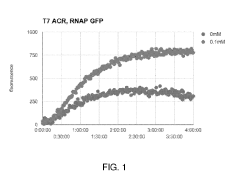
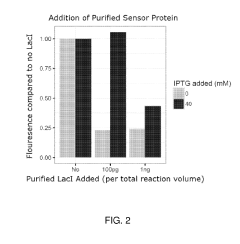
Investigating enzyme efficiency in cell-free biomanufacturing systems.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Enzyme Efficiency Background and Objectives
Cell-free biomanufacturing represents a paradigm shift in biotechnology, offering a simplified approach to producing valuable compounds without the constraints of living cells. The evolution of this technology dates back to the early 20th century with pioneering work on cell extracts, but has gained significant momentum in the past two decades due to advances in synthetic biology, protein engineering, and analytical techniques. The trajectory of development has moved from simple proof-of-concept demonstrations to increasingly sophisticated systems capable of producing complex molecules, pharmaceuticals, and materials.
Enzyme efficiency stands as the cornerstone of successful cell-free systems, directly impacting productivity, cost-effectiveness, and commercial viability. Historical challenges in this domain have included limited enzyme stability, suboptimal activity in artificial environments, and inefficient cofactor regeneration. Recent breakthroughs in protein engineering and directed evolution have begun addressing these limitations, yet substantial opportunities for improvement remain.
The primary objective of investigating enzyme efficiency in cell-free biomanufacturing systems is to develop robust methodologies that maximize catalytic performance while minimizing resource inputs. This entails comprehensive characterization of enzyme kinetics under various conditions, identification of rate-limiting steps, and development of strategies to overcome these bottlenecks. Additionally, the research aims to establish standardized metrics for comparing enzyme performance across different cell-free platforms.
A critical goal is to achieve multi-fold improvements in turnover numbers, operational stability, and substrate conversion rates compared to current benchmarks. This includes extending enzyme half-life from hours to days or weeks, increasing catalytic efficiency by orders of magnitude, and enabling sustained production over extended timeframes. Such advancements would dramatically reduce production costs and expand the range of commercially viable bioproducts.
The technological trajectory suggests several promising directions, including the development of enzyme cascades with optimized spatial organization, creation of artificial metabolons, and integration of non-natural cofactors to expand reaction capabilities. Computational approaches, including machine learning algorithms for predicting enzyme behavior and designing improved variants, represent an emerging frontier with significant potential impact.
Success in this domain would enable transformative applications across multiple industries, including on-demand pharmaceutical production, sustainable chemical manufacturing, and point-of-use biosensing. The ultimate vision is to create highly efficient, modular cell-free systems that can be rapidly deployed for diverse applications, from addressing global health challenges to enabling distributed biomanufacturing in resource-limited settings.
Enzyme efficiency stands as the cornerstone of successful cell-free systems, directly impacting productivity, cost-effectiveness, and commercial viability. Historical challenges in this domain have included limited enzyme stability, suboptimal activity in artificial environments, and inefficient cofactor regeneration. Recent breakthroughs in protein engineering and directed evolution have begun addressing these limitations, yet substantial opportunities for improvement remain.
The primary objective of investigating enzyme efficiency in cell-free biomanufacturing systems is to develop robust methodologies that maximize catalytic performance while minimizing resource inputs. This entails comprehensive characterization of enzyme kinetics under various conditions, identification of rate-limiting steps, and development of strategies to overcome these bottlenecks. Additionally, the research aims to establish standardized metrics for comparing enzyme performance across different cell-free platforms.
A critical goal is to achieve multi-fold improvements in turnover numbers, operational stability, and substrate conversion rates compared to current benchmarks. This includes extending enzyme half-life from hours to days or weeks, increasing catalytic efficiency by orders of magnitude, and enabling sustained production over extended timeframes. Such advancements would dramatically reduce production costs and expand the range of commercially viable bioproducts.
The technological trajectory suggests several promising directions, including the development of enzyme cascades with optimized spatial organization, creation of artificial metabolons, and integration of non-natural cofactors to expand reaction capabilities. Computational approaches, including machine learning algorithms for predicting enzyme behavior and designing improved variants, represent an emerging frontier with significant potential impact.
