Lithium Hydroxide Purification: Techniques For High Purity
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Purification Background and Objectives
Lithium hydroxide (LiOH) has emerged as a critical material in the global energy transition, particularly for its role in high-performance lithium-ion batteries that power electric vehicles and energy storage systems. The evolution of lithium hydroxide purification techniques spans several decades, with significant advancements occurring in response to increasing demand for higher purity grades required by modern battery technologies.
Historically, lithium hydroxide production began with relatively simple precipitation methods from lithium carbonate in the mid-20th century. The technical landscape has since evolved dramatically, moving from basic chemical processes to sophisticated multi-stage purification systems incorporating advanced separation technologies, membrane filtration, and crystallization techniques.
The current technological trajectory is driven by the battery industry's stringent requirements for battery-grade lithium hydroxide with purity levels exceeding 99.5%, with particular emphasis on minimizing metallic impurities such as sodium, potassium, calcium, and transition metals that can significantly impact battery performance and longevity.
Market forecasts indicate that demand for high-purity lithium hydroxide will grow at a CAGR of approximately 18-20% through 2030, outpacing supply capabilities and creating a technological imperative for more efficient purification methods. This growth is primarily fueled by the electric vehicle revolution and grid-scale energy storage deployments worldwide.
The primary technical objectives in lithium hydroxide purification center around achieving several critical parameters: increasing final product purity to 99.9% or higher, reducing energy consumption in purification processes, minimizing chemical reagent usage, developing environmentally sustainable methods, and establishing economically viable recovery of lithium from secondary sources including battery recycling streams.
Recent innovations have focused on continuous flow processing systems, advanced ion exchange technologies, electrochemical purification methods, and supercritical fluid extraction techniques. These approaches aim to overcome traditional bottlenecks in batch processing while reducing environmental impacts associated with conventional chemical purification routes.
Regulatory frameworks are increasingly influencing technical development in this field, with particular emphasis on reducing water consumption, minimizing hazardous waste generation, and lowering the carbon footprint of purification processes. This regulatory pressure is accelerating research into green chemistry approaches and closed-loop production systems.
The ultimate technical goal remains the development of scalable, energy-efficient purification technologies capable of consistently producing battery-grade lithium hydroxide from diverse feedstocks, including both primary lithium sources and recycled materials, to support the circular economy objectives of the clean energy transition.
Historically, lithium hydroxide production began with relatively simple precipitation methods from lithium carbonate in the mid-20th century. The technical landscape has since evolved dramatically, moving from basic chemical processes to sophisticated multi-stage purification systems incorporating advanced separation technologies, membrane filtration, and crystallization techniques.
The current technological trajectory is driven by the battery industry's stringent requirements for battery-grade lithium hydroxide with purity levels exceeding 99.5%, with particular emphasis on minimizing metallic impurities such as sodium, potassium, calcium, and transition metals that can significantly impact battery performance and longevity.
Market forecasts indicate that demand for high-purity lithium hydroxide will grow at a CAGR of approximately 18-20% through 2030, outpacing supply capabilities and creating a technological imperative for more efficient purification methods. This growth is primarily fueled by the electric vehicle revolution and grid-scale energy storage deployments worldwide.
The primary technical objectives in lithium hydroxide purification center around achieving several critical parameters: increasing final product purity to 99.9% or higher, reducing energy consumption in purification processes, minimizing chemical reagent usage, developing environmentally sustainable methods, and establishing economically viable recovery of lithium from secondary sources including battery recycling streams.
Recent innovations have focused on continuous flow processing systems, advanced ion exchange technologies, electrochemical purification methods, and supercritical fluid extraction techniques. These approaches aim to overcome traditional bottlenecks in batch processing while reducing environmental impacts associated with conventional chemical purification routes.
Regulatory frameworks are increasingly influencing technical development in this field, with particular emphasis on reducing water consumption, minimizing hazardous waste generation, and lowering the carbon footprint of purification processes. This regulatory pressure is accelerating research into green chemistry approaches and closed-loop production systems.
The ultimate technical goal remains the development of scalable, energy-efficient purification technologies capable of consistently producing battery-grade lithium hydroxide from diverse feedstocks, including both primary lithium sources and recycled materials, to support the circular economy objectives of the clean energy transition.
Market Demand Analysis for High-Purity Lithium Hydroxide
The global market for high-purity lithium hydroxide has experienced exponential growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry. As of 2023, the market size for high-purity lithium hydroxide exceeds $7 billion globally, with projections indicating a compound annual growth rate of 18-20% through 2030, potentially reaching $25 billion by the end of the decade.
