MS instrumentation is often suboptimal, creating workflow discontinuities that compromise overall throughput efficiency. The interface between sample preparation and analysis frequently requires manual intervention, introducing delays and potential contamination risks.
Matrix effects and spectral interferences become increasingly problematic at higher throughput rates. The reduced dwell times and faster scanning speeds necessary for high-throughput applications limit the effectiveness of collision/reaction cell technologies in resolving complex interferences. This challenge is particularly acute when analyzing diverse sample matrices in rapid succession without adequate washout periods.
Regulatory compliance and quality assurance protocols impose additional constraints on throughput optimization. Validation requirements for pharmaceutical and clinical applications often mandate extensive system suitability testing and quality control measures that are difficult to streamline without compromising regulatory compliance.
Consumable limitations also impact sustained high-throughput operations, with rapid sample introduction accelerating wear on components like nebulizers, torches, and cones. The frequent maintenance requirements and associated downtime significantly affect the practical throughput achievable in routine laboratory settings.
Current HTS Implementation Strategies for ICP-MS
01 Automated sample introduction systems for ICP-MS
Automated sample introduction systems can significantly increase the throughput of ICP-MS analysis. These systems include autosampling devices, automated dilution systems, and integrated sample preparation platforms that reduce manual handling. By automating the sample introduction process, laboratories can analyze more samples in less time while maintaining analytical precision and reducing operator intervention.- Automated sample introduction systems: Automated sample introduction systems are crucial for enhancing ICP-MS throughput. These systems include autosampling devices, automated dilution systems, and robotic sample handling that minimize manual intervention. By automating the sample preparation and introduction process, laboratories can analyze more samples in less time while maintaining analytical precision and reducing operator error. These systems often incorporate intelligent scheduling algorithms to optimize sample queuing and processing.
- Multi-element analysis optimization: Techniques for optimizing multi-element analysis significantly improve ICP-MS throughput. These include specialized scanning methods, simultaneous multi-isotope detection, and advanced collision/reaction cell technologies that allow for the concurrent analysis of multiple elements. By reducing the time needed for individual element analysis while maintaining accuracy, these approaches enable faster sample processing and higher sample throughput. Optimization of dwell times, integration periods, and mass scanning parameters further enhances analytical efficiency.
- High-throughput sample preparation methods: Innovative sample preparation methods specifically designed for high-throughput applications reduce the overall analysis time in ICP-MS workflows. These include microwave-assisted digestion, ultrasonic extraction, and batch processing techniques that can prepare multiple samples simultaneously. Miniaturization of sample preparation steps and development of rapid digestion protocols minimize the sample preparation bottleneck, which is often the rate-limiting step in ICP-MS analysis. These methods are particularly valuable for laboratories processing large sample volumes.
- Data processing and analysis automation: Advanced data processing and analysis automation systems enhance ICP-MS throughput by streamlining the post-acquisition workflow. These systems incorporate machine learning algorithms for automated peak identification, calibration, and quality control assessment. Real-time data processing capabilities allow for immediate results interpretation and decision-making. Integration with laboratory information management systems (LIMS) further improves efficiency by automating data transfer, report generation, and archiving processes, reducing the time from sample analysis to result reporting.
- Integrated high-throughput ICP-MS systems: Fully integrated high-throughput ICP-MS systems combine hardware and software innovations to maximize analytical efficiency. These systems feature optimized plasma generation, ion optics, and detector technologies specifically designed for rapid analysis. They incorporate parallel processing capabilities, reduced washout times between samples, and intelligent system management to minimize downtime. Some systems include inline dilution capabilities, automated quality control protocols, and self-optimization features that adapt to different sample matrices, enabling continuous high-throughput operation across diverse analytical applications.
