Latest Trends in Zirconia-Based Biomaterials
Zirconia Biomaterials Evolution and Objectives
Zirconia-based biomaterials have emerged as a significant advancement in the field of biomedical engineering, particularly in dental and orthopedic applications. The evolution of these materials can be traced back to the 1960s when zirconia was first introduced as a potential alternative to alumina in biomedical applications. Since then, the development of zirconia-based biomaterials has been driven by the need for materials with superior mechanical properties, biocompatibility, and aesthetic appeal.
The primary objective in the development of zirconia-based biomaterials has been to create materials that can withstand the demanding conditions of the human body while maintaining long-term stability and functionality. This has led to extensive research into various aspects of zirconia, including its crystal structure, phase transformations, and surface properties. The focus has been on enhancing the material's strength, toughness, and wear resistance, while simultaneously improving its biocompatibility and reducing the risk of adverse reactions in the body.
One of the key milestones in the evolution of zirconia biomaterials was the development of yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) in the 1970s. This material offered significantly improved mechanical properties compared to earlier zirconia formulations, making it suitable for load-bearing applications such as dental implants and joint replacements. The introduction of Y-TZP marked the beginning of a new era in zirconia-based biomaterials, leading to extensive research into optimizing its composition and processing techniques.
In recent years, the focus has shifted towards developing zirconia-based composites and nanostructured materials. These advanced materials aim to combine the excellent mechanical properties of zirconia with enhanced bioactivity and osseointegration capabilities. Researchers are exploring various approaches, such as incorporating bioactive glass or hydroxyapatite into zirconia matrices, to create materials that can actively promote bone growth and tissue regeneration.
The current objectives in zirconia biomaterials research are multifaceted. There is a strong emphasis on improving the long-term stability of zirconia in physiological environments, addressing concerns related to low-temperature degradation, and enhancing the material's resistance to aging. Additionally, researchers are working on developing zirconia-based materials with tailored surface properties to promote better cell adhesion and tissue integration.
Another important goal is to expand the application range of zirconia-based biomaterials beyond dental and orthopedic fields. This includes exploring their potential in soft tissue engineering, drug delivery systems, and even as components in advanced medical devices. The development of 3D printing techniques for zirconia-based materials is also a key objective, as it could enable the production of custom-designed, patient-specific implants and scaffolds.
Market Analysis for Zirconia-Based Medical Devices
The global market for zirconia-based medical devices has experienced significant growth in recent years, driven by the increasing demand for dental implants, orthopedic implants, and other biomedical applications. This market segment is expected to continue its upward trajectory due to several key factors.
Firstly, the aging population in many developed countries is contributing to a rise in demand for dental and orthopedic procedures. As people live longer, there is a greater need for durable and biocompatible materials in medical implants. Zirconia-based biomaterials, with their excellent mechanical properties and biocompatibility, are well-positioned to meet this growing demand.
The dental implant sector represents a substantial portion of the zirconia-based medical device market. The shift towards metal-free dentistry and the aesthetic advantages of zirconia over traditional materials have led to increased adoption in dental crowns, bridges, and implants. This trend is expected to continue, with dental professionals and patients alike recognizing the benefits of zirconia-based solutions.
In the orthopedic segment, zirconia-based materials are gaining traction in hip and knee replacements. The material's wear resistance and low friction properties make it an attractive option for articulating surfaces in joint replacements. As surgical techniques advance and long-term clinical data becomes available, the use of zirconia in orthopedic applications is likely to expand.
The market is also being driven by technological advancements in manufacturing processes. Improved sintering techniques and the development of nano-zirconia materials are enhancing the performance characteristics of zirconia-based devices. These innovations are opening up new possibilities for applications in load-bearing implants and other high-stress environments within the body.
Geographically, North America and Europe currently dominate the zirconia-based medical device market, owing to their advanced healthcare infrastructure and higher adoption rates of new medical technologies. However, the Asia-Pacific region is emerging as a significant growth market, fueled by improving healthcare access, rising disposable incomes, and increasing awareness of advanced medical treatments.
Despite the positive outlook, the market faces challenges such as the high cost of zirconia-based materials compared to traditional alternatives and the need for long-term clinical studies to fully establish the efficacy and safety of these devices in various applications. Regulatory hurdles and the time required for product approval also impact market growth.
In conclusion, the market for zirconia-based medical devices shows promising growth potential, driven by demographic trends, technological advancements, and expanding applications in dentistry and orthopedics. As research continues and manufacturing processes improve, zirconia-based biomaterials are likely to play an increasingly important role in the medical device industry.
Current Challenges in Zirconia Biomaterial Development
Despite significant advancements in zirconia-based biomaterials, several challenges persist in their development and application. One of the primary concerns is the long-term stability of zirconia in physiological environments. While zirconia exhibits excellent initial mechanical properties, its tendency to undergo low-temperature degradation (LTD) in the presence of water molecules remains a significant hurdle. This aging process can lead to surface roughening, microcracking, and potential implant failure over time.
Another challenge lies in optimizing the balance between strength and toughness in zirconia biomaterials. While high-strength zirconia compositions are desirable for load-bearing applications, they often come at the cost of reduced fracture toughness. Developing zirconia-based materials that maintain both high strength and adequate toughness remains an ongoing research focus.
The bioactivity of zirconia surfaces presents another area of concern. Unlike some other ceramic biomaterials, zirconia is relatively bioinert, which can limit its ability to form strong bonds with surrounding tissues. Enhancing the bioactivity of zirconia surfaces without compromising its mechanical properties is a complex task that researchers are actively pursuing.
Manufacturing challenges also persist in the field of zirconia biomaterials. Achieving consistent and reproducible properties across batches, especially for complex geometries, remains difficult. Issues such as residual stresses, non-uniform densification, and the presence of defects during the sintering process can significantly impact the final product's performance and reliability.
The development of zirconia-based composites and hybrid materials introduces additional complexities. While these approaches offer potential solutions to some of the aforementioned challenges, they also bring new hurdles in terms of material design, processing, and characterization. Ensuring uniform distribution of secondary phases or reinforcements, optimizing interfacial properties, and maintaining overall biocompatibility are critical aspects that require further investigation.
Lastly, the regulatory landscape for zirconia biomaterials continues to evolve, presenting challenges in terms of standardization and approval processes. As new compositions and manufacturing techniques emerge, establishing appropriate testing protocols and safety standards becomes increasingly important to ensure the reliable and safe use of these materials in clinical applications.
State-of-the-Art Zirconia Biomaterial Solutions
01 Zirconia-based dental materials
Zirconia-based materials are widely used in dental applications due to their excellent mechanical properties and biocompatibility. These materials are used for dental implants, crowns, and bridges. The high strength and toughness of zirconia make it an ideal material for load-bearing dental applications. Various techniques are employed to improve the aesthetics and performance of zirconia-based dental materials.- Zirconia-based dental materials: Zirconia is widely used in dental applications due to its excellent mechanical properties and biocompatibility. These materials are used for dental implants, crowns, and bridges. The formulations often include stabilizers to maintain the desired crystal structure and enhance durability.
- Zirconia nanoparticles for biomedical applications: Zirconia nanoparticles are utilized in various biomedical applications, including drug delivery systems, imaging contrast agents, and tissue engineering scaffolds. The nanoparticles can be surface-modified to improve their functionality and biocompatibility.
- Zirconia-based composites for orthopedic implants: Zirconia is combined with other materials like alumina or polymers to create composite biomaterials for orthopedic implants. These composites offer improved wear resistance, mechanical strength, and biocompatibility compared to traditional implant materials.
- Surface modification of zirconia biomaterials: Various surface modification techniques are applied to zirconia biomaterials to enhance their biological performance. These include plasma treatment, chemical etching, and coating with bioactive materials to improve cell adhesion, osseointegration, and overall biocompatibility.