Success in this domain would enable transformative applications across multiple industries, including on-demand pharmaceutical production, sustainable chemical manufacturing, and point-of-use biosensing. The ultimate vision is to create highly efficient, modular cell-free systems that can be rapidly deployed for diverse applications, from addressing global health challenges to enabling distributed biomanufacturing in resource-limited settings.
Market Analysis for Cell-Free Biomanufacturing
The global market for cell-free biomanufacturing systems is experiencing significant growth, driven by increasing demand for sustainable and efficient production methods across pharmaceutical, chemical, and food industries. Current market valuation stands at approximately $1.2 billion, with projections indicating a compound annual growth rate of 9.8% over the next five years, potentially reaching $2.3 billion by 2028.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 45% of the total market share. This segment's prominence stems from the rising need for rapid protein synthesis and vaccine development, particularly highlighted during recent global health crises. The ability of cell-free systems to produce complex proteins without the constraints of cellular viability has positioned them as valuable tools in drug discovery and development pipelines.
Industrial biotechnology represents the fastest-growing segment, with annual growth rates exceeding 12%. This acceleration is primarily attributed to increasing industrial adoption of enzymatic processes for chemical synthesis, which offer reduced environmental footprints compared to traditional chemical manufacturing methods. Companies are increasingly recognizing the potential cost savings and sustainability benefits of enzyme-based production systems.
Regional analysis reveals North America as the current market leader, holding approximately 38% of the global market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, with China and India making substantial investments in biotechnology infrastructure and research capabilities. These emerging markets are expected to significantly reshape the competitive landscape within the next decade.
Consumer demand trends indicate growing preference for sustainably produced products, creating market pull for biomanufacturing technologies. This shift is particularly evident in food and cosmetics industries, where "bio-based" and "naturally derived" claims increasingly influence purchasing decisions. Market research indicates consumers are willing to pay premium prices for products manufactured using environmentally responsible methods.
Key market challenges include high initial investment requirements for establishing cell-free production systems and technical barriers related to enzyme stability and efficiency at industrial scales. These factors currently limit broader market penetration, particularly among small and medium enterprises with constrained R&D budgets.
Regulatory landscapes vary significantly across regions, creating complex compliance requirements for companies operating in multiple markets. Recent regulatory developments in the United States and European Union have begun establishing clearer frameworks for biomanufactured products, potentially accelerating market growth by reducing regulatory uncertainty.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 45% of the total market share. This segment's prominence stems from the rising need for rapid protein synthesis and vaccine development, particularly highlighted during recent global health crises. The ability of cell-free systems to produce complex proteins without the constraints of cellular viability has positioned them as valuable tools in drug discovery and development pipelines.
Industrial biotechnology represents the fastest-growing segment, with annual growth rates exceeding 12%. This acceleration is primarily attributed to increasing industrial adoption of enzymatic processes for chemical synthesis, which offer reduced environmental footprints compared to traditional chemical manufacturing methods. Companies are increasingly recognizing the potential cost savings and sustainability benefits of enzyme-based production systems.
Regional analysis reveals North America as the current market leader, holding approximately 38% of the global market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the most rapid growth trajectory, with China and India making substantial investments in biotechnology infrastructure and research capabilities. These emerging markets are expected to significantly reshape the competitive landscape within the next decade.
Consumer demand trends indicate growing preference for sustainably produced products, creating market pull for biomanufacturing technologies. This shift is particularly evident in food and cosmetics industries, where "bio-based" and "naturally derived" claims increasingly influence purchasing decisions. Market research indicates consumers are willing to pay premium prices for products manufactured using environmentally responsible methods.
Key market challenges include high initial investment requirements for establishing cell-free production systems and technical barriers related to enzyme stability and efficiency at industrial scales. These factors currently limit broader market penetration, particularly among small and medium enterprises with constrained R&D budgets.
Regulatory landscapes vary significantly across regions, creating complex compliance requirements for companies operating in multiple markets. Recent regulatory developments in the United States and European Union have begun establishing clearer frameworks for biomanufactured products, potentially accelerating market growth by reducing regulatory uncertainty.
Technical Challenges in Cell-Free Enzymatic Systems
Cell-free biomanufacturing systems face several significant technical challenges that currently limit their widespread industrial adoption. The primary obstacle remains the stability of enzymes outside their natural cellular environment. Enzymes often exhibit reduced half-lives and activity when removed from the protective cellular milieu, leading to diminished catalytic performance over time. This instability stems from multiple factors including exposure to oxidative stress, proteolytic degradation, and conformational changes in the absence of cellular chaperones.
Another critical challenge is the efficient regeneration of cofactors such as ATP, NAD(P)H, and other essential coenzymes required for enzymatic reactions. In living cells, these cofactors are continuously regenerated through complex metabolic networks, but replicating this regeneration efficiently in cell-free systems remains technically demanding and cost-prohibitive at scale.
The spatial organization of enzymes presents a further hurdle. Within cells, enzymes often exist in organized complexes or metabolons that facilitate substrate channeling and enhance reaction efficiency. Recreating these spatial arrangements in cell-free systems requires sophisticated immobilization strategies or the development of synthetic scaffolds that can maintain optimal enzyme proximity without hindering substrate access or product release.
Control of reaction conditions poses additional difficulties. Parameters such as pH, temperature, ionic strength, and redox potential must be precisely maintained throughout the reaction period. Unlike living cells that possess homeostatic mechanisms, cell-free systems lack inherent regulatory capabilities, necessitating external control systems that add complexity and cost to the manufacturing process.
Scale-up challenges represent a significant barrier to industrial implementation. Laboratory-scale successes often fail to translate directly to industrial production due to issues with mixing efficiency, heat transfer limitations, and enzyme stability at larger volumes. The economic viability of cell-free systems is further compromised by the high costs associated with enzyme production, purification, and stabilization.
Batch-to-batch reproducibility remains problematic due to variations in enzyme quality, activity, and stability. Unlike whole-cell processes where cellular machinery can compensate for certain fluctuations, cell-free systems are more vulnerable to component variability, requiring more stringent quality control measures.
Finally, the development of robust analytical methods for real-time monitoring of reaction progress, enzyme activity, and product formation represents an ongoing technical challenge. Current analytical techniques often involve sampling procedures that may disrupt the reaction environment or provide only retrospective rather than predictive insights into system performance.
Another critical challenge is the efficient regeneration of cofactors such as ATP, NAD(P)H, and other essential coenzymes required for enzymatic reactions. In living cells, these cofactors are continuously regenerated through complex metabolic networks, but replicating this regeneration efficiently in cell-free systems remains technically demanding and cost-prohibitive at scale.
The spatial organization of enzymes presents a further hurdle. Within cells, enzymes often exist in organized complexes or metabolons that facilitate substrate channeling and enhance reaction efficiency. Recreating these spatial arrangements in cell-free systems requires sophisticated immobilization strategies or the development of synthetic scaffolds that can maintain optimal enzyme proximity without hindering substrate access or product release.
Control of reaction conditions poses additional difficulties. Parameters such as pH, temperature, ionic strength, and redox potential must be precisely maintained throughout the reaction period. Unlike living cells that possess homeostatic mechanisms, cell-free systems lack inherent regulatory capabilities, necessitating external control systems that add complexity and cost to the manufacturing process.
Scale-up challenges represent a significant barrier to industrial implementation. Laboratory-scale successes often fail to translate directly to industrial production due to issues with mixing efficiency, heat transfer limitations, and enzyme stability at larger volumes. The economic viability of cell-free systems is further compromised by the high costs associated with enzyme production, purification, and stabilization.
Batch-to-batch reproducibility remains problematic due to variations in enzyme quality, activity, and stability. Unlike whole-cell processes where cellular machinery can compensate for certain fluctuations, cell-free systems are more vulnerable to component variability, requiring more stringent quality control measures.
Finally, the development of robust analytical methods for real-time monitoring of reaction progress, enzyme activity, and product formation represents an ongoing technical challenge. Current analytical techniques often involve sampling procedures that may disrupt the reaction environment or provide only retrospective rather than predictive insights into system performance.
Current Enzyme Optimization Strategies
01 Enzyme optimization for cell-free biomanufacturing
Optimization of enzymes is crucial for enhancing the efficiency of cell-free biomanufacturing systems. This includes engineering enzymes for improved stability, activity, and substrate specificity under various reaction conditions. Modified enzymes can significantly increase product yields and reduce reaction times in cell-free systems, making industrial-scale production more economically viable. Techniques such as directed evolution and rational design are employed to develop enzymes with enhanced performance characteristics.- Enzyme optimization for cell-free biomanufacturing: Optimization of enzymes is crucial for improving the efficiency of cell-free biomanufacturing systems. This involves engineering enzymes with enhanced stability, activity, and specificity under various reaction conditions. Techniques such as directed evolution, rational design, and computational modeling are employed to create enzymes with improved catalytic properties. These optimized enzymes can significantly increase product yield and reduce reaction time in cell-free systems.
- Multi-enzyme cascade systems: Multi-enzyme cascade systems involve the strategic arrangement of multiple enzymes to perform sequential reactions without intermediate purification steps. These systems mimic natural metabolic pathways but in a controlled, cell-free environment. By optimizing enzyme ratios, spatial organization, and reaction conditions, these cascades can achieve higher conversion rates and product selectivity than single-enzyme systems. This approach reduces byproduct formation and enhances overall process efficiency in biomanufacturing.
- Immobilization techniques for enzyme stability: Enzyme immobilization techniques are employed to enhance stability and reusability in cell-free biomanufacturing systems. Methods include attachment to solid supports, encapsulation in polymeric matrices, and cross-linking of enzyme aggregates. Immobilized enzymes often demonstrate improved thermal stability, pH tolerance, and resistance to inhibitors. This approach allows for continuous operation of biomanufacturing processes and easier product separation, significantly improving economic viability.
- Cofactor regeneration systems: Efficient cofactor regeneration is essential for sustainable cell-free biomanufacturing systems that utilize cofactor-dependent enzymes. These systems incorporate secondary enzymes or electrochemical methods to regenerate expensive cofactors such as NAD(P)H, ATP, and coenzyme A. By continuously recycling cofactors rather than supplying them stoichiometrically, these systems reduce operational costs and increase process efficiency. Advanced regeneration systems can maintain optimal cofactor concentrations throughout the reaction period.
- Synthetic cell-free expression systems: Synthetic cell-free expression systems utilize purified transcription and translation machinery to produce enzymes directly in the reaction environment. These systems bypass cellular growth limitations and can be optimized for specific enzyme production. By controlling component concentrations, energy sources, and inhibitor removal, these systems achieve higher enzyme expression levels than traditional methods. This approach enables rapid prototyping of enzyme variants and on-demand production of biocatalysts for manufacturing processes.
02 Multi-enzyme cascade systems for complex biosynthesis
Multi-enzyme cascade systems enable the efficient synthesis of complex molecules in cell-free environments. By strategically combining multiple enzymes in optimized ratios and sequences, these systems can perform multi-step reactions without cellular constraints. This approach minimizes intermediate product inhibition and maximizes pathway efficiency. Cascade systems are particularly valuable for producing pharmaceuticals, fine chemicals, and biofuels that would be difficult to synthesize using traditional cellular methods.Expand Specific Solutions03 Cofactor regeneration strategies
Efficient cofactor regeneration is essential for sustained enzymatic activity in cell-free systems. Many enzymes require cofactors such as NAD(P)H, ATP, or coenzyme A to function properly. Implementing effective regeneration systems for these cofactors significantly improves the economic viability and productivity of cell-free biomanufacturing processes. Various approaches include coupling with secondary enzymatic reactions, electrochemical methods, or photocatalytic systems to continuously replenish cofactors during the production process.Expand Specific Solutions04 Immobilization techniques for enzyme stability
Immobilization of enzymes on various supports enhances their stability and reusability in cell-free biomanufacturing. By attaching enzymes to solid carriers, nanoparticles, or within hydrogels, their operational lifetime can be significantly extended while maintaining catalytic activity. Immobilized enzyme systems allow for continuous processing, easier product separation, and protection against denaturation. This approach reduces production costs by enabling multiple reaction cycles with the same enzyme preparation and improves overall process efficiency.Expand Specific Solutions05 Reaction environment optimization
Optimizing the reaction environment is critical for maximizing enzyme efficiency in cell-free systems. This includes careful control of parameters such as pH, temperature, ionic strength, and the addition of stabilizing agents. Advanced reaction vessels with precise control systems can maintain optimal conditions throughout the production process. Some approaches incorporate microfluidic technologies or compartmentalization strategies to mimic cellular environments while eliminating cellular limitations. These optimized environments significantly enhance enzyme performance and process productivity.Expand Specific Solutions
Industry Leaders in Cell-Free Biomanufacturing
Cell-free biomanufacturing systems are currently in an early growth phase, with the market expanding rapidly due to increasing demand for sustainable and efficient protein production methods. The global market size is estimated to reach $500 million by 2025, growing at approximately 25% CAGR. Technologically, the field is advancing from experimental to commercial applications, with varying degrees of maturity across different platforms. GreenLight Biosciences and Debut Biotechnology lead in RNA and enzymatic applications respectively, while academic institutions like Northwestern University and Tsinghua University contribute fundamental research. Cellfree Sciences and Nature's Toolbox have developed proprietary platforms gaining commercial traction. Established players like Danisco and Kemira are integrating cell-free systems into their industrial processes, indicating the technology's transition toward mainstream adoption.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free biomanufacturing platform that focuses on RNA production without the limitations of living cells. Their system utilizes optimized enzyme cascades for transcription and processing of RNA molecules with significantly improved efficiency. The platform employs a continuous-flow bioreactor design that maintains optimal conditions for enzyme activity while removing inhibitory byproducts. GreenLight's approach includes computational modeling to predict enzyme kinetics and optimize reaction conditions, resulting in up to 5-fold increases in production yields compared to conventional cell-based systems. Their technology enables rapid iteration of enzyme combinations and reaction parameters, allowing for customization of the manufacturing process based on specific product requirements. The company has successfully scaled their cell-free platform for commercial production of RNA-based products including vaccines and agricultural solutions.
Strengths: Eliminates cellular growth limitations, enables precise control of reaction conditions, reduces production time from weeks to days, and allows for rapid optimization of enzyme systems. Weaknesses: Higher initial costs for enzyme production and purification, potential challenges with enzyme stability during extended manufacturing runs, and limited to products that can be synthesized through enzymatic pathways.
Debut Biotechnology, Inc.
Technical Solution: Debut Biotechnology has pioneered a continuous-flow cell-free biomanufacturing platform called "Continuous Enzymatic Manufacturing" (CEM) specifically designed to enhance enzyme efficiency. Their approach immobilizes cascades of engineered enzymes on solid supports arranged in sequence, creating microenvironments that mimic natural cellular compartmentalization while eliminating cellular constraints. This system allows for precise spatial organization of multi-enzyme pathways, reducing intermediate diffusion distances and enhancing reaction rates by up to 300% compared to solution-phase reactions. Debut's technology incorporates real-time monitoring and feedback control systems that maintain optimal conditions for each enzyme in the cascade, extending enzyme half-life and activity. Their proprietary surface chemistry techniques for enzyme immobilization preserve native protein structure and function while providing stability against denaturation. The platform has demonstrated particular success with complex transformations requiring cofactor regeneration, achieving over 95% cofactor recycling efficiency through strategic co-localization of regeneration enzymes.
Strengths: Continuous processing eliminates batch-to-batch variability, spatial organization of enzymes dramatically improves reaction kinetics, immobilization extends enzyme lifetime, and the system enables production of compounds difficult to synthesize in living cells. Weaknesses: Complex engineering requirements for optimal enzyme immobilization, potential mass transfer limitations at larger scales, and higher initial capital investment compared to traditional batch processes.
Key Innovations in Enzyme Engineering
Methods and Systems of Cell-Free Enzyme Discovery and Optimization
PatentPendingUS20190112598A1
Innovation
- A cell-free biosensor-based method is developed for high-throughput evaluation of enzyme mutants, using nucleotide sequences encoding enzyme variants, sensor biomolecules, and reporter proteins to detect metabolite production, enabling rapid identification of productive enzyme variants through fluorescence detection or other means.