02 High-throughput multi-element analysis techniques
Advanced techniques for multi-element analysis enable higher throughput in ICP-MS applications. These include simultaneous detection of multiple elements, optimized scanning protocols, and specialized software algorithms for rapid data acquisition and processing. These approaches allow for comprehensive elemental analysis in a single run, significantly reducing the time required per sample while maintaining sensitivity and accuracy.Expand Specific Solutions03 Microfluidic and flow injection systems
Microfluidic platforms and flow injection systems enhance ICP-MS throughput by reducing sample volume requirements and enabling rapid, sequential sample introduction. These systems incorporate miniaturized components, precise flow control, and optimized sample transport mechanisms to minimize analysis time and washout periods between samples. The integration of these technologies with ICP-MS allows for high-speed screening applications and improved sample throughput.Expand Specific Solutions04 Advanced data processing and instrument control software
Specialized software solutions for ICP-MS improve throughput through enhanced data processing capabilities, automated quality control procedures, and intelligent instrument control. These software platforms enable real-time data analysis, automated calibration, and adaptive measurement protocols that optimize acquisition parameters based on sample characteristics. By streamlining data handling and instrument operation, these systems reduce overall analysis time and increase laboratory productivity.Expand Specific Solutions05 Sample preparation automation and high-throughput digestion methods
Automated sample preparation techniques and high-throughput digestion methods significantly improve overall ICP-MS workflow efficiency. These include parallel digestion systems, microwave-assisted extraction, automated filtration, and integrated sample tracking. By reducing the time required for sample preparation while ensuring consistent sample quality, these approaches address a major bottleneck in ICP-MS analysis and enable higher sample throughput from start to finish.Expand Specific Solutions
Key Industry Players in High-Throughput ICP-MS
The High Throughput Screening (HTS) in ICP-MS market is currently in a growth phase, with increasing adoption across pharmaceutical, environmental, and materials science sectors. The global market size is estimated to exceed $1.5 billion, driven by demand for rapid multi-element analysis. Leading players include Thermo Fisher Scientific and Agilent Technologies, who dominate with comprehensive instrument portfolios and advanced automation solutions. Mid-tier competitors like PerkinElmer (Revvity) and Shimadzu are gaining market share through specialized applications. Technology maturity varies significantly, with established players offering highly automated systems while emerging companies like Kimia Analytics and AmberGen focus on innovative sample introduction systems and specialized screening methodologies. Academic institutions including MIT and Swiss Federal Institute of Technology contribute significantly to advancing fundamental technologies, particularly in high-throughput data processing algorithms and novel sample preparation techniques.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced ICP-MS systems featuring their patented Triple Quadrupole (TQ-ICP-MS) technology for high throughput screening. Their iCAP TQ ICP-MS platform incorporates intelligent sample introduction systems with automated dilution capabilities and collision/reaction cell technology to eliminate spectral interferences. The system utilizes their Qtegra Intelligent Scientific Data Solution software that enables automated quality control protocols, customizable templates, and comprehensive data processing algorithms. Their high-throughput solution includes integrated autosampling systems capable of handling hundreds of samples per batch with minimal carryover (<0.1%) between analyses[1]. Additionally, they've implemented rapid sample-to-sample analysis times of <90 seconds while maintaining detection limits in the sub-ppt range for most elements[3]. Their systems feature kinetic energy discrimination (KED) mode for polyatomic interference removal without significant sensitivity loss.
Strengths: Superior interference management through triple quadrupole technology allowing for improved accuracy in complex matrices; comprehensive software integration enabling automated workflows and data processing; high sample throughput with minimal cross-contamination. Weaknesses: Higher initial investment cost compared to competitors; more complex system requiring specialized training; potentially higher operating costs due to additional gas requirements for collision/reaction cell technology.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered high throughput screening in ICP-MS through their ICP-QQQ (triple quadrupole) systems, particularly the 8900 and 8800 series. Their approach integrates the Ultra High Matrix Introduction (UHMI) technology, allowing direct analysis of samples with up to 25% total dissolved solids without significant dilution[2]. The company's high throughput solution incorporates their Integrated Sample Introduction System (ISIS 3) that enables discrete sampling with uptake/stabilization times as low as 3 seconds and rinse times of 3-7 seconds between samples[4]. This dramatically reduces the analysis cycle to approximately 30-60 seconds per sample. Agilent's systems feature their proprietary Octopole Reaction System (ORS) with helium collision mode and MS/MS capabilities for interference removal. Their automated software platform includes intelligent Quality Control protocols that can automatically identify and flag problematic samples, adjust dilution factors, and rerun samples when necessary, minimizing operator intervention[5]. The systems also incorporate automated tuning procedures that optimize performance for specific applications.