- Zirconia-based biosensors and diagnostic devices: Zirconia materials are used in the development of biosensors and diagnostic devices due to their chemical stability and unique surface properties. These devices can be used for detecting various biomolecules and monitoring physiological parameters in medical applications.
02 Zirconia-based orthopedic implants
Zirconia-based materials are utilized in orthopedic implants, particularly for joint replacements. These materials offer high wear resistance, low friction, and excellent biocompatibility. Researchers are developing new compositions and manufacturing techniques to enhance the longevity and performance of zirconia-based orthopedic implants.Expand Specific Solutions03 Surface modification of zirconia biomaterials
Various surface modification techniques are applied to zirconia-based biomaterials to improve their biological and mechanical properties. These techniques include surface roughening, coating with bioactive materials, and creating nanostructured surfaces. The goal is to enhance osseointegration, reduce bacterial adhesion, and improve overall biocompatibility.Expand Specific Solutions04 Zirconia-based composites for biomedical applications
Researchers are developing zirconia-based composite materials by incorporating other ceramics, polymers, or metals. These composites aim to combine the beneficial properties of zirconia with those of other materials, resulting in improved mechanical strength, toughness, and bioactivity. Such composites find applications in various biomedical fields, including dentistry and orthopedics.Expand Specific Solutions05 Manufacturing processes for zirconia biomaterials
Advanced manufacturing processes are being developed and optimized for zirconia-based biomaterials. These include 3D printing, injection molding, and novel sintering techniques. The focus is on achieving precise control over the material's microstructure, density, and final shape, which are crucial for the performance of biomedical devices.Expand Specific Solutions
Key Players in Zirconia Biomaterials Industry
The zirconia-based biomaterials market is in a growth phase, driven by increasing demand in dental and orthopedic applications. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, zirconia-based biomaterials are reaching maturity, with ongoing innovations focused on enhancing properties and expanding applications. Key players like 3M Innovative Properties Co., Kyocera Corp., and Straumann Holding AG are leading in research and development, while emerging companies such as Hangzhou Ranran Technology Co., Ltd. and Aidite (Qinhuangdao) Technology Co., Ltd. are contributing to market dynamism. The competitive landscape is characterized by a mix of established global corporations and innovative startups, driving continuous advancements in material properties, manufacturing processes, and clinical applications.
3M Innovative Properties Co.
Aidite (Qinhuangdao) Technology Co., Ltd.
Breakthrough Innovations in Zirconia Biomaterials
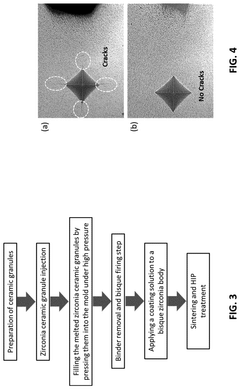
- A method involving ceramic injection molding of yttria-stabilized zirconia powder with a thermoplastic organic binder, followed by heat treatment, application of a tantalum or niobium-containing solution to form a functional gradient coating layer, and hot isostatic pressing to enhance flexural strength and fracture toughness.
- Development of alumina ceramic matrix composites incorporating zirconia, chromia, strontium carbonate, and strontium niobate additives, processed through planetary ball milling, uniaxial and cold isostatic pressing, and hot isostatic pressing to achieve high hardness, fracture toughness, and improved surface wettability, reducing porosity and enhancing wear resistance.
Biocompatibility and Safety Regulations
Zirconia-based biomaterials have gained significant attention in the medical field due to their excellent biocompatibility and mechanical properties. As these materials continue to evolve and find new applications, it is crucial to understand and adhere to the biocompatibility and safety regulations governing their use in medical devices and implants.
The biocompatibility of zirconia-based biomaterials is primarily assessed through a series of standardized tests outlined by international regulatory bodies such as the International Organization for Standardization (ISO) and the Food and Drug Administration (FDA). These tests evaluate the material's potential to cause adverse biological reactions when in contact with living tissues, cells, and bodily fluids.
One of the key aspects of biocompatibility testing for zirconia-based biomaterials is cytotoxicity evaluation. This involves exposing cell cultures to the material or its extracts to determine if it causes cell death or inhibits cell growth. Recent studies have shown that zirconia-based materials generally exhibit low cytotoxicity, making them suitable for various biomedical applications.
In addition to cytotoxicity, genotoxicity and carcinogenicity tests are conducted to ensure that zirconia-based biomaterials do not cause genetic mutations or promote tumor formation. These tests are particularly important for long-term implants and devices that remain in the body for extended periods.
Safety regulations also require the assessment of local tissue responses to zirconia-based biomaterials through in vivo studies. These studies evaluate the material's potential to cause inflammation, foreign body reactions, or tissue necrosis when implanted in animal models. Recent trends in this area focus on developing more predictive and ethically sound in vitro alternatives to reduce animal testing.
The mechanical stability and wear resistance of zirconia-based biomaterials are crucial factors in their safety profile, especially for load-bearing applications such as dental and orthopedic implants. Regulatory bodies require extensive testing to ensure that these materials maintain their structural integrity and do not release potentially harmful particles over time.
As nanotechnology advances, there is growing interest in nanostructured zirconia-based biomaterials. This has led to the development of new safety guidelines specifically addressing the potential risks associated with nanomaterials, including their ability to penetrate biological barriers and interact with cellular components.
Recent trends in biocompatibility and safety regulations for zirconia-based biomaterials also emphasize the importance of considering the entire life cycle of the material, from manufacturing to disposal. This includes evaluating the environmental impact of production processes and the potential for material degradation products to affect ecosystems.
Regulatory bodies are increasingly focusing on harmonizing international standards for biocompatibility and safety testing of zirconia-based biomaterials. This effort aims to streamline the approval process for new medical devices and implants while ensuring consistent safety standards across different regions.
Sustainability in Zirconia Biomaterial Production
Sustainability in zirconia biomaterial production has become a critical focus in recent years, driven by increasing environmental concerns and the need for more eco-friendly manufacturing processes. The production of zirconia-based biomaterials traditionally involves energy-intensive processes and the use of potentially harmful chemicals, prompting researchers and manufacturers to explore more sustainable alternatives.
One of the key trends in sustainable zirconia biomaterial production is the development of low-temperature synthesis methods. These approaches aim to reduce energy consumption during the manufacturing process, thereby decreasing the overall carbon footprint. Hydrothermal synthesis and sol-gel methods are gaining traction as they allow for the production of high-quality zirconia materials at lower temperatures compared to conventional high-temperature calcination techniques.
Another significant area of focus is the use of renewable precursors and green solvents in zirconia synthesis. Researchers are investigating bio-based sources of zirconium, such as zircon sand from sustainable mining practices, as alternatives to traditional synthetic precursors. Additionally, the replacement of toxic organic solvents with environmentally benign alternatives, like water or supercritical CO2, is being explored to minimize the environmental impact of production processes.
The concept of circular economy is also being applied to zirconia biomaterial production. This involves developing recycling and recovery methods for zirconia materials at the end of their lifecycle. Advanced separation techniques and reprocessing methods are being investigated to reclaim zirconia from discarded medical devices and industrial applications, reducing the demand for raw materials and minimizing waste.
Additive manufacturing technologies, such as 3D printing, are revolutionizing the production of zirconia biomaterials. These techniques offer the potential for more efficient material use, reduced waste, and the ability to create complex, customized structures with minimal material loss. The integration of additive manufacturing with sustainable material feedstocks is an emerging area of research that promises to further enhance the sustainability of zirconia biomaterial production.
Lastly, the development of multifunctional zirconia biomaterials is contributing to sustainability efforts. By creating materials that serve multiple purposes or exhibit enhanced performance, researchers aim to reduce the overall material consumption and improve the longevity of zirconia-based products. This approach not only conserves resources but also potentially reduces the frequency of replacements or interventions in medical applications.