The demand for battery-grade lithium hydroxide (minimum 99.5% purity) has become particularly acute as manufacturers shift toward nickel-rich cathode materials for high-performance lithium-ion batteries. These advanced cathode formulations, such as NMC 811 (nickel-manganese-cobalt in 8:1:1 ratio) and NCA (nickel-cobalt-aluminum), require exceptionally pure lithium hydroxide rather than lithium carbonate due to superior electrochemical performance and thermal stability characteristics.
Major automotive manufacturers including Tesla, Volkswagen Group, and BYD have announced significant production increases for electric vehicles, with combined targets exceeding 15 million units annually by 2025. This manufacturing scale-up directly translates to heightened demand for high-purity lithium hydroxide, with each electric vehicle requiring between 40-80 kg of lithium chemicals depending on battery capacity.
Beyond the automotive sector, emerging applications in grid-scale energy storage systems are creating additional demand vectors. Utility companies worldwide are deploying large-scale battery installations to support renewable energy integration, with installed capacity growing at approximately 30% annually. These systems increasingly specify high-purity lithium hydroxide-based batteries for their superior cycle life and energy density.
Regional analysis reveals China currently dominates both production and consumption of high-purity lithium hydroxide, accounting for approximately 60% of global manufacturing capacity. However, significant market diversification is underway, with major investments in production facilities across Australia, North America, and Europe aimed at reducing supply chain vulnerabilities.
The price dynamics for high-purity lithium hydroxide demonstrate considerable volatility, with spot prices ranging from $15,000 to $80,000 per metric ton over the past three years. This volatility underscores the critical supply-demand imbalance and highlights the strategic importance of developing more efficient purification technologies to increase production capacity and stabilize market conditions.
Industry surveys indicate that battery manufacturers are willing to pay premium prices for ultra-high-purity lithium hydroxide (99.9%+) that exceeds current industry standards, as even minor impurities can significantly impact battery performance, safety, and longevity. This quality-driven premium segment represents a particularly attractive market opportunity for advanced purification technologies.
The demand for battery-grade lithium hydroxide (minimum 99.5% purity) has become particularly acute as manufacturers shift toward nickel-rich cathode materials for high-performance lithium-ion batteries. These advanced cathode formulations, such as NMC 811 (nickel-manganese-cobalt in 8:1:1 ratio) and NCA (nickel-cobalt-aluminum), require exceptionally pure lithium hydroxide rather than lithium carbonate due to superior electrochemical performance and thermal stability characteristics.
Major automotive manufacturers including Tesla, Volkswagen Group, and BYD have announced significant production increases for electric vehicles, with combined targets exceeding 15 million units annually by 2025. This manufacturing scale-up directly translates to heightened demand for high-purity lithium hydroxide, with each electric vehicle requiring between 40-80 kg of lithium chemicals depending on battery capacity.
Beyond the automotive sector, emerging applications in grid-scale energy storage systems are creating additional demand vectors. Utility companies worldwide are deploying large-scale battery installations to support renewable energy integration, with installed capacity growing at approximately 30% annually. These systems increasingly specify high-purity lithium hydroxide-based batteries for their superior cycle life and energy density.
Regional analysis reveals China currently dominates both production and consumption of high-purity lithium hydroxide, accounting for approximately 60% of global manufacturing capacity. However, significant market diversification is underway, with major investments in production facilities across Australia, North America, and Europe aimed at reducing supply chain vulnerabilities.
The price dynamics for high-purity lithium hydroxide demonstrate considerable volatility, with spot prices ranging from $15,000 to $80,000 per metric ton over the past three years. This volatility underscores the critical supply-demand imbalance and highlights the strategic importance of developing more efficient purification technologies to increase production capacity and stabilize market conditions.
Industry surveys indicate that battery manufacturers are willing to pay premium prices for ultra-high-purity lithium hydroxide (99.9%+) that exceeds current industry standards, as even minor impurities can significantly impact battery performance, safety, and longevity. This quality-driven premium segment represents a particularly attractive market opportunity for advanced purification technologies.
Current Purification Technologies and Challenges
The lithium hydroxide purification landscape is currently dominated by several established technologies, each with specific advantages and limitations. Conventional precipitation methods remain widely utilized, involving the addition of chemical reagents to form insoluble compounds with impurities. This approach, while cost-effective, often struggles to achieve the ultra-high purity levels (>99.9%) required for advanced battery applications, particularly in removing elements like sodium, potassium, and calcium that share similar chemical properties with lithium.
Ion exchange technologies have gained significant traction in recent years, offering superior selectivity for impurity removal. These systems utilize specialized resins that preferentially bind to specific contaminants while allowing lithium ions to pass through. However, challenges persist in resin degradation over multiple regeneration cycles, particularly when processing solutions with high concentrations of competing ions, leading to diminished efficiency and increased operational costs over time.