Strengths: Exceptional matrix tolerance through UHMI technology allowing direct analysis of complex samples; superior speed with ISIS 3 technology enabling very short sample-to-sample times; comprehensive interference management through MS/MS capabilities. Weaknesses: Higher gas consumption compared to some competitors; more complex system requiring specialized training for optimal operation; premium pricing structure that may be prohibitive for smaller laboratories.
Core Innovations in Sample Introduction Systems
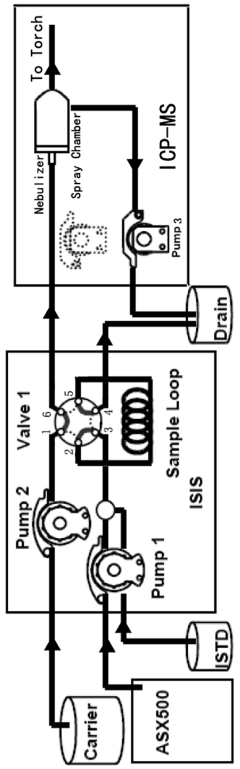
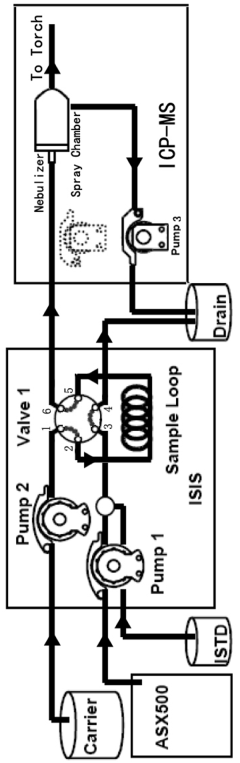
ICP-ms method for multi-element determination by direct sample injection of high salt water
PatentActiveNL2033445A
Innovation
- A Discrete Flow Injection Sampling (DFIS) method using a six-way valve and peristaltic pumps to directly inject seawater samples into an ICP-MS system, optimizing parameters like sample loop diameter, carrier acidity, and pumping speed to minimize salt deposition and ensure stable sample delivery, allowing simultaneous analysis of multiple elements.
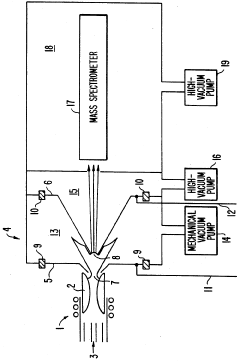
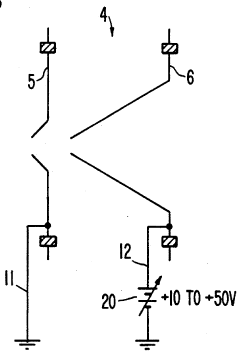
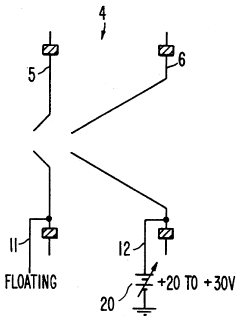
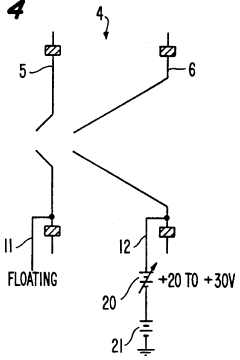
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Regulatory Compliance for High-Throughput Analysis
High-throughput screening in ICP-MS must adhere to stringent regulatory frameworks that vary across different regions and industries. In the United States, the FDA's Good Laboratory Practice (GLP) regulations and the EPA's Method 6020 provide comprehensive guidelines for ICP-MS analysis, particularly emphasizing data integrity, instrument validation, and quality control procedures. Similarly, the European Union enforces compliance through directives like 2004/9/EC and ISO/IEC 17025 standards, which establish requirements for testing laboratories.
For pharmaceutical applications, high-throughput ICP-MS analysis must comply with ICH Q3D guidelines for elemental impurities, requiring robust validation protocols and documentation systems. These regulations mandate specific detection limits, calibration procedures, and quality assurance measures that must be integrated into high-throughput workflows without compromising analytical performance.
Data management presents significant compliance challenges in high-throughput environments. 21 CFR Part 11 regulations in the US and Annex 11 in the EU establish requirements for electronic records and signatures, necessitating secure data storage systems with audit trails, access controls, and data integrity features. High-throughput ICP-MS systems must incorporate these capabilities while maintaining operational efficiency.
Sample chain-of-custody documentation becomes increasingly complex with higher sample volumes. Regulatory bodies require comprehensive tracking from collection through analysis and reporting, creating logistical challenges that must be addressed through automated sample handling systems with integrated barcode tracking and electronic documentation.
Method validation for high-throughput protocols requires additional considerations beyond traditional ICP-MS methods. Regulatory agencies expect demonstration that accelerated procedures maintain equivalent accuracy, precision, and detection limits. This typically involves comparative studies between standard and high-throughput methods, with statistical analysis proving equivalence.
Emerging regulations around nanomaterials and novel pharmaceutical compounds are introducing new compliance requirements for ICP-MS analysis. Organizations implementing high-throughput screening must establish regulatory intelligence systems to monitor evolving standards and adapt their analytical protocols accordingly.
Ultimately, successful regulatory compliance for high-throughput ICP-MS requires a balanced approach that integrates automated compliance tools, staff training programs, and quality management systems designed specifically for high-volume analytical environments.
For pharmaceutical applications, high-throughput ICP-MS analysis must comply with ICH Q3D guidelines for elemental impurities, requiring robust validation protocols and documentation systems. These regulations mandate specific detection limits, calibration procedures, and quality assurance measures that must be integrated into high-throughput workflows without compromising analytical performance.
Data management presents significant compliance challenges in high-throughput environments. 21 CFR Part 11 regulations in the US and Annex 11 in the EU establish requirements for electronic records and signatures, necessitating secure data storage systems with audit trails, access controls, and data integrity features. High-throughput ICP-MS systems must incorporate these capabilities while maintaining operational efficiency.
Sample chain-of-custody documentation becomes increasingly complex with higher sample volumes. Regulatory bodies require comprehensive tracking from collection through analysis and reporting, creating logistical challenges that must be addressed through automated sample handling systems with integrated barcode tracking and electronic documentation.
Method validation for high-throughput protocols requires additional considerations beyond traditional ICP-MS methods. Regulatory agencies expect demonstration that accelerated procedures maintain equivalent accuracy, precision, and detection limits. This typically involves comparative studies between standard and high-throughput methods, with statistical analysis proving equivalence.
Emerging regulations around nanomaterials and novel pharmaceutical compounds are introducing new compliance requirements for ICP-MS analysis. Organizations implementing high-throughput screening must establish regulatory intelligence systems to monitor evolving standards and adapt their analytical protocols accordingly.
Ultimately, successful regulatory compliance for high-throughput ICP-MS requires a balanced approach that integrates automated compliance tools, staff training programs, and quality management systems designed specifically for high-volume analytical environments.
Cost-Benefit Analysis of HTS ICP-MS Implementation
The implementation of High Throughput Screening (HTS) in Inductively Coupled Plasma Mass Spectrometry (ICP-MS) represents a significant investment that requires thorough financial evaluation. Initial capital expenditure for HTS ICP-MS systems typically ranges from $300,000 to $750,000, depending on the level of automation, sample handling capabilities, and analytical performance specifications. This includes costs for the instrument itself, automated sample introduction systems, and specialized software for high-throughput data processing.
Operational expenses must also be factored into the analysis, with annual maintenance contracts averaging $20,000-$40,000, consumables (argon gas, standards, reagents) costing approximately $30,000-$50,000 annually, and specialized staff training requirements adding $5,000-$10,000 per analyst initially.
However, these substantial investments can yield significant returns through increased analytical throughput. Traditional ICP-MS methods typically process 20-30 samples per hour, while HTS implementations can achieve 100-200 samples per hour, representing a 4-7x improvement in productivity. This translates to reduced cost per sample, with estimates showing a decrease from $15-25 per sample to $5-8 per sample in high-volume operations.
Labor efficiency gains are equally impressive, with automated sample preparation and introduction systems reducing hands-on time by 60-75%. A single analyst can effectively manage significantly larger sample loads, potentially reducing staffing requirements or allowing reallocation of skilled personnel to higher-value activities.
Quality improvements represent another benefit dimension, as automated systems demonstrate enhanced reproducibility with typical relative standard deviations (RSDs) decreasing from 3-5% to 1-2%. This improved precision can reduce the need for repeat analyses, further enhancing throughput and cost efficiency.
The return on investment timeline varies by implementation scale and utilization rates. Organizations processing >10,000 samples annually typically achieve ROI within 2-3 years, while smaller operations may require 4-5 years to fully recoup costs. Sensitivity analysis indicates that sample volume is the most critical factor affecting ROI calculations, followed by labor costs and instrument utilization rates.
Risk factors that may impact the cost-benefit equation include potential downtime during implementation and integration with existing laboratory information management systems (LIMS), which can temporarily reduce productivity. Additionally, rapid technological advancement in the field may lead to faster-than-expected obsolescence, potentially shortening the effective lifespan of the investment.
Operational expenses must also be factored into the analysis, with annual maintenance contracts averaging $20,000-$40,000, consumables (argon gas, standards, reagents) costing approximately $30,000-$50,000 annually, and specialized staff training requirements adding $5,000-$10,000 per analyst initially.
However, these substantial investments can yield significant returns through increased analytical throughput. Traditional ICP-MS methods typically process 20-30 samples per hour, while HTS implementations can achieve 100-200 samples per hour, representing a 4-7x improvement in productivity. This translates to reduced cost per sample, with estimates showing a decrease from $15-25 per sample to $5-8 per sample in high-volume operations.
Labor efficiency gains are equally impressive, with automated sample preparation and introduction systems reducing hands-on time by 60-75%. A single analyst can effectively manage significantly larger sample loads, potentially reducing staffing requirements or allowing reallocation of skilled personnel to higher-value activities.
Quality improvements represent another benefit dimension, as automated systems demonstrate enhanced reproducibility with typical relative standard deviations (RSDs) decreasing from 3-5% to 1-2%. This improved precision can reduce the need for repeat analyses, further enhancing throughput and cost efficiency.
The return on investment timeline varies by implementation scale and utilization rates. Organizations processing >10,000 samples annually typically achieve ROI within 2-3 years, while smaller operations may require 4-5 years to fully recoup costs. Sensitivity analysis indicates that sample volume is the most critical factor affecting ROI calculations, followed by labor costs and instrument utilization rates.
Risk factors that may impact the cost-benefit equation include potential downtime during implementation and integration with existing laboratory information management systems (LIMS), which can temporarily reduce productivity. Additionally, rapid technological advancement in the field may lead to faster-than-expected obsolescence, potentially shortening the effective lifespan of the investment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!