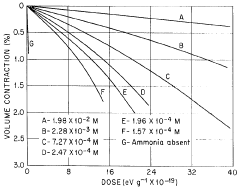
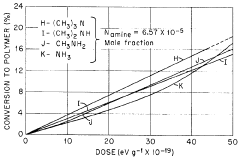
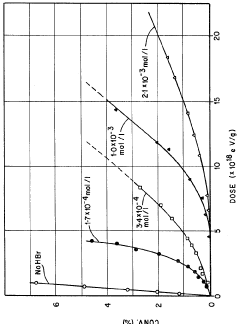
Lewis Acid in Controlled Radical Polymerization
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lewis Acid CRP Background and Objectives
Controlled Radical Polymerization (CRP) has revolutionized polymer science since its emergence in the 1990s, enabling unprecedented control over polymer architecture, molecular weight distribution, and functionality. Within this field, the incorporation of Lewis acids has emerged as a significant advancement, offering enhanced control mechanisms and expanded synthetic possibilities.
Lewis acids, defined as electron pair acceptors, have a rich history in organic synthesis dating back to the early 20th century. Their application in polymerization processes began to gain traction in the 1980s, primarily in cationic polymerization systems. However, their integration into radical polymerization techniques represents a more recent development that has opened new avenues for polymer design and synthesis.
The evolution of Lewis acid applications in CRP has followed several distinct phases. Initially, they were employed as simple additives to modify reaction kinetics. This progressed to their use as catalysts in atom transfer radical polymerization (ATRP) systems, where they significantly enhanced control over polymerization rates and polymer properties. Most recently, researchers have developed sophisticated Lewis acid-mediated CRP techniques that enable polymerization of previously challenging monomers.
Current technological trends indicate growing interest in developing more environmentally benign Lewis acid catalysts, reducing metal content in final polymers, and expanding the range of compatible functional groups. The field is moving toward more precise spatial and temporal control over polymerization processes, enabling advanced materials with programmable properties.
The primary objectives of Lewis acid CRP research encompass several dimensions. Fundamentally, researchers aim to elucidate the mechanistic interactions between Lewis acids and radical intermediates, providing deeper understanding of these complex systems. From an application perspective, goals include developing more efficient catalytic systems with lower catalyst loadings, broader monomer compatibility, and enhanced control over polymer architecture.
Additional objectives include the design of stimuli-responsive polymerization systems where Lewis acid activity can be triggered by external stimuli such as light, temperature, or pH changes. There is also significant interest in creating heterogeneous Lewis acid catalysts that can be easily recovered and recycled, addressing sustainability concerns in polymer production.
The ultimate technological goal remains the development of versatile, robust Lewis acid CRP systems capable of producing polymers with precisely defined structures and properties for advanced applications in medicine, electronics, and materials science. This requires overcoming current limitations in catalyst efficiency, monomer scope, and reaction conditions.
Lewis acids, defined as electron pair acceptors, have a rich history in organic synthesis dating back to the early 20th century. Their application in polymerization processes began to gain traction in the 1980s, primarily in cationic polymerization systems. However, their integration into radical polymerization techniques represents a more recent development that has opened new avenues for polymer design and synthesis.
The evolution of Lewis acid applications in CRP has followed several distinct phases. Initially, they were employed as simple additives to modify reaction kinetics. This progressed to their use as catalysts in atom transfer radical polymerization (ATRP) systems, where they significantly enhanced control over polymerization rates and polymer properties. Most recently, researchers have developed sophisticated Lewis acid-mediated CRP techniques that enable polymerization of previously challenging monomers.
Current technological trends indicate growing interest in developing more environmentally benign Lewis acid catalysts, reducing metal content in final polymers, and expanding the range of compatible functional groups. The field is moving toward more precise spatial and temporal control over polymerization processes, enabling advanced materials with programmable properties.
The primary objectives of Lewis acid CRP research encompass several dimensions. Fundamentally, researchers aim to elucidate the mechanistic interactions between Lewis acids and radical intermediates, providing deeper understanding of these complex systems. From an application perspective, goals include developing more efficient catalytic systems with lower catalyst loadings, broader monomer compatibility, and enhanced control over polymer architecture.
Additional objectives include the design of stimuli-responsive polymerization systems where Lewis acid activity can be triggered by external stimuli such as light, temperature, or pH changes. There is also significant interest in creating heterogeneous Lewis acid catalysts that can be easily recovered and recycled, addressing sustainability concerns in polymer production.
The ultimate technological goal remains the development of versatile, robust Lewis acid CRP systems capable of producing polymers with precisely defined structures and properties for advanced applications in medicine, electronics, and materials science. This requires overcoming current limitations in catalyst efficiency, monomer scope, and reaction conditions.
Market Analysis for Lewis Acid-Mediated Polymerization
The global market for Lewis acid-mediated controlled radical polymerization has experienced significant growth over the past decade, driven by increasing demand for specialty polymers with precise architectures and properties. This technology enables the production of polymers with controlled molecular weight, narrow polydispersity, and defined end-group functionality, making it valuable across multiple industries.
The polymer market utilizing Lewis acid catalysis is currently valued at approximately $4.2 billion, with a compound annual growth rate (CAGR) of 6.8% projected through 2028. This growth outpaces the broader polymer additives market, which maintains a CAGR of 4.5%, indicating the increasing adoption of advanced polymerization techniques.
Key market segments benefiting from Lewis acid-mediated polymerization include coatings and adhesives (38% market share), healthcare and pharmaceuticals (24%), electronics (17%), automotive (12%), and other applications (9%). The coatings sector dominates due to the need for polymers with specific surface properties and durability characteristics that can be precisely engineered through controlled polymerization.
Regionally, North America and Europe currently lead the market with 35% and 32% shares respectively, owing to their established chemical industries and research infrastructure. However, Asia-Pacific represents the fastest-growing region with a 9.2% CAGR, driven by expanding manufacturing capabilities in China, Japan, and South Korea, and increasing investment in specialty chemical production.
Market demand is increasingly focused on sustainable polymerization processes, with 63% of end-users expressing preference for catalytic systems that reduce waste and energy consumption. Lewis acid catalysts that can operate under mild conditions or that can be recovered and reused are gaining particular attention, commanding premium pricing of 15-20% above conventional alternatives.
The competitive landscape features both specialty chemical companies and polymer manufacturers. Major players include BASF (16% market share), Dow Chemical (14%), Mitsubishi Chemical (11%), and Solvay (9%), alongside emerging specialized firms focusing exclusively on advanced polymerization technologies.
Customer pain points driving market growth include the need for polymers with enhanced performance characteristics (cited by 78% of customers), cost-effective production methods (65%), and environmentally sustainable processes (57%). Lewis acid-mediated polymerization addresses these concerns by enabling precise control over polymer architecture while potentially reducing production steps and waste generation.
The polymer market utilizing Lewis acid catalysis is currently valued at approximately $4.2 billion, with a compound annual growth rate (CAGR) of 6.8% projected through 2028. This growth outpaces the broader polymer additives market, which maintains a CAGR of 4.5%, indicating the increasing adoption of advanced polymerization techniques.
Key market segments benefiting from Lewis acid-mediated polymerization include coatings and adhesives (38% market share), healthcare and pharmaceuticals (24%), electronics (17%), automotive (12%), and other applications (9%). The coatings sector dominates due to the need for polymers with specific surface properties and durability characteristics that can be precisely engineered through controlled polymerization.
Regionally, North America and Europe currently lead the market with 35% and 32% shares respectively, owing to their established chemical industries and research infrastructure. However, Asia-Pacific represents the fastest-growing region with a 9.2% CAGR, driven by expanding manufacturing capabilities in China, Japan, and South Korea, and increasing investment in specialty chemical production.
Market demand is increasingly focused on sustainable polymerization processes, with 63% of end-users expressing preference for catalytic systems that reduce waste and energy consumption. Lewis acid catalysts that can operate under mild conditions or that can be recovered and reused are gaining particular attention, commanding premium pricing of 15-20% above conventional alternatives.
The competitive landscape features both specialty chemical companies and polymer manufacturers. Major players include BASF (16% market share), Dow Chemical (14%), Mitsubishi Chemical (11%), and Solvay (9%), alongside emerging specialized firms focusing exclusively on advanced polymerization technologies.
Customer pain points driving market growth include the need for polymers with enhanced performance characteristics (cited by 78% of customers), cost-effective production methods (65%), and environmentally sustainable processes (57%). Lewis acid-mediated polymerization addresses these concerns by enabling precise control over polymer architecture while potentially reducing production steps and waste generation.
Current Challenges in Lewis Acid CRP Technology
Despite significant advancements in Lewis acid-mediated controlled radical polymerization (CRP), several critical challenges continue to impede broader industrial adoption and technological advancement. One primary obstacle remains the sensitivity of many Lewis acid catalysts to oxygen and moisture, necessitating stringent reaction conditions that complicate scalability and increase production costs. This sensitivity often requires specialized equipment and handling protocols that are impractical for large-scale manufacturing environments.
Another significant challenge lies in catalyst efficiency and loading requirements. Current Lewis acid systems frequently demand relatively high catalyst concentrations to achieve desired polymerization control, raising concerns about cost-effectiveness and product purification. The presence of metal residues in the final polymer products can adversely affect material properties and limit applications in sensitive fields such as biomedical devices and electronics.
Selectivity issues present additional complications, as Lewis acids can interact with various functional groups beyond those intended for polymerization control. This non-specific reactivity often leads to side reactions, chain termination events, and reduced control over molecular weight distribution. Consequently, the synthesis of complex architectures like block copolymers and star polymers remains challenging with existing Lewis acid CRP systems.
Temperature sensitivity constitutes another barrier, with many Lewis acid catalysts exhibiting optimal performance only within narrow temperature ranges. This limitation restricts process flexibility and complicates heat management during scale-up, particularly for exothermic polymerizations where temperature control is critical for maintaining reaction control.
The mechanistic understanding of Lewis acid interactions in radical polymerization systems remains incomplete, hampering rational catalyst design and optimization. The complex interplay between Lewis acids, monomers, and growing polymer chains involves subtle electronic and steric effects that are difficult to predict and model accurately.
Recyclability and catalyst recovery represent persistent economic and environmental concerns. Most current systems do not allow for efficient catalyst separation and reuse, leading to increased waste generation and higher production costs. This limitation contradicts growing industry emphasis on sustainable manufacturing practices and circular economy principles.
Finally, compatibility issues with diverse monomer classes restrict the versatility of Lewis acid CRP technology. While certain acid systems work effectively with specific monomer families, developing universal catalysts capable of controlling polymerization across a broad spectrum of vinyl monomers remains an elusive goal for researchers in this field.
Another significant challenge lies in catalyst efficiency and loading requirements. Current Lewis acid systems frequently demand relatively high catalyst concentrations to achieve desired polymerization control, raising concerns about cost-effectiveness and product purification. The presence of metal residues in the final polymer products can adversely affect material properties and limit applications in sensitive fields such as biomedical devices and electronics.
Selectivity issues present additional complications, as Lewis acids can interact with various functional groups beyond those intended for polymerization control. This non-specific reactivity often leads to side reactions, chain termination events, and reduced control over molecular weight distribution. Consequently, the synthesis of complex architectures like block copolymers and star polymers remains challenging with existing Lewis acid CRP systems.
Temperature sensitivity constitutes another barrier, with many Lewis acid catalysts exhibiting optimal performance only within narrow temperature ranges. This limitation restricts process flexibility and complicates heat management during scale-up, particularly for exothermic polymerizations where temperature control is critical for maintaining reaction control.
The mechanistic understanding of Lewis acid interactions in radical polymerization systems remains incomplete, hampering rational catalyst design and optimization. The complex interplay between Lewis acids, monomers, and growing polymer chains involves subtle electronic and steric effects that are difficult to predict and model accurately.
Recyclability and catalyst recovery represent persistent economic and environmental concerns. Most current systems do not allow for efficient catalyst separation and reuse, leading to increased waste generation and higher production costs. This limitation contradicts growing industry emphasis on sustainable manufacturing practices and circular economy principles.
Finally, compatibility issues with diverse monomer classes restrict the versatility of Lewis acid CRP technology. While certain acid systems work effectively with specific monomer families, developing universal catalysts capable of controlling polymerization across a broad spectrum of vinyl monomers remains an elusive goal for researchers in this field.
