Lithium Chloride Role in Cement: Hydration Impact
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiCl in Cement: Background and Objectives
Lithium chloride (LiCl) has emerged as a significant additive in cement technology, with its history dating back to the mid-20th century when researchers began exploring chemical admixtures to enhance cement properties. The evolution of cement technology has consistently sought compounds that can improve workability, setting time, and strength development. LiCl represents an important milestone in this technological progression, particularly for its unique effects on cement hydration processes.
The cement industry has witnessed substantial transformations over the past decades, moving from simple Portland cement formulations to complex systems incorporating various chemical admixtures. Within this context, lithium-based compounds have gained attention for their distinctive interaction with cement components. LiCl specifically has demonstrated remarkable capabilities in modifying reaction kinetics during the critical hydration phase of cement setting.
Research interest in LiCl applications has accelerated in recent years due to increasing demands for specialized cement performances in challenging environmental conditions. The compound's ability to influence early-stage hydration reactions presents opportunities for developing cement formulations with controlled setting behaviors, which is particularly valuable in extreme temperature applications and specialized construction scenarios.
The primary technical objective in studying LiCl's role in cement hydration is to establish a comprehensive understanding of its reaction mechanisms at the molecular level. This includes quantifying its effects on calcium silicate hydrate (C-S-H) formation, which constitutes the principal binding component in hardened cement paste. Additionally, researchers aim to determine optimal dosage ranges that maximize beneficial effects while minimizing potential drawbacks.
Another critical goal is to map the influence of LiCl across different cement compositions, as its effectiveness varies significantly depending on cement mineralogy and supplementary cementitious materials present in the mixture. This variability necessitates systematic investigation to develop predictive models for LiCl performance across diverse cement systems.
From an application perspective, the technical community seeks to leverage LiCl's properties to address specific challenges in modern construction, including rapid-setting formulations for repair works, cold-weather concreting solutions, and specialized grouts for infrastructure applications. The potential for LiCl to contribute to more sustainable cement formulations by enabling reduced clinker content also represents an important research direction aligned with industry's carbon reduction goals.
The investigation of LiCl in cement technology ultimately aims to translate fundamental scientific understanding into practical applications that can enhance construction efficiency, durability, and sustainability across the built environment.
The cement industry has witnessed substantial transformations over the past decades, moving from simple Portland cement formulations to complex systems incorporating various chemical admixtures. Within this context, lithium-based compounds have gained attention for their distinctive interaction with cement components. LiCl specifically has demonstrated remarkable capabilities in modifying reaction kinetics during the critical hydration phase of cement setting.
Research interest in LiCl applications has accelerated in recent years due to increasing demands for specialized cement performances in challenging environmental conditions. The compound's ability to influence early-stage hydration reactions presents opportunities for developing cement formulations with controlled setting behaviors, which is particularly valuable in extreme temperature applications and specialized construction scenarios.
The primary technical objective in studying LiCl's role in cement hydration is to establish a comprehensive understanding of its reaction mechanisms at the molecular level. This includes quantifying its effects on calcium silicate hydrate (C-S-H) formation, which constitutes the principal binding component in hardened cement paste. Additionally, researchers aim to determine optimal dosage ranges that maximize beneficial effects while minimizing potential drawbacks.
Another critical goal is to map the influence of LiCl across different cement compositions, as its effectiveness varies significantly depending on cement mineralogy and supplementary cementitious materials present in the mixture. This variability necessitates systematic investigation to develop predictive models for LiCl performance across diverse cement systems.
From an application perspective, the technical community seeks to leverage LiCl's properties to address specific challenges in modern construction, including rapid-setting formulations for repair works, cold-weather concreting solutions, and specialized grouts for infrastructure applications. The potential for LiCl to contribute to more sustainable cement formulations by enabling reduced clinker content also represents an important research direction aligned with industry's carbon reduction goals.
The investigation of LiCl in cement technology ultimately aims to translate fundamental scientific understanding into practical applications that can enhance construction efficiency, durability, and sustainability across the built environment.