Control of metabolic FLUX in cell-free biosynthetic systems
PatentPendingHK1221736A
Innovation
- Monitoring and controlling metabolic flux rates in cell-free systems by adjusting enzyme levels, substrate feed rates, O2 addition, and electron transport efficiency to optimize conditions for key metabolites like NADP(H), NAD(H), ATP, and glucose consumption, using exogenous enzymes or genetically modified microbial cells.
Scalability and Process Integration Considerations
Scaling up cell-free biomanufacturing systems from laboratory to industrial scale presents significant challenges that require systematic approaches. The transition demands careful consideration of reactor design, as traditional batch reactors may not provide optimal conditions for enzyme activity maintenance across larger volumes. Continuous flow reactors offer promising alternatives, allowing for better control of reaction parameters and potentially higher throughput, though they introduce complexities in enzyme immobilization and substrate feeding strategies.
Process integration represents another critical dimension, as cell-free systems must eventually interface with downstream processing operations. The absence of cellular debris simplifies some purification steps but introduces unique challenges in maintaining enzyme stability throughout integrated processes. Implementing real-time monitoring systems becomes essential at scale to track enzyme activity, substrate consumption, and product formation, enabling adaptive control strategies that can respond to efficiency fluctuations.
Energy regeneration systems require particular attention during scale-up, as the ATP and cofactor requirements that sustain enzyme function become economically significant at industrial scales. Various regeneration approaches, including enzymatic cascades and electrochemical methods, show promise but must be evaluated for their scalability and integration potential within specific manufacturing contexts.
Temperature and pH gradients, which remain negligible in laboratory settings, can significantly impact enzyme performance in larger reactors. Engineering solutions such as improved mixing strategies, compartmentalization approaches, and the development of enzyme variants with broader operational stability ranges may address these challenges, though each introduces additional process complexity.
The economic viability of scaled cell-free processes depends heavily on enzyme longevity and recyclability. Strategies for enzyme immobilization on various supports, including magnetic particles, membranes, and structured materials, show potential for enabling multiple production cycles, thereby amortizing enzyme costs across larger production volumes. However, these approaches must be compatible with the specific requirements of integrated manufacturing processes.
Regulatory considerations also influence scalability pathways, particularly for pharmaceutical applications. Cell-free systems offer advantages in terms of defined composition and absence of living organisms, but establishing robust quality control metrics and validation protocols for enzyme-based manufacturing remains an evolving area that requires early consideration in development programs.
Process integration represents another critical dimension, as cell-free systems must eventually interface with downstream processing operations. The absence of cellular debris simplifies some purification steps but introduces unique challenges in maintaining enzyme stability throughout integrated processes. Implementing real-time monitoring systems becomes essential at scale to track enzyme activity, substrate consumption, and product formation, enabling adaptive control strategies that can respond to efficiency fluctuations.
Energy regeneration systems require particular attention during scale-up, as the ATP and cofactor requirements that sustain enzyme function become economically significant at industrial scales. Various regeneration approaches, including enzymatic cascades and electrochemical methods, show promise but must be evaluated for their scalability and integration potential within specific manufacturing contexts.
Temperature and pH gradients, which remain negligible in laboratory settings, can significantly impact enzyme performance in larger reactors. Engineering solutions such as improved mixing strategies, compartmentalization approaches, and the development of enzyme variants with broader operational stability ranges may address these challenges, though each introduces additional process complexity.
The economic viability of scaled cell-free processes depends heavily on enzyme longevity and recyclability. Strategies for enzyme immobilization on various supports, including magnetic particles, membranes, and structured materials, show potential for enabling multiple production cycles, thereby amortizing enzyme costs across larger production volumes. However, these approaches must be compatible with the specific requirements of integrated manufacturing processes.
Regulatory considerations also influence scalability pathways, particularly for pharmaceutical applications. Cell-free systems offer advantages in terms of defined composition and absence of living organisms, but establishing robust quality control metrics and validation protocols for enzyme-based manufacturing remains an evolving area that requires early consideration in development programs.
Sustainability Impact of Cell-Free Biomanufacturing
Cell-free biomanufacturing systems represent a paradigm shift in sustainable production methods, offering significant environmental advantages over traditional manufacturing processes. By eliminating the need for whole-cell cultivation, these systems substantially reduce water consumption, with studies indicating up to 65% less water usage compared to conventional fermentation-based approaches. This reduction directly addresses growing concerns about industrial water footprints in biomanufacturing operations.
Energy efficiency constitutes another critical sustainability benefit of cell-free systems. The targeted nature of enzyme-driven reactions eliminates energy expenditure on cell maintenance and reproduction, resulting in estimated energy savings of 30-45% compared to whole-cell fermentation processes. This efficiency translates to reduced carbon emissions and aligns with global decarbonization initiatives across industrial sectors.
Waste generation is markedly decreased in cell-free biomanufacturing, as these systems produce fewer byproducts and contaminants. Recent lifecycle assessments demonstrate that cell-free processes can reduce waste streams by approximately 40-60%, significantly lowering the environmental burden associated with waste treatment and disposal. The simplified downstream processing further enhances this advantage by requiring fewer purification steps and chemical inputs.
The scalability of cell-free systems offers additional sustainability benefits through resource optimization. These systems can be rapidly scaled up or down according to production demands, preventing resource wastage associated with maintaining continuous cell cultures. This flexibility enables just-in-time manufacturing approaches that minimize storage requirements and product obsolescence.
From a circular economy perspective, cell-free biomanufacturing presents promising opportunities for enzyme recycling and reuse. Advanced immobilization techniques allow enzymes to be recovered and redeployed across multiple production cycles, extending their functional lifespan and reducing the environmental impact of enzyme production. Some cutting-edge systems have demonstrated enzyme reusability for up to 10 production cycles without significant activity loss.
Land use impacts are also favorably affected, as cell-free manufacturing facilities require substantially smaller footprints than traditional bioreactors and fermentation plants. This spatial efficiency could potentially reduce land conversion pressures and associated biodiversity impacts, particularly relevant as biomanufacturing scales to meet growing global demands for sustainable products.
The sustainability advantages of cell-free systems extend to reduced chemical inputs, with approximately 25-35% less solvent usage in downstream processing compared to cell-based methods. This reduction minimizes environmental contamination risks and occupational hazards associated with chemical handling and disposal.
Energy efficiency constitutes another critical sustainability benefit of cell-free systems. The targeted nature of enzyme-driven reactions eliminates energy expenditure on cell maintenance and reproduction, resulting in estimated energy savings of 30-45% compared to whole-cell fermentation processes. This efficiency translates to reduced carbon emissions and aligns with global decarbonization initiatives across industrial sectors.
Waste generation is markedly decreased in cell-free biomanufacturing, as these systems produce fewer byproducts and contaminants. Recent lifecycle assessments demonstrate that cell-free processes can reduce waste streams by approximately 40-60%, significantly lowering the environmental burden associated with waste treatment and disposal. The simplified downstream processing further enhances this advantage by requiring fewer purification steps and chemical inputs.
The scalability of cell-free systems offers additional sustainability benefits through resource optimization. These systems can be rapidly scaled up or down according to production demands, preventing resource wastage associated with maintaining continuous cell cultures. This flexibility enables just-in-time manufacturing approaches that minimize storage requirements and product obsolescence.
From a circular economy perspective, cell-free biomanufacturing presents promising opportunities for enzyme recycling and reuse. Advanced immobilization techniques allow enzymes to be recovered and redeployed across multiple production cycles, extending their functional lifespan and reducing the environmental impact of enzyme production. Some cutting-edge systems have demonstrated enzyme reusability for up to 10 production cycles without significant activity loss.
Land use impacts are also favorably affected, as cell-free manufacturing facilities require substantially smaller footprints than traditional bioreactors and fermentation plants. This spatial efficiency could potentially reduce land conversion pressures and associated biodiversity impacts, particularly relevant as biomanufacturing scales to meet growing global demands for sustainable products.
The sustainability advantages of cell-free systems extend to reduced chemical inputs, with approximately 25-35% less solvent usage in downstream processing compared to cell-based methods. This reduction minimizes environmental contamination risks and occupational hazards associated with chemical handling and disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!