Membrane-based separation processes, including nanofiltration and electrodialysis, represent another important purification avenue. These technologies leverage size exclusion and charge-based separation principles to isolate lithium hydroxide from contaminants. While offering excellent scalability and continuous operation capabilities, membrane fouling remains a persistent challenge, especially when processing solutions derived from diverse lithium sources with varying impurity profiles.
Solvent extraction has emerged as a promising technique for achieving high-purity lithium hydroxide, utilizing selective organic extractants to separate lithium from impurities. This method demonstrates exceptional selectivity for lithium over sodium and magnesium, critical for battery-grade applications. However, the technology faces implementation barriers related to solvent loss, environmental concerns regarding organic solvents, and complex multi-stage processing requirements.
Crystallization techniques, particularly fractional crystallization, leverage the differential solubility of lithium hydroxide and its impurities across temperature gradients. While capable of producing high-purity products, these methods are energy-intensive and face challenges in controlling crystal morphology and size distribution, which directly impact downstream processing and final product quality.
A significant industry-wide challenge remains the development of purification technologies capable of handling the increasingly diverse lithium feedstocks entering the supply chain. Traditional processes optimized for conventional brine or mineral sources often perform suboptimally when processing recycled materials or unconventional resources, which contain different impurity profiles and concentrations. This feedstock variability necessitates more adaptive and robust purification approaches.
Additionally, current purification technologies face mounting pressure to reduce environmental footprints, particularly regarding water consumption, chemical usage, and energy requirements. The industry is actively seeking more sustainable alternatives that maintain high purity standards while minimizing resource intensity and waste generation.
Ion exchange technologies have gained significant traction in recent years, offering superior selectivity for impurity removal. These systems utilize specialized resins that preferentially bind to specific contaminants while allowing lithium ions to pass through. However, challenges persist in resin degradation over multiple regeneration cycles, particularly when processing solutions with high concentrations of competing ions, leading to diminished efficiency and increased operational costs over time.
Membrane-based separation processes, including nanofiltration and electrodialysis, represent another important purification avenue. These technologies leverage size exclusion and charge-based separation principles to isolate lithium hydroxide from contaminants. While offering excellent scalability and continuous operation capabilities, membrane fouling remains a persistent challenge, especially when processing solutions derived from diverse lithium sources with varying impurity profiles.
Solvent extraction has emerged as a promising technique for achieving high-purity lithium hydroxide, utilizing selective organic extractants to separate lithium from impurities. This method demonstrates exceptional selectivity for lithium over sodium and magnesium, critical for battery-grade applications. However, the technology faces implementation barriers related to solvent loss, environmental concerns regarding organic solvents, and complex multi-stage processing requirements.
Crystallization techniques, particularly fractional crystallization, leverage the differential solubility of lithium hydroxide and its impurities across temperature gradients. While capable of producing high-purity products, these methods are energy-intensive and face challenges in controlling crystal morphology and size distribution, which directly impact downstream processing and final product quality.
A significant industry-wide challenge remains the development of purification technologies capable of handling the increasingly diverse lithium feedstocks entering the supply chain. Traditional processes optimized for conventional brine or mineral sources often perform suboptimally when processing recycled materials or unconventional resources, which contain different impurity profiles and concentrations. This feedstock variability necessitates more adaptive and robust purification approaches.
Additionally, current purification technologies face mounting pressure to reduce environmental footprints, particularly regarding water consumption, chemical usage, and energy requirements. The industry is actively seeking more sustainable alternatives that maintain high purity standards while minimizing resource intensity and waste generation.
Mainstream Purification Techniques and Processes
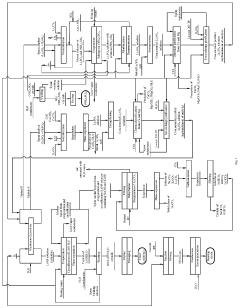
01 Purification methods for lithium hydroxide
Various methods are employed to purify lithium hydroxide to achieve high purity levels. These include crystallization, filtration, and washing processes to remove impurities. Advanced purification techniques can involve multiple stages of processing to progressively eliminate contaminants, resulting in battery-grade lithium hydroxide suitable for high-performance applications.- Purification methods for lithium hydroxide: Various methods are employed to purify lithium hydroxide, including crystallization, filtration, and washing processes. These techniques help remove impurities such as sodium, potassium, calcium, and other metal ions that can affect the quality of lithium hydroxide. Advanced purification methods may involve multiple crystallization steps, selective precipitation, or ion exchange to achieve high purity levels required for battery-grade applications.
- Battery-grade lithium hydroxide specifications: Battery-grade lithium hydroxide requires specific purity levels, typically exceeding 99.5% with strict limits on impurities. For use in high-performance lithium-ion batteries, particularly important are low levels of sodium, potassium, calcium, iron, and other transition metals that can negatively impact battery performance and lifespan. Specifications often include maximum allowable concentrations for each impurity element and minimum overall purity requirements.