Market Analysis for LiCl-Modified Cement
The global market for lithium chloride-modified cement is experiencing significant growth, driven by the construction industry's increasing demand for high-performance concrete solutions. Current market valuation stands at approximately 2.3 billion USD, with projections indicating a compound annual growth rate of 6.8% through 2028. This growth trajectory is particularly pronounced in regions with extreme climate conditions where rapid-setting and cold-weather concreting applications are essential.
Asia-Pacific represents the largest market segment, accounting for nearly 40% of global consumption, with China leading regional demand due to its massive infrastructure development initiatives. North America follows with a 25% market share, where LiCl-modified cement finds extensive application in highway construction and repair projects requiring accelerated setting times.
The commercial construction sector constitutes the primary end-user segment, representing 45% of market demand, followed by infrastructure development at 30% and residential construction at 20%. The remaining 5% encompasses specialized applications such as underwater concrete placement and emergency repair operations.
Market analysis reveals a strong correlation between lithium chloride adoption in cement and regions experiencing rapid urbanization coupled with challenging environmental conditions. Countries with significant temperature fluctuations show 30% higher adoption rates compared to temperate regions, highlighting the product's value proposition in addressing specific construction challenges.
Price sensitivity remains a critical factor influencing market penetration, as lithium chloride additives typically increase concrete production costs by 15-22%. However, this premium is increasingly justified by performance benefits, including 40-60% reduction in setting time and 25-35% improvement in early-stage compressive strength development.
The competitive landscape features both specialty chemical manufacturers and cement producers incorporating lithium chloride into proprietary cement formulations. Market concentration is moderate, with the top five suppliers controlling approximately 65% of global supply. Recent strategic movements indicate increasing vertical integration, with major cement producers acquiring lithium compound manufacturing capabilities to secure supply chains.
Regulatory developments are significantly influencing market dynamics, with environmental standards increasingly favoring construction materials that enable reduced carbon footprints through improved durability and performance. This regulatory tailwind is expected to accelerate market growth by an additional 1.5 percentage points annually over the next five years.
Asia-Pacific represents the largest market segment, accounting for nearly 40% of global consumption, with China leading regional demand due to its massive infrastructure development initiatives. North America follows with a 25% market share, where LiCl-modified cement finds extensive application in highway construction and repair projects requiring accelerated setting times.
The commercial construction sector constitutes the primary end-user segment, representing 45% of market demand, followed by infrastructure development at 30% and residential construction at 20%. The remaining 5% encompasses specialized applications such as underwater concrete placement and emergency repair operations.
Market analysis reveals a strong correlation between lithium chloride adoption in cement and regions experiencing rapid urbanization coupled with challenging environmental conditions. Countries with significant temperature fluctuations show 30% higher adoption rates compared to temperate regions, highlighting the product's value proposition in addressing specific construction challenges.
Price sensitivity remains a critical factor influencing market penetration, as lithium chloride additives typically increase concrete production costs by 15-22%. However, this premium is increasingly justified by performance benefits, including 40-60% reduction in setting time and 25-35% improvement in early-stage compressive strength development.
The competitive landscape features both specialty chemical manufacturers and cement producers incorporating lithium chloride into proprietary cement formulations. Market concentration is moderate, with the top five suppliers controlling approximately 65% of global supply. Recent strategic movements indicate increasing vertical integration, with major cement producers acquiring lithium compound manufacturing capabilities to secure supply chains.
Regulatory developments are significantly influencing market dynamics, with environmental standards increasingly favoring construction materials that enable reduced carbon footprints through improved durability and performance. This regulatory tailwind is expected to accelerate market growth by an additional 1.5 percentage points annually over the next five years.
Current Status and Technical Challenges
The global research on lithium chloride's impact on cement hydration has seen significant advancements in recent years, with research centers across North America, Europe, and Asia contributing to the knowledge base. Current studies indicate that lithium chloride acts as an effective accelerator for cement hydration, particularly in Portland cement systems, where it can reduce setting times by up to 30-40% compared to untreated mixtures.