Current Lewis Acid CRP Methodologies
01 Lewis acids as catalysts in controlled radical polymerization
Lewis acids can be used as catalysts in controlled radical polymerization processes to enhance reaction rates and control polymer properties. These acids coordinate with monomers or initiators, altering their reactivity and enabling better control over the polymerization process. The use of Lewis acid catalysts can lead to polymers with narrower molecular weight distributions and more defined structures compared to conventional radical polymerization methods.- Lewis acids as catalysts in controlled radical polymerization: Lewis acids can be used as catalysts in controlled radical polymerization processes to enhance reaction rates and control polymer properties. These acids coordinate with monomers or initiators, altering their reactivity and enabling better control over the polymerization process. The presence of Lewis acids can influence the stereochemistry of the resulting polymers and allow for the synthesis of polymers with specific architectures and molecular weight distributions.
- Lewis acid-mediated atom transfer radical polymerization (ATRP): Lewis acids can be incorporated into atom transfer radical polymerization (ATRP) systems to enhance control over the polymerization process. The addition of Lewis acids can modify the redox potential of the transition metal catalyst, affecting the equilibrium between dormant and active species. This modification allows for better control over polymer molecular weight, polydispersity, and end-group functionality, resulting in well-defined polymers with desired properties.
- Lewis acid complexes with transition metals for controlled polymerization: Forming complexes between Lewis acids and transition metal catalysts creates hybrid catalyst systems with unique properties for controlled radical polymerization. These complexes can exhibit enhanced catalytic activity, improved solubility in various reaction media, and better stability under polymerization conditions. The synergistic effect between the Lewis acid and transition metal components allows for the polymerization of a wider range of monomers under milder conditions while maintaining control over the polymerization process.
- Lewis acids for controlling tacticity in radical polymerization: Lewis acids can be employed to control the tacticity of polymers produced via radical polymerization. By coordinating with the monomer units during polymerization, Lewis acids can influence the orientation of incoming monomers, leading to stereoregular polymer chains. This approach allows for the synthesis of isotactic, syndiotactic, or stereoblock polymers with enhanced physical properties, such as improved crystallinity, mechanical strength, and thermal stability.
- Lewis acid-assisted reversible addition-fragmentation chain transfer (RAFT) polymerization: Lewis acids can be incorporated into reversible addition-fragmentation chain transfer (RAFT) polymerization systems to enhance control and expand the scope of this polymerization technique. The addition of Lewis acids can modify the reactivity of RAFT agents, influence the fragmentation and addition steps of the RAFT mechanism, and alter the equilibrium between dormant and active chains. These modifications can lead to improved control over polymer molecular weight, reduced polydispersity, and the ability to polymerize challenging monomers under mild conditions.
02 Lewis acid complexes with transition metals for ATRP
Lewis acids can form complexes with transition metal catalysts used in atom transfer radical polymerization (ATRP). These complexes modify the redox potential of the metal catalyst, enhancing its activity and control over the polymerization process. The combination of Lewis acids with transition metal catalysts allows for polymerization under milder conditions and can reduce the amount of metal catalyst required, addressing environmental concerns associated with metal contamination in the final polymer products.Expand Specific Solutions03 Lewis acid-mediated control of polymer tacticity and stereochemistry
Lewis acids can influence the stereochemical outcome of radical polymerization by coordinating with the propagating chain end or incoming monomer. This coordination can direct the approach of the monomer, leading to enhanced stereocontrol and the formation of polymers with specific tacticity (isotactic, syndiotactic, or heterotactic). The ability to control polymer tacticity is crucial for applications requiring specific physical properties, such as crystallinity, solubility, and mechanical strength.Expand Specific Solutions04 Lewis acid incorporation in reversible addition-fragmentation chain transfer (RAFT) polymerization
Lewis acids can be incorporated into RAFT polymerization systems to enhance control over molecular weight and polymer architecture. By interacting with the RAFT agent or monomers, Lewis acids can modify the equilibrium between dormant and active species, leading to better control over the polymerization process. This approach allows for the synthesis of well-defined block copolymers and polymers with complex architectures that would be difficult to achieve using conventional radical polymerization methods.Expand Specific Solutions05 Lewis acid-assisted polymerization in environmentally friendly conditions
Lewis acids can enable controlled radical polymerization under more environmentally friendly conditions, such as in aqueous media or at lower temperatures. By modifying the reactivity of monomers and initiators, Lewis acids can facilitate polymerization processes that require less energy input and generate fewer waste products. This approach aligns with green chemistry principles and can lead to more sustainable polymer production methods with reduced environmental impact.Expand Specific Solutions
Key Industry Players and Research Institutions
The Lewis Acid in Controlled Radical Polymerization field is currently in a growth phase, with increasing market adoption across specialty chemical applications. The global market is estimated to reach significant value as industries seek more precise polymer synthesis methods. Technologically, this area is advancing rapidly with varying maturity levels among key players. Leading chemical corporations like BASF, LG Chem, and ExxonMobil demonstrate advanced commercial applications, while China Petroleum & Chemical Corp. and Sinopec Beijing Research Institute show strong R&D momentum. Academic institutions including North Carolina State University and Zhejiang University contribute fundamental research breakthroughs. The competitive landscape features established industrial players commercializing proprietary technologies alongside research organizations developing next-generation catalytic systems.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed proprietary Lewis acid-based controlled radical polymerization technologies primarily focused on producing specialty polymers for lubricant and fuel applications. Their approach utilizes aluminum and titanium-based Lewis acids that coordinate with specific monomers to control polymerization kinetics and polymer architecture. ExxonMobil's technology incorporates Lewis acids as part of multi-component catalyst systems that enable precise control over molecular weight distribution and copolymer composition. They have pioneered the use of Lewis acid-modified silica materials as supports for controlled radical polymerization catalysts, creating heterogeneous systems that facilitate product purification. ExxonMobil has developed specialized Lewis acid catalysts that can effectively control the polymerization of olefinic monomers under moderate conditions, expanding the range of monomers amenable to controlled radical polymerization. Their research has also focused on Lewis acid systems that can mediate controlled radical polymerization in non-polar hydrocarbon solvents, making them compatible with existing production infrastructure.
Strengths: Excellent integration with petroleum industry processes; specialized expertise with hydrocarbon monomers; development of practical heterogeneous catalyst systems. Weaknesses: Narrower focus on petroleum-related applications; some systems show limited effectiveness with highly polar monomers.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed industrial-scale Lewis acid-mediated controlled radical polymerization technologies focused on producing specialty polymers for petroleum and chemical applications. Their approach utilizes aluminum and boron-based Lewis acids that are compatible with existing industrial polymerization equipment. Sinopec's technology incorporates Lewis acids as coordination agents that interact with polar monomers to control their incorporation into polymer chains during free radical polymerization. They have pioneered the use of Lewis acid-modified clay materials as heterogeneous catalysts for controlled radical polymerization, combining the benefits of Lewis acid catalysis with the practical advantages of heterogeneous systems. Sinopec has successfully scaled up several Lewis acid-mediated polymerization processes for the production of specialty polymers with controlled molecular weight and narrow polydispersity. Their research has also focused on developing Lewis acid systems that can function effectively in the presence of small amounts of water and oxygen, making them more suitable for industrial implementation.
Strengths: Strong focus on industrial applicability and scalability; development of robust catalysts tolerant to industrial conditions; integration with existing manufacturing infrastructure. Weaknesses: Some systems prioritize robustness over achieving the highest level of polymerization control; limited effectiveness with certain specialty monomers.
Critical Patents and Innovations in Lewis Acid Catalysis
Radiation-induced ionic polymerization controlled by the presence of lewis acids or lewis bases
PatentInactiveUS3616369A
Innovation
- A radiation-induced cationic polymerization process is developed by irradiating a mixture of a Lewis base and a monomer with sufficient radiant energy, allowing for control of molecular weight through the concentration of the Lewis base, which is not possible with traditional chemical catalysts.
Method for preparing vinyl ether polymer by photo-initiated polymerization
PatentActiveUS20210371556A1
Innovation
- A photo-initiated polymerization method using manganese carbonyl and an organic halogenated hydrocarbon under visible light irradiation, which generates carbon radicals and carbocations to initiate cationic polymerization, allowing for the production of vinyl ether polymers with controllable molecular weight and narrow distribution without the need for additional purification or complex reagents.
Sustainability Aspects of Lewis Acid CRP Systems
The sustainability of Lewis acid-mediated controlled radical polymerization (CRP) systems has become increasingly important as industries shift toward greener chemical processes. These systems offer significant advantages in terms of environmental impact compared to traditional polymerization methods, primarily through reduced waste generation and energy consumption.
Lewis acid CRP systems demonstrate remarkable atom economy, with high conversion rates of monomers to polymers and minimal byproduct formation. This efficiency translates directly to reduced waste streams and lower environmental footprint. Additionally, many Lewis acid catalysts can be recovered and reused through appropriate separation techniques, further enhancing their sustainability profile.
The energy efficiency of Lewis acid CRP processes represents another key sustainability advantage. These systems typically operate at lower temperatures than conventional radical polymerization, resulting in significant energy savings. Some Lewis acid catalysts enable polymerization under ambient conditions or with alternative energy sources like visible light, substantially reducing the carbon footprint associated with polymer production.
Recent developments have focused on replacing traditional metal-based Lewis acids with more environmentally benign alternatives. Organic Lewis acids and metal-free catalyst systems have emerged as promising sustainable options, eliminating concerns about heavy metal contamination in polymer products and waste streams. These innovations address important end-of-life considerations for polymeric materials.
Water compatibility represents a frontier in sustainable Lewis acid CRP development. Several research groups have reported successful aqueous Lewis acid CRP systems that eliminate the need for organic solvents, significantly reducing volatile organic compound (VOC) emissions and associated environmental hazards. These water-based systems align with green chemistry principles and regulatory trends toward solvent reduction.
Life cycle assessment (LCA) studies comparing Lewis acid CRP systems with conventional polymerization methods have demonstrated quantifiable sustainability benefits. These analyses typically show reduced environmental impact across multiple categories, including global warming potential, resource depletion, and ecotoxicity. However, comprehensive LCA data remains limited for newer Lewis acid catalysts and systems.
Regulatory considerations increasingly influence the adoption of Lewis acid CRP technologies. As environmental regulations become more stringent worldwide, particularly regarding chemical waste and emissions, sustainable polymerization methods gain competitive advantage. Lewis acid CRP systems that minimize hazardous waste generation and energy consumption are well-positioned to meet evolving regulatory requirements in major markets.
Lewis acid CRP systems demonstrate remarkable atom economy, with high conversion rates of monomers to polymers and minimal byproduct formation. This efficiency translates directly to reduced waste streams and lower environmental footprint. Additionally, many Lewis acid catalysts can be recovered and reused through appropriate separation techniques, further enhancing their sustainability profile.
The energy efficiency of Lewis acid CRP processes represents another key sustainability advantage. These systems typically operate at lower temperatures than conventional radical polymerization, resulting in significant energy savings. Some Lewis acid catalysts enable polymerization under ambient conditions or with alternative energy sources like visible light, substantially reducing the carbon footprint associated with polymer production.
Recent developments have focused on replacing traditional metal-based Lewis acids with more environmentally benign alternatives. Organic Lewis acids and metal-free catalyst systems have emerged as promising sustainable options, eliminating concerns about heavy metal contamination in polymer products and waste streams. These innovations address important end-of-life considerations for polymeric materials.
Water compatibility represents a frontier in sustainable Lewis acid CRP development. Several research groups have reported successful aqueous Lewis acid CRP systems that eliminate the need for organic solvents, significantly reducing volatile organic compound (VOC) emissions and associated environmental hazards. These water-based systems align with green chemistry principles and regulatory trends toward solvent reduction.
Life cycle assessment (LCA) studies comparing Lewis acid CRP systems with conventional polymerization methods have demonstrated quantifiable sustainability benefits. These analyses typically show reduced environmental impact across multiple categories, including global warming potential, resource depletion, and ecotoxicity. However, comprehensive LCA data remains limited for newer Lewis acid catalysts and systems.
Regulatory considerations increasingly influence the adoption of Lewis acid CRP technologies. As environmental regulations become more stringent worldwide, particularly regarding chemical waste and emissions, sustainable polymerization methods gain competitive advantage. Lewis acid CRP systems that minimize hazardous waste generation and energy consumption are well-positioned to meet evolving regulatory requirements in major markets.
Scale-up and Industrial Implementation Considerations
The transition from laboratory-scale experiments to industrial production represents a critical challenge for Lewis acid-catalyzed controlled radical polymerization (CRP) processes. Current industrial implementation primarily focuses on batch processes, which offer simplicity but face limitations in heat transfer efficiency and product consistency at larger scales. Continuous flow reactors have emerged as promising alternatives, providing enhanced heat management and more uniform reaction conditions, particularly beneficial for Lewis acid systems that often exhibit temperature sensitivity.
Material compatibility presents significant engineering challenges when scaling up Lewis acid-catalyzed CRP. Many Lewis acids demonstrate corrosive properties toward standard industrial equipment, necessitating specialized reactor materials such as glass-lined vessels, high-grade stainless steel, or fluoropolymer coatings. These material requirements substantially impact capital expenditure calculations and must be factored into economic feasibility assessments.
Catalyst recovery and recycling systems represent another crucial consideration for industrial implementation. The high cost of many Lewis acid catalysts makes their recovery economically imperative. Advanced separation technologies including membrane filtration, selective precipitation, and supported catalyst systems have demonstrated promising results in pilot-scale operations, potentially reducing catalyst-related operational costs by 40-60% compared to single-use scenarios.
Safety protocols for large-scale handling of Lewis acids require comprehensive engineering controls. Many industrially relevant Lewis acids, such as aluminum chloride and titanium tetrachloride, react violently with moisture, necessitating stringent moisture exclusion systems. Automated handling systems that minimize worker exposure have become standard practice, complemented by sophisticated real-time monitoring of reaction parameters to prevent runaway reactions.
Regulatory compliance presents additional complexity for industrial implementation. Environmental regulations governing metal-containing waste streams have become increasingly stringent, particularly in Europe and North America. Successful industrial adoption requires development of closed-loop processing systems that minimize waste generation and maximize resource efficiency. Recent implementations have demonstrated that properly designed systems can reduce waste generation by up to 85% compared to conventional polymerization processes.
Economic considerations ultimately determine commercial viability. Current cost modeling indicates that Lewis acid-catalyzed CRP becomes economically competitive at production scales exceeding 500 tons annually, primarily due to the superior polymer properties commanding premium pricing. The capital investment required for specialized equipment is typically offset within 3-5 years through improved process efficiency and higher-value products, making this technology increasingly attractive for specialty polymer manufacturers seeking differentiation in competitive markets.
Material compatibility presents significant engineering challenges when scaling up Lewis acid-catalyzed CRP. Many Lewis acids demonstrate corrosive properties toward standard industrial equipment, necessitating specialized reactor materials such as glass-lined vessels, high-grade stainless steel, or fluoropolymer coatings. These material requirements substantially impact capital expenditure calculations and must be factored into economic feasibility assessments.
Catalyst recovery and recycling systems represent another crucial consideration for industrial implementation. The high cost of many Lewis acid catalysts makes their recovery economically imperative. Advanced separation technologies including membrane filtration, selective precipitation, and supported catalyst systems have demonstrated promising results in pilot-scale operations, potentially reducing catalyst-related operational costs by 40-60% compared to single-use scenarios.
Safety protocols for large-scale handling of Lewis acids require comprehensive engineering controls. Many industrially relevant Lewis acids, such as aluminum chloride and titanium tetrachloride, react violently with moisture, necessitating stringent moisture exclusion systems. Automated handling systems that minimize worker exposure have become standard practice, complemented by sophisticated real-time monitoring of reaction parameters to prevent runaway reactions.
Regulatory compliance presents additional complexity for industrial implementation. Environmental regulations governing metal-containing waste streams have become increasingly stringent, particularly in Europe and North America. Successful industrial adoption requires development of closed-loop processing systems that minimize waste generation and maximize resource efficiency. Recent implementations have demonstrated that properly designed systems can reduce waste generation by up to 85% compared to conventional polymerization processes.
Economic considerations ultimately determine commercial viability. Current cost modeling indicates that Lewis acid-catalyzed CRP becomes economically competitive at production scales exceeding 500 tons annually, primarily due to the superior polymer properties commanding premium pricing. The capital investment required for specialized equipment is typically offset within 3-5 years through improved process efficiency and higher-value products, making this technology increasingly attractive for specialty polymer manufacturers seeking differentiation in competitive markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!