- Analytical methods for purity determination: Various analytical techniques are used to determine the purity of lithium hydroxide, including inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy (AAS), and titration methods. These techniques allow for precise measurement of lithium content and detection of trace impurities. Quality control protocols often involve multiple complementary analytical methods to ensure comprehensive purity assessment and consistency across production batches.
- Production of high-purity lithium hydroxide from lithium sources: High-purity lithium hydroxide can be produced from various lithium sources including brines, spodumene, and recycled lithium materials. The production processes typically involve extraction, conversion, and purification steps designed to minimize impurities. Advanced techniques such as direct lithium extraction from brines, optimized roasting and leaching of lithium-containing minerals, and specialized conversion processes help achieve higher purity levels while improving production efficiency.
- Impact of impurities on lithium hydroxide applications: The presence of impurities in lithium hydroxide can significantly impact its performance in various applications, particularly in battery manufacturing. Impurities can affect electrochemical stability, cycle life, and safety of lithium-ion batteries. For example, sodium and calcium impurities may reduce battery capacity and increase internal resistance, while transition metal impurities can catalyze unwanted side reactions. Understanding these impacts drives the development of increasingly stringent purity specifications for different applications.
02 Battery-grade lithium hydroxide specifications
Battery-grade lithium hydroxide requires specific purity levels to ensure optimal performance in lithium-ion batteries. Typically, battery-grade specifications demand purity exceeding 99.5%, with strict limits on metallic impurities such as sodium, potassium, calcium, and heavy metals. These specifications are critical for battery performance, longevity, and safety in electric vehicle and energy storage applications.Expand Specific Solutions03 Analytical methods for purity determination
Various analytical techniques are used to determine the purity of lithium hydroxide. These include titration methods, atomic absorption spectroscopy, inductively coupled plasma mass spectrometry (ICP-MS), and X-ray fluorescence. These methods allow for precise quantification of lithium hydroxide content and detection of trace impurities at parts per million levels, ensuring quality control in production processes.Expand Specific Solutions04 Production of high-purity lithium hydroxide from lithium resources
High-purity lithium hydroxide can be produced from various lithium resources including brines, spodumene, and recycled materials. The production processes typically involve extraction, conversion, and purification steps. Advanced technologies focus on minimizing environmental impact while maximizing purity, with some methods achieving purity levels exceeding 99.9% through optimized process parameters and innovative extraction techniques.Expand Specific Solutions05 Impurity control in lithium hydroxide production
Controlling impurities during lithium hydroxide production is critical for achieving high purity. This involves careful selection of raw materials, process optimization to prevent contamination, and implementation of quality control measures throughout the production chain. Specific techniques include selective precipitation of impurities, ion exchange processes, and membrane separation technologies to remove unwanted elements and compounds.Expand Specific Solutions
Key Industry Players in Lithium Hydroxide Production
The lithium hydroxide purification market is currently in a growth phase, driven by increasing demand for high-purity materials in electric vehicle batteries. The global market size is expanding rapidly, projected to reach several billion dollars by 2030 with a CAGR exceeding 15%. Technologically, the field shows varying maturity levels, with established players like Ganfeng Lithium, Tianqi Lithium, and POSCO Holdings demonstrating advanced purification capabilities. Companies such as Sumitomo Metal Mining and BASF are leveraging their chemical expertise to develop proprietary purification methods, while newer entrants like Nemaska Lithium and Terralithium are introducing innovative techniques. The competitive landscape features both traditional mining companies expanding downstream and specialized chemical firms focusing on high-purity processing technologies, creating a dynamic environment for technological advancement.
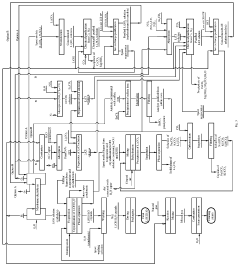
Ganfeng Lithium Group Co., Ltd.
Technical Solution: Ganfeng Lithium employs a multi-stage purification process for high-purity lithium hydroxide production. Their technology combines solvent extraction and ion exchange methods with advanced crystallization techniques. The process begins with lithium-rich brine or ore processing, followed by primary impurity removal using selective precipitation. The intermediate product undergoes ion exchange purification where specialized resins selectively capture lithium ions while rejecting impurities. Their proprietary crystallization technology then produces battery-grade lithium hydroxide (>99.5% purity) through controlled temperature and pressure conditions. Ganfeng has also developed membrane filtration systems that can remove metallic impurities down to parts-per-billion levels, essential for high-performance battery applications. Their closed-loop water recycling system reduces environmental impact while maintaining product consistency.
Strengths: Integrated supply chain from raw material to finished product enables quality control throughout the process; advanced automation systems ensure consistent high purity levