Improving Electrochemical Performance of Lithium Iron Phosphate Batteries
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Evolution
Lithium iron phosphate (LiFePO4 or LFP) batteries have undergone significant evolution since their introduction in the late 1990s. Initially developed as a safer alternative to lithium cobalt oxide (LCO) batteries, LFP technology has seen continuous improvements in performance, cost-effectiveness, and manufacturing processes.
The early 2000s marked the beginning of commercial LFP battery production, with companies like A123 Systems and BYD leading the way. These first-generation LFP batteries offered improved safety and longer cycle life compared to other lithium-ion chemistries but suffered from lower energy density and power output.
As research progressed, scientists focused on enhancing the intrinsic properties of LFP materials. One major breakthrough came with the development of nano-sized LFP particles, which significantly improved the material's conductivity and rate capability. This innovation allowed for faster charging and discharging, making LFP batteries more suitable for high-power applications.
The mid-2010s saw advancements in electrode design and manufacturing techniques. Carbon coating of LFP particles became a standard practice, further enhancing conductivity and overall battery performance. Additionally, improvements in electrolyte formulations and separator technologies contributed to increased energy density and better low-temperature performance.
In recent years, the focus has shifted towards optimizing cell and pack designs. The introduction of cell-to-pack (CTP) technology has been a game-changer, allowing for higher energy density at the pack level by reducing the number of structural components. This innovation has made LFP batteries more competitive in the electric vehicle market, where energy density is crucial.
The latest developments in LFP technology include the exploration of doping strategies to enhance conductivity and capacity. Researchers are investigating the incorporation of various elements such as vanadium, manganese, and nickel into the LFP crystal structure to improve its electrochemical properties.
Looking ahead, the evolution of LFP batteries is expected to continue with a focus on further increasing energy density, improving fast-charging capabilities, and extending cycle life. Emerging technologies like solid-state electrolytes and advanced manufacturing processes, such as 3D printing of electrodes, may play a significant role in the next generation of LFP batteries.
As environmental concerns and raw material availability become increasingly important, the sustainability advantages of LFP batteries are likely to drive further research and development efforts. The absence of cobalt and nickel in LFP chemistry positions it favorably in terms of long-term sustainability and cost stability.
The early 2000s marked the beginning of commercial LFP battery production, with companies like A123 Systems and BYD leading the way. These first-generation LFP batteries offered improved safety and longer cycle life compared to other lithium-ion chemistries but suffered from lower energy density and power output.
As research progressed, scientists focused on enhancing the intrinsic properties of LFP materials. One major breakthrough came with the development of nano-sized LFP particles, which significantly improved the material's conductivity and rate capability. This innovation allowed for faster charging and discharging, making LFP batteries more suitable for high-power applications.
The mid-2010s saw advancements in electrode design and manufacturing techniques. Carbon coating of LFP particles became a standard practice, further enhancing conductivity and overall battery performance. Additionally, improvements in electrolyte formulations and separator technologies contributed to increased energy density and better low-temperature performance.
In recent years, the focus has shifted towards optimizing cell and pack designs. The introduction of cell-to-pack (CTP) technology has been a game-changer, allowing for higher energy density at the pack level by reducing the number of structural components. This innovation has made LFP batteries more competitive in the electric vehicle market, where energy density is crucial.
The latest developments in LFP technology include the exploration of doping strategies to enhance conductivity and capacity. Researchers are investigating the incorporation of various elements such as vanadium, manganese, and nickel into the LFP crystal structure to improve its electrochemical properties.
Looking ahead, the evolution of LFP batteries is expected to continue with a focus on further increasing energy density, improving fast-charging capabilities, and extending cycle life. Emerging technologies like solid-state electrolytes and advanced manufacturing processes, such as 3D printing of electrodes, may play a significant role in the next generation of LFP batteries.
As environmental concerns and raw material availability become increasingly important, the sustainability advantages of LFP batteries are likely to drive further research and development efforts. The absence of cobalt and nickel in LFP chemistry positions it favorably in terms of long-term sustainability and cost stability.
Market Demand Analysis
The market demand for improved electrochemical performance in lithium iron phosphate (LiFePO4) batteries has been steadily increasing, driven by the growing adoption of electric vehicles (EVs) and renewable energy storage systems. As governments worldwide push for cleaner energy solutions and stricter emissions regulations, the demand for high-performance, safe, and cost-effective energy storage solutions has surged.
In the EV sector, LiFePO4 batteries are gaining traction due to their superior safety profile, longer cycle life, and lower cost compared to other lithium-ion chemistries. Major automakers are increasingly incorporating LiFePO4 batteries into their electric vehicle lineups, particularly in the mid-range and economy segments. This trend is expected to continue, with the global EV market projected to grow at a compound annual growth rate (CAGR) of over 20% in the coming years.
The renewable energy sector is another key driver for improved LiFePO4 battery performance. As solar and wind power installations increase, the need for efficient and reliable energy storage systems grows proportionally. LiFePO4 batteries are well-suited for grid-scale energy storage applications due to their stability, long lifespan, and ability to withstand frequent charge-discharge cycles.
Consumer electronics represent another significant market for enhanced LiFePO4 batteries. With the increasing power demands of portable devices and the push for longer-lasting, faster-charging batteries, there is a growing interest in LiFePO4 technology as an alternative to traditional lithium-ion batteries in certain applications.
The industrial sector, including material handling equipment and backup power systems, is also contributing to the demand for improved LiFePO4 batteries. These applications require batteries with high power density, rapid charging capabilities, and long operational lifetimes – characteristics that align well with the potential of enhanced LiFePO4 technology.
Market analysts predict that the global LiFePO4 battery market will experience substantial growth in the coming years. This growth is expected to be fueled by advancements in battery technology, increasing adoption in various applications, and the ongoing shift towards sustainable energy solutions.
However, to fully capitalize on this market potential, several key performance improvements are necessary. These include enhancing energy density to compete more effectively with other lithium-ion chemistries, improving low-temperature performance, and further reducing production costs. Addressing these challenges could significantly expand the market opportunities for LiFePO4 batteries across various industries and applications.
In the EV sector, LiFePO4 batteries are gaining traction due to their superior safety profile, longer cycle life, and lower cost compared to other lithium-ion chemistries. Major automakers are increasingly incorporating LiFePO4 batteries into their electric vehicle lineups, particularly in the mid-range and economy segments. This trend is expected to continue, with the global EV market projected to grow at a compound annual growth rate (CAGR) of over 20% in the coming years.
The renewable energy sector is another key driver for improved LiFePO4 battery performance. As solar and wind power installations increase, the need for efficient and reliable energy storage systems grows proportionally. LiFePO4 batteries are well-suited for grid-scale energy storage applications due to their stability, long lifespan, and ability to withstand frequent charge-discharge cycles.
Consumer electronics represent another significant market for enhanced LiFePO4 batteries. With the increasing power demands of portable devices and the push for longer-lasting, faster-charging batteries, there is a growing interest in LiFePO4 technology as an alternative to traditional lithium-ion batteries in certain applications.
The industrial sector, including material handling equipment and backup power systems, is also contributing to the demand for improved LiFePO4 batteries. These applications require batteries with high power density, rapid charging capabilities, and long operational lifetimes – characteristics that align well with the potential of enhanced LiFePO4 technology.
Market analysts predict that the global LiFePO4 battery market will experience substantial growth in the coming years. This growth is expected to be fueled by advancements in battery technology, increasing adoption in various applications, and the ongoing shift towards sustainable energy solutions.
However, to fully capitalize on this market potential, several key performance improvements are necessary. These include enhancing energy density to compete more effectively with other lithium-ion chemistries, improving low-temperature performance, and further reducing production costs. Addressing these challenges could significantly expand the market opportunities for LiFePO4 batteries across various industries and applications.
Technical Challenges
Lithium iron phosphate (LiFePO4) batteries face several technical challenges that hinder their widespread adoption and performance optimization. One of the primary issues is the inherently low electronic conductivity of LiFePO4, which limits the battery's rate capability and overall performance. This characteristic restricts the material's ability to efficiently transport electrons during charge and discharge cycles, resulting in reduced power output and slower charging times.
Another significant challenge is the relatively low energy density of LiFePO4 batteries compared to other lithium-ion chemistries. This limitation stems from the lower operating voltage and specific capacity of LiFePO4 cathodes, which directly impacts the battery's overall energy storage capacity. As a result, LiFePO4 batteries may struggle to meet the demanding energy requirements of certain applications, particularly in the automotive and portable electronics sectors.
The synthesis and manufacturing processes of LiFePO4 materials also present technical hurdles. Achieving uniform particle size distribution and optimal crystal structure is crucial for maximizing battery performance. However, controlling these parameters during large-scale production can be challenging, leading to inconsistencies in battery quality and performance across batches.
Furthermore, the stability of the LiFePO4 cathode-electrolyte interface remains a concern. Although LiFePO4 batteries are known for their excellent thermal stability, the formation and growth of solid electrolyte interphase (SEI) layers can still occur over time, potentially leading to capacity fade and increased internal resistance. Developing effective strategies to mitigate SEI formation and maintain long-term cycling stability is an ongoing challenge for researchers and manufacturers.
The low-temperature performance of LiFePO4 batteries is another area that requires improvement. These batteries tend to exhibit reduced capacity and power output at low temperatures, limiting their effectiveness in cold climates or applications with wide temperature fluctuations. Enhancing the low-temperature electrochemical kinetics of LiFePO4 materials is crucial for expanding their usability across diverse environmental conditions.
Lastly, balancing the trade-offs between different performance parameters poses a significant challenge. Efforts to improve one aspect of battery performance, such as energy density, may come at the expense of other desirable characteristics like cycle life or safety. Finding the optimal balance that meets the specific requirements of various applications while maintaining the inherent advantages of LiFePO4 chemistry is a complex task that requires continuous research and innovation.
Addressing these technical challenges is essential for advancing the electrochemical performance of LiFePO4 batteries and expanding their potential applications. Overcoming these hurdles will not only enhance the competitiveness of LiFePO4 technology but also contribute to the broader development of sustainable energy storage solutions.
Another significant challenge is the relatively low energy density of LiFePO4 batteries compared to other lithium-ion chemistries. This limitation stems from the lower operating voltage and specific capacity of LiFePO4 cathodes, which directly impacts the battery's overall energy storage capacity. As a result, LiFePO4 batteries may struggle to meet the demanding energy requirements of certain applications, particularly in the automotive and portable electronics sectors.
The synthesis and manufacturing processes of LiFePO4 materials also present technical hurdles. Achieving uniform particle size distribution and optimal crystal structure is crucial for maximizing battery performance. However, controlling these parameters during large-scale production can be challenging, leading to inconsistencies in battery quality and performance across batches.
Furthermore, the stability of the LiFePO4 cathode-electrolyte interface remains a concern. Although LiFePO4 batteries are known for their excellent thermal stability, the formation and growth of solid electrolyte interphase (SEI) layers can still occur over time, potentially leading to capacity fade and increased internal resistance. Developing effective strategies to mitigate SEI formation and maintain long-term cycling stability is an ongoing challenge for researchers and manufacturers.
The low-temperature performance of LiFePO4 batteries is another area that requires improvement. These batteries tend to exhibit reduced capacity and power output at low temperatures, limiting their effectiveness in cold climates or applications with wide temperature fluctuations. Enhancing the low-temperature electrochemical kinetics of LiFePO4 materials is crucial for expanding their usability across diverse environmental conditions.
Lastly, balancing the trade-offs between different performance parameters poses a significant challenge. Efforts to improve one aspect of battery performance, such as energy density, may come at the expense of other desirable characteristics like cycle life or safety. Finding the optimal balance that meets the specific requirements of various applications while maintaining the inherent advantages of LiFePO4 chemistry is a complex task that requires continuous research and innovation.
Addressing these technical challenges is essential for advancing the electrochemical performance of LiFePO4 batteries and expanding their potential applications. Overcoming these hurdles will not only enhance the competitiveness of LiFePO4 technology but also contribute to the broader development of sustainable energy storage solutions.
Current LFP Solutions
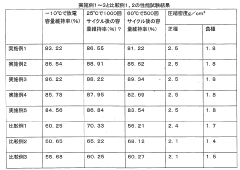
01 Electrode material composition optimization
Improving the electrochemical performance of lithium iron phosphate batteries by optimizing the composition of electrode materials. This includes doping with various elements, adjusting particle sizes, and creating composite materials to enhance conductivity, capacity, and cycling stability.- Electrode material composition optimization: Improving the electrochemical performance of lithium iron phosphate batteries by optimizing the composition of electrode materials. This includes doping with various elements, adjusting particle sizes, and modifying surface properties to enhance conductivity, capacity, and cycling stability.
- Electrolyte formulation enhancement: Developing advanced electrolyte formulations to improve the electrochemical performance of lithium iron phosphate batteries. This involves using novel additives, adjusting electrolyte concentrations, and exploring alternative solvents to enhance ionic conductivity and reduce interfacial resistance.
- Nanostructured electrode design: Utilizing nanostructured electrode designs to enhance the electrochemical performance of lithium iron phosphate batteries. This includes developing nanoparticles, nanofibers, and hierarchical structures to increase active material utilization and improve charge transfer kinetics.
- Carbon coating and conductive additives: Improving the electrochemical performance of lithium iron phosphate batteries by incorporating carbon coatings and conductive additives. This approach enhances electronic conductivity, reduces polarization, and improves rate capability of the electrode materials.
- Advanced manufacturing techniques: Employing advanced manufacturing techniques to enhance the electrochemical performance of lithium iron phosphate batteries. This includes using novel synthesis methods, optimizing production parameters, and developing innovative cell assembly processes to improve overall battery performance and consistency.
02 Electrolyte formulation enhancement
Developing advanced electrolyte formulations to improve the electrochemical performance of lithium iron phosphate batteries. This involves using novel additives, optimizing electrolyte concentrations, and exploring alternative electrolyte systems to enhance ionic conductivity and interface stability.Expand Specific Solutions03 Nanostructured electrode design
Utilizing nanostructured electrode designs to enhance the electrochemical performance of lithium iron phosphate batteries. This includes developing nanoparticles, nanofibers, and 3D nanostructures to increase surface area, improve lithium-ion diffusion, and enhance overall battery performance.Expand Specific Solutions04 Surface modification techniques
Applying various surface modification techniques to improve the electrochemical performance of lithium iron phosphate batteries. This involves coating electrode materials with conductive layers, creating core-shell structures, and surface functionalization to enhance conductivity and stability.Expand Specific Solutions05 Advanced manufacturing processes
Developing and implementing advanced manufacturing processes to enhance the electrochemical performance of lithium iron phosphate batteries. This includes exploring novel synthesis methods, optimizing production parameters, and utilizing innovative assembly techniques to improve battery quality and consistency.Expand Specific Solutions
Key Industry Players
The market for improving electrochemical performance of lithium iron phosphate (LFP) batteries is in a growth phase, driven by increasing demand for electric vehicles and energy storage systems. The global LFP battery market is expected to expand significantly in the coming years, with major players like BYD, CATL, and LG Energy Solution leading the charge. Technological advancements are rapidly evolving, with companies focusing on enhancing energy density, charging speed, and cycle life. Established manufacturers such as Panasonic and emerging players like Guangdong Brunp Recycling Technology are investing heavily in R&D to overcome current limitations of LFP chemistry. The competitive landscape is intensifying as automotive giants and battery specialists race to develop next-generation LFP technologies.
BYD Co., Ltd.
Technical Solution: BYD has developed its proprietary Blade Battery technology, which utilizes LFP chemistry in a novel cell design. The Blade Battery features long and thin cells that are arranged in a parallel configuration, maximizing space utilization and energy density. This design reportedly increases volumetric energy density by up to 50% compared to conventional LFP batteries[6]. BYD has also implemented advanced thermal management systems to enhance safety and performance. Their research includes optimizing particle size and morphology of LFP materials to improve capacity and rate capability, as well as exploring new electrolyte additives to enhance cycling stability and low-temperature performance[7].
Strengths: High energy density, improved safety, efficient space utilization. Weaknesses: Potential challenges in mass production scalability, limited flexibility in pack design.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has focused on developing advanced LFP cathode materials with improved conductivity and structural stability. They have implemented a proprietary carbon-coating process that enhances the electronic conductivity of LFP particles, resulting in improved rate capability and low-temperature performance[4]. Additionally, LG has explored the use of conductive additives and optimized electrolyte formulations to enhance the overall electrochemical performance of their LFP batteries. Their research also includes the development of silicon-graphite composite anodes to pair with LFP cathodes, aiming to increase energy density while maintaining the cost and safety benefits of LFP chemistry[5].
Strengths: Enhanced conductivity, improved low-temperature performance, potential for higher energy density. Weaknesses: Complexity in manufacturing process, potential increase in production costs.
Core LFP Innovations
Lithium iron phosphate battery and its preparation method
PatentInactiveJP2022527237A
Innovation
- Incorporation of carbon nanotubes, graphene, fullerene, nanowires, and nanotitanium into the electrode materials, along with a specific electrolyte composition containing catechol diacetate, to enhance the wettability and improve the battery's performance.
Material Advancements
Material advancements have played a crucial role in improving the electrochemical performance of lithium iron phosphate (LiFePO4) batteries. Over the past decade, researchers have focused on enhancing the intrinsic properties of LiFePO4 cathode materials to overcome their limitations in electronic conductivity and lithium-ion diffusion.
One significant advancement has been the development of carbon coating techniques. By applying a thin layer of conductive carbon to LiFePO4 particles, researchers have successfully increased the electronic conductivity of the cathode material. This carbon coating not only improves the overall conductivity but also helps to prevent particle agglomeration during cycling, leading to better capacity retention and rate capability.
Nanostructuring of LiFePO4 particles has emerged as another promising approach. By reducing the particle size to nanoscale dimensions, researchers have significantly shortened the diffusion path for lithium ions, resulting in improved charge-discharge kinetics. Various morphologies, such as nanoplates, nanorods, and hollow spheres, have been explored to optimize the surface area and enhance the electrochemical performance.
Doping strategies have also been extensively investigated to modify the electronic structure of LiFePO4. The incorporation of small amounts of foreign elements, such as Mg, Zn, or Nb, into the crystal lattice has shown to enhance the intrinsic conductivity and structural stability of the material. These dopants can create defects or alter the band structure, facilitating faster electron and ion transport.
Surface modification techniques have been developed to improve the interface between the cathode material and the electrolyte. By applying coatings of metal oxides or phosphates, researchers have successfully mitigated unwanted side reactions and enhanced the cycling stability of LiFePO4 batteries. These surface modifications also contribute to better rate performance by reducing the charge transfer resistance at the electrode-electrolyte interface.
Recent advancements in composite materials have shown great promise. Combining LiFePO4 with conductive additives, such as graphene or carbon nanotubes, has resulted in synergistic effects that enhance both electronic and ionic conductivity. These composite structures provide a conductive network throughout the electrode, facilitating faster charge transport and improving the overall battery performance.
The development of hierarchical structures has gained attention as a means to optimize the porosity and surface area of LiFePO4 cathodes. By creating multi-level architectures, researchers have achieved improved electrolyte penetration and enhanced lithium-ion accessibility, leading to better rate capability and cycling stability.
One significant advancement has been the development of carbon coating techniques. By applying a thin layer of conductive carbon to LiFePO4 particles, researchers have successfully increased the electronic conductivity of the cathode material. This carbon coating not only improves the overall conductivity but also helps to prevent particle agglomeration during cycling, leading to better capacity retention and rate capability.
Nanostructuring of LiFePO4 particles has emerged as another promising approach. By reducing the particle size to nanoscale dimensions, researchers have significantly shortened the diffusion path for lithium ions, resulting in improved charge-discharge kinetics. Various morphologies, such as nanoplates, nanorods, and hollow spheres, have been explored to optimize the surface area and enhance the electrochemical performance.
Doping strategies have also been extensively investigated to modify the electronic structure of LiFePO4. The incorporation of small amounts of foreign elements, such as Mg, Zn, or Nb, into the crystal lattice has shown to enhance the intrinsic conductivity and structural stability of the material. These dopants can create defects or alter the band structure, facilitating faster electron and ion transport.
Surface modification techniques have been developed to improve the interface between the cathode material and the electrolyte. By applying coatings of metal oxides or phosphates, researchers have successfully mitigated unwanted side reactions and enhanced the cycling stability of LiFePO4 batteries. These surface modifications also contribute to better rate performance by reducing the charge transfer resistance at the electrode-electrolyte interface.
Recent advancements in composite materials have shown great promise. Combining LiFePO4 with conductive additives, such as graphene or carbon nanotubes, has resulted in synergistic effects that enhance both electronic and ionic conductivity. These composite structures provide a conductive network throughout the electrode, facilitating faster charge transport and improving the overall battery performance.
The development of hierarchical structures has gained attention as a means to optimize the porosity and surface area of LiFePO4 cathodes. By creating multi-level architectures, researchers have achieved improved electrolyte penetration and enhanced lithium-ion accessibility, leading to better rate capability and cycling stability.
Environmental Impact
The environmental impact of improving the electrochemical performance of lithium iron phosphate (LiFePO4) batteries is a critical consideration in the development and adoption of this technology. As these batteries become more efficient and widespread, their lifecycle environmental footprint becomes increasingly significant.
One of the primary environmental benefits of enhancing LiFePO4 battery performance is the potential reduction in raw material consumption. Improved energy density and longer cycle life mean fewer batteries are needed for the same energy storage capacity, reducing the demand for lithium, iron, and phosphorus. This can lead to decreased mining activities and associated environmental disruptions.
The manufacturing process of high-performance LiFePO4 batteries may require more advanced techniques and potentially more energy-intensive processes. However, the overall environmental impact could be mitigated if these processes result in batteries with significantly longer lifespans and higher efficiency. The trade-off between manufacturing complexity and extended product life needs careful evaluation.
Improved electrochemical performance often translates to better energy efficiency during the use phase. This means less energy waste during charging and discharging cycles, potentially reducing the overall carbon footprint associated with battery-powered applications. In electric vehicles, for instance, this could lead to longer driving ranges and less frequent charging, indirectly reducing the load on power grids.
End-of-life considerations are crucial in assessing the environmental impact. Enhanced LiFePO4 batteries with longer lifespans reduce the frequency of battery replacements, thereby decreasing electronic waste. Moreover, the improved stability and safety of these batteries can facilitate easier and safer recycling processes, potentially increasing the recovery rates of valuable materials.
The use of advanced LiFePO4 batteries in renewable energy storage systems can have far-reaching environmental benefits. By enabling more efficient storage of intermittent renewable energy sources like solar and wind, these batteries can contribute to a reduction in reliance on fossil fuels for electricity generation, leading to lower greenhouse gas emissions on a systemic level.
However, it's important to note that the increased performance and potential widespread adoption of LiFePO4 batteries could lead to a rebound effect. Improved efficiency might encourage more extensive use of battery-powered devices and systems, potentially offsetting some of the environmental gains. This underscores the need for holistic environmental assessments that consider not just the direct impacts of the technology, but also its broader implications on consumption patterns and energy use.
One of the primary environmental benefits of enhancing LiFePO4 battery performance is the potential reduction in raw material consumption. Improved energy density and longer cycle life mean fewer batteries are needed for the same energy storage capacity, reducing the demand for lithium, iron, and phosphorus. This can lead to decreased mining activities and associated environmental disruptions.
The manufacturing process of high-performance LiFePO4 batteries may require more advanced techniques and potentially more energy-intensive processes. However, the overall environmental impact could be mitigated if these processes result in batteries with significantly longer lifespans and higher efficiency. The trade-off between manufacturing complexity and extended product life needs careful evaluation.
Improved electrochemical performance often translates to better energy efficiency during the use phase. This means less energy waste during charging and discharging cycles, potentially reducing the overall carbon footprint associated with battery-powered applications. In electric vehicles, for instance, this could lead to longer driving ranges and less frequent charging, indirectly reducing the load on power grids.
End-of-life considerations are crucial in assessing the environmental impact. Enhanced LiFePO4 batteries with longer lifespans reduce the frequency of battery replacements, thereby decreasing electronic waste. Moreover, the improved stability and safety of these batteries can facilitate easier and safer recycling processes, potentially increasing the recovery rates of valuable materials.
The use of advanced LiFePO4 batteries in renewable energy storage systems can have far-reaching environmental benefits. By enabling more efficient storage of intermittent renewable energy sources like solar and wind, these batteries can contribute to a reduction in reliance on fossil fuels for electricity generation, leading to lower greenhouse gas emissions on a systemic level.
However, it's important to note that the increased performance and potential widespread adoption of LiFePO4 batteries could lead to a rebound effect. Improved efficiency might encourage more extensive use of battery-powered devices and systems, potentially offsetting some of the environmental gains. This underscores the need for holistic environmental assessments that consider not just the direct impacts of the technology, but also its broader implications on consumption patterns and energy use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!