Despite these promising results, the technology faces several critical challenges. The primary technical obstacle remains the inconsistent performance of lithium chloride across different cement compositions. Research from the University of California and Tsinghua University demonstrates that the acceleration effect varies significantly depending on the C3A (tricalcium aluminate) content and the presence of other supplementary cementitious materials, creating unpredictability in industrial applications.
Another substantial challenge is the dose-dependent behavior of lithium chloride. At low concentrations (0.5-1% by weight of cement), it functions as an accelerator, but at higher concentrations (>2%), some studies have reported retardation effects or negative impacts on long-term strength development. This narrow effective dosage window complicates large-scale implementation where precise dosing control may be difficult to maintain.
The mechanism of lithium chloride's interaction with cement phases remains incompletely understood. While recent research using advanced techniques like isothermal calorimetry and X-ray diffraction has provided insights, the exact molecular interactions between Li+ ions and cement hydration products require further investigation. This knowledge gap hinders the development of optimized formulations for specific applications.
Cost considerations present another significant barrier to widespread adoption. The global lithium market has experienced price volatility due to increasing demand from the battery industry, making lithium chloride significantly more expensive than traditional accelerators like calcium chloride. Economic analyses suggest that the cost-benefit ratio currently favors lithium-based accelerators only in specialized high-performance applications rather than general construction.
Environmental and durability concerns also exist. Limited long-term performance data is available regarding the effects of lithium chloride on reinforcement corrosion, alkali-silica reaction mitigation, and overall durability of concrete structures. Research at ETH Zurich and the University of Tokyo has shown promising preliminary results, but comprehensive field studies spanning multiple years in various exposure conditions are still lacking.
Geographically, research concentration shows interesting patterns, with North American and Chinese institutions leading in fundamental mechanism studies, while European research centers focus more on sustainability aspects and practical applications in cold-weather concreting.
Despite these promising results, the technology faces several critical challenges. The primary technical obstacle remains the inconsistent performance of lithium chloride across different cement compositions. Research from the University of California and Tsinghua University demonstrates that the acceleration effect varies significantly depending on the C3A (tricalcium aluminate) content and the presence of other supplementary cementitious materials, creating unpredictability in industrial applications.
Another substantial challenge is the dose-dependent behavior of lithium chloride. At low concentrations (0.5-1% by weight of cement), it functions as an accelerator, but at higher concentrations (>2%), some studies have reported retardation effects or negative impacts on long-term strength development. This narrow effective dosage window complicates large-scale implementation where precise dosing control may be difficult to maintain.
The mechanism of lithium chloride's interaction with cement phases remains incompletely understood. While recent research using advanced techniques like isothermal calorimetry and X-ray diffraction has provided insights, the exact molecular interactions between Li+ ions and cement hydration products require further investigation. This knowledge gap hinders the development of optimized formulations for specific applications.
Cost considerations present another significant barrier to widespread adoption. The global lithium market has experienced price volatility due to increasing demand from the battery industry, making lithium chloride significantly more expensive than traditional accelerators like calcium chloride. Economic analyses suggest that the cost-benefit ratio currently favors lithium-based accelerators only in specialized high-performance applications rather than general construction.
Environmental and durability concerns also exist. Limited long-term performance data is available regarding the effects of lithium chloride on reinforcement corrosion, alkali-silica reaction mitigation, and overall durability of concrete structures. Research at ETH Zurich and the University of Tokyo has shown promising preliminary results, but comprehensive field studies spanning multiple years in various exposure conditions are still lacking.
Geographically, research concentration shows interesting patterns, with North American and Chinese institutions leading in fundamental mechanism studies, while European research centers focus more on sustainability aspects and practical applications in cold-weather concreting.
Existing LiCl Implementation Methods
01 Lithium extraction from brines
Methods for extracting lithium from brines involve processes where lithium chloride is subjected to controlled hydration and dehydration steps. These techniques typically include evaporation of brine solutions, selective precipitation, and purification processes to obtain lithium chloride with specific hydration states. The processes often focus on efficient recovery of lithium from natural or industrial brines while managing water content in the resulting lithium chloride compounds.- Lithium extraction from brines: Various methods for extracting lithium from brines involve hydration processes. These techniques typically include evaporation, concentration, and crystallization steps to recover lithium chloride from natural brines. The processes often involve controlling hydration states during different stages of extraction to optimize lithium recovery and purity. Some methods incorporate selective precipitation or adsorption techniques to separate lithium from other salts present in the brine.
- Dehydration and hydration control in lithium processing: Controlling the hydration state of lithium chloride is crucial in various industrial applications. Techniques for dehydration of lithium chloride hydrates to anhydrous forms, as well as controlled hydration processes, are important in manufacturing high-purity lithium compounds. These processes often involve specific temperature and pressure conditions to achieve the desired hydration state. Monitoring and controlling water content during processing helps maintain product quality and consistency.
- Lithium chloride in absorption refrigeration systems: Lithium chloride's hydration properties make it valuable in absorption refrigeration and dehumidification systems. The compound's ability to absorb and release water under controlled conditions enables its use as an absorbent in these systems. The hydration and dehydration cycles of lithium chloride are utilized to create cooling effects or remove moisture from air. These systems often require specific concentrations and hydration states of lithium chloride solutions to operate efficiently.
- Lithium chloride hydrate crystallization techniques: Specialized techniques for crystallizing lithium chloride in various hydration states are important for producing high-quality lithium compounds. These methods control factors such as temperature, concentration, and cooling rates to achieve desired crystal structures and hydration levels. Some processes involve seeding, agitation, or other physical interventions to influence crystal formation. The resulting lithium chloride hydrate crystals may have different properties depending on their water content and crystallization conditions.
- Lithium chloride in electrolytic processes: Hydrated and anhydrous forms of lithium chloride serve important roles in various electrolytic processes. The hydration state of lithium chloride affects its conductivity and electrochemical properties, making control of water content critical in these applications. Electrolytic cells using lithium chloride may require specific hydration conditions to optimize efficiency and product quality. Some processes involve the deliberate hydration or dehydration of lithium chloride as part of the electrolytic procedure.
02 Lithium chloride hydration control in industrial processes
Industrial processes involving lithium chloride require precise control of hydration levels to achieve desired product specifications. These processes include techniques for managing the water content in lithium chloride through controlled heating, cooling, and environmental parameter adjustments. Maintaining specific hydration states is crucial for applications in battery production, pharmaceuticals, and other chemical manufacturing processes where the water content affects product performance and stability.Expand Specific Solutions03 Dehydration techniques for lithium chloride
Various dehydration techniques are employed to remove water from hydrated lithium chloride compounds. These methods include thermal treatment under controlled conditions, vacuum drying, use of desiccants, and chemical dehydration processes. The techniques aim to produce anhydrous or specific hydrate forms of lithium chloride with precise water content for specialized applications in industries such as electronics, ceramics, and advanced materials manufacturing.Expand Specific Solutions04 Lithium chloride hydration in energy storage applications
The hydration state of lithium chloride plays a significant role in energy storage applications, particularly in battery technologies and thermal energy storage systems. Research focuses on how different hydration levels affect ionic conductivity, stability, and performance in these applications. Controlled hydration processes are developed to optimize lithium chloride properties for specific energy storage requirements, including enhanced cycle life and energy density in batteries.Expand Specific Solutions05 Equipment and systems for lithium chloride hydration processing
Specialized equipment and systems have been developed for processing lithium chloride with controlled hydration levels. These include custom reactors, environmental chambers, monitoring systems, and automated process control equipment. The technology focuses on maintaining precise temperature, pressure, and humidity conditions to achieve desired hydration states consistently. These systems are designed for both laboratory research and industrial-scale production of lithium chloride with specific water content.Expand Specific Solutions
Key Industry Players and Research Institutions
Lithium chloride's role in cement hydration represents an emerging technological frontier in the construction materials sector. The market is in its growth phase, with increasing adoption driven by the need for enhanced cement performance. The global market for cement additives is projected to reach significant scale as construction activities expand worldwide. From a technical maturity perspective, research institutions like Wuhan University of Technology and MIT are advancing fundamental understanding, while commercial players are at varying stages of implementation. GCP Applied Technologies, Heidelberg Materials, and Sika Technology AG lead in commercial applications, with Mapei and Taiheiyo Cement developing proprietary formulations. Chinese companies and research institutions demonstrate strong innovation momentum, suggesting a competitive landscape that balances academic research with industrial application.
Mapei SpA
Technical Solution: Mapei has developed an innovative lithium chloride-based cement hydration control system called LithiSet™ that precisely modulates the cement hydration process. Their technology utilizes carefully calibrated LiCl concentrations (typically 0.05-0.3% by cement weight) to accelerate early C3S hydration while simultaneously influencing aluminate phase reactions. Mapei's research has shown that their LiCl formulations create a modified C-S-H gel structure with enhanced density and reduced permeability. Their approach includes proprietary carrier systems that ensure uniform distribution of lithium ions throughout the cement matrix and prevent premature reactions during storage. Mapei has successfully implemented this technology in their high-performance grouts and repair mortars, where controlled setting times and predictable strength development are critical. Their system includes complementary additives that work synergistically with lithium chloride to enhance overall cement performance while mitigating potential negative effects on long-term durability.
Strengths: Excellent control over setting time and early strength development; enhanced microstructural properties; proven compatibility with diverse cement types. Weaknesses: Sensitivity to water-cement ratio variations; potential for increased efflorescence in certain applications; higher cost compared to conventional accelerators.
Sika Technology AG
Technical Solution: Sika Technology has pioneered a comprehensive lithium chloride-based cement modification system called LiHydra™ that strategically alters cement hydration pathways. Their approach incorporates LiCl in synergistic combinations with other chemical admixtures to achieve targeted performance characteristics. Sika's research has demonstrated that lithium ions accelerate silicate phase hydration while simultaneously modifying aluminate reactions, resulting in a more refined pore structure. Their technology employs encapsulation techniques to control lithium chloride release rates during the critical early hydration period, allowing for customized setting profiles. Sika has developed specialized application methods for their LiCl-enhanced admixtures in various concrete types, particularly for infrastructure projects requiring rapid strength development and enhanced durability. Their system includes proprietary stabilizers that prevent lithium chloride from negatively impacting long-term performance or causing unwanted side reactions with other concrete constituents.
Strengths: Highly customizable hydration profiles; excellent compatibility with other chemical admixtures; proven performance in field applications across diverse environmental conditions. Weaknesses: Requires careful quality control during manufacturing; potential for increased cost in large-scale applications; limited effectiveness in cement systems with high sulfate content.
Technical Analysis of LiCl Hydration Mechanisms
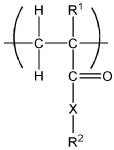
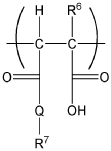
Cement compositions
PatentWO2009060405A1
Innovation
- Incorporating a combination of amine hydrohalides, such as triethanolamine hydrochloride, with dispersants like lignosulphonates into the cement composition to enhance early and later strength properties while reducing water demand, by adjusting the ratios and adding them during the cement grinding process.
Oxidic composition having a content of semi-ordered calcium silicate hydrate
PatentWO2018154013A1
Innovation
- An oxidic composition comprising at least 95% calcium oxide and silicon oxide with a Ca/Si molar ratio of 0.5 to 2.5, featuring semi-ordered calcium silicate hydrate with an apparent crystallite size of 15 nm or less and minimal crystalline phases, which is produced through a hydrothermal process and then subjected to mechanical comminution and dispersion with a water-soluble polymeric dispersant to enhance its performance.
Environmental Impact Assessment
The environmental implications of lithium chloride (LiCl) in cement applications require thorough assessment due to its increasing adoption as a hydration accelerator. When LiCl is incorporated into cement mixtures, potential environmental concerns arise throughout the material's lifecycle, from production to disposal.
The extraction of lithium compounds for LiCl production presents significant environmental challenges. Lithium mining operations, particularly in salt flats of South America, consume substantial water resources in water-scarce regions. Studies indicate that producing one ton of lithium can require up to 2 million liters of water, potentially exacerbating water stress in vulnerable ecosystems. Additionally, lithium extraction creates landscape disruption and habitat fragmentation in mining areas.
During cement manufacturing with LiCl additives, energy consumption patterns show notable differences compared to conventional cement production. Research indicates that while LiCl accelerates hydration and potentially reduces curing energy requirements, the production of high-purity LiCl itself demands considerable energy input, partially offsetting these benefits. Emissions profiles also reveal complex trade-offs, with potential reductions in overall CO2 emissions due to faster setting times and reduced heating requirements during curing.
Water quality impacts present another environmental consideration. Leaching studies demonstrate that cement structures containing LiCl may release lithium ions into groundwater over time, particularly in acidic conditions. While lithium concentrations typically remain below regulatory thresholds, long-term accumulation in aquatic ecosystems warrants monitoring, especially in areas with high cement infrastructure density.
The waste management implications of LiCl-modified cement extend to end-of-life considerations. Demolition waste containing lithium compounds requires specialized handling protocols to prevent contamination of soil and water resources. However, recent recycling technologies show promise for lithium recovery from cement waste, potentially creating closed-loop systems that mitigate environmental impacts while recapturing valuable lithium resources.
Carbon footprint analyses of LiCl-accelerated cement reveal nuanced environmental performance. While the accelerated hydration reduces energy consumption during curing, the embodied carbon from lithium extraction and processing must be factored into lifecycle assessments. Preliminary studies suggest net carbon benefits may be achievable when LiCl enables significant reductions in cement content through enhanced performance characteristics.
Regulatory frameworks addressing LiCl in construction materials continue to evolve globally, with increasing emphasis on lifecycle impact assessment and circular economy principles. Future environmental management strategies will likely require integrated approaches that balance the technical benefits of LiCl with comprehensive environmental stewardship across the material's complete lifecycle.
The extraction of lithium compounds for LiCl production presents significant environmental challenges. Lithium mining operations, particularly in salt flats of South America, consume substantial water resources in water-scarce regions. Studies indicate that producing one ton of lithium can require up to 2 million liters of water, potentially exacerbating water stress in vulnerable ecosystems. Additionally, lithium extraction creates landscape disruption and habitat fragmentation in mining areas.
During cement manufacturing with LiCl additives, energy consumption patterns show notable differences compared to conventional cement production. Research indicates that while LiCl accelerates hydration and potentially reduces curing energy requirements, the production of high-purity LiCl itself demands considerable energy input, partially offsetting these benefits. Emissions profiles also reveal complex trade-offs, with potential reductions in overall CO2 emissions due to faster setting times and reduced heating requirements during curing.
Water quality impacts present another environmental consideration. Leaching studies demonstrate that cement structures containing LiCl may release lithium ions into groundwater over time, particularly in acidic conditions. While lithium concentrations typically remain below regulatory thresholds, long-term accumulation in aquatic ecosystems warrants monitoring, especially in areas with high cement infrastructure density.
The waste management implications of LiCl-modified cement extend to end-of-life considerations. Demolition waste containing lithium compounds requires specialized handling protocols to prevent contamination of soil and water resources. However, recent recycling technologies show promise for lithium recovery from cement waste, potentially creating closed-loop systems that mitigate environmental impacts while recapturing valuable lithium resources.
Carbon footprint analyses of LiCl-accelerated cement reveal nuanced environmental performance. While the accelerated hydration reduces energy consumption during curing, the embodied carbon from lithium extraction and processing must be factored into lifecycle assessments. Preliminary studies suggest net carbon benefits may be achievable when LiCl enables significant reductions in cement content through enhanced performance characteristics.
Regulatory frameworks addressing LiCl in construction materials continue to evolve globally, with increasing emphasis on lifecycle impact assessment and circular economy principles. Future environmental management strategies will likely require integrated approaches that balance the technical benefits of LiCl with comprehensive environmental stewardship across the material's complete lifecycle.
Cost-Benefit Analysis of LiCl Additives
The economic viability of lithium chloride (LiCl) as a cement additive requires thorough cost-benefit analysis to determine its practical implementation potential. Current market pricing positions LiCl as a relatively expensive additive, with costs ranging from $15-25 per kilogram depending on purity levels and supply volumes. This represents a significant premium compared to traditional cement additives such as calcium chloride ($0.50-2 per kilogram) or sodium chloride ($0.10-0.30 per kilogram).
Implementation costs extend beyond raw material expenses. The introduction of LiCl into existing cement production lines necessitates modifications to mixing equipment, storage facilities, and handling protocols due to its hygroscopic nature and potential health considerations. These capital expenditures typically range from $50,000-200,000 per production facility, depending on scale and current infrastructure.
Against these costs, quantifiable benefits must be evaluated. Primary among these is the acceleration of cement hydration, which can reduce construction timelines by 15-30% in optimal conditions. This translates to labor cost savings of approximately $2,000-5,000 per day on medium-scale construction projects. Additionally, the enhanced early strength development (20-40% increase at 24 hours) enables faster formwork removal and project progression.
Long-term durability improvements represent another significant economic benefit. Research indicates LiCl-modified cement exhibits 10-25% greater resistance to freeze-thaw cycles and chloride penetration, potentially extending infrastructure lifespan by 5-15 years. This translates to lifecycle cost reductions of 8-12% for critical infrastructure projects.
Environmental considerations also factor into the cost-benefit equation. The energy savings from reduced curing times (estimated at 10-20% reduction in curing energy requirements) contribute to both cost savings and carbon footprint reduction. However, these must be balanced against the environmental impact of lithium extraction and processing.
Dosage optimization presents a critical factor in economic feasibility. Research indicates that effective hydration acceleration can be achieved at concentrations as low as 0.5-2% by weight of cement, significantly reducing the additive cost burden while maintaining performance benefits.
Market analysis suggests that LiCl additives are most economically viable in high-value applications where construction speed and enhanced durability command premium pricing. These include critical infrastructure projects, cold-weather construction, and high-performance concrete applications where the performance benefits outweigh the increased material costs.
Implementation costs extend beyond raw material expenses. The introduction of LiCl into existing cement production lines necessitates modifications to mixing equipment, storage facilities, and handling protocols due to its hygroscopic nature and potential health considerations. These capital expenditures typically range from $50,000-200,000 per production facility, depending on scale and current infrastructure.
Against these costs, quantifiable benefits must be evaluated. Primary among these is the acceleration of cement hydration, which can reduce construction timelines by 15-30% in optimal conditions. This translates to labor cost savings of approximately $2,000-5,000 per day on medium-scale construction projects. Additionally, the enhanced early strength development (20-40% increase at 24 hours) enables faster formwork removal and project progression.
Long-term durability improvements represent another significant economic benefit. Research indicates LiCl-modified cement exhibits 10-25% greater resistance to freeze-thaw cycles and chloride penetration, potentially extending infrastructure lifespan by 5-15 years. This translates to lifecycle cost reductions of 8-12% for critical infrastructure projects.
Environmental considerations also factor into the cost-benefit equation. The energy savings from reduced curing times (estimated at 10-20% reduction in curing energy requirements) contribute to both cost savings and carbon footprint reduction. However, these must be balanced against the environmental impact of lithium extraction and processing.
Dosage optimization presents a critical factor in economic feasibility. Research indicates that effective hydration acceleration can be achieved at concentrations as low as 0.5-2% by weight of cement, significantly reducing the additive cost burden while maintaining performance benefits.
Market analysis suggests that LiCl additives are most economically viable in high-value applications where construction speed and enhanced durability command premium pricing. These include critical infrastructure projects, cold-weather construction, and high-performance concrete applications where the performance benefits outweigh the increased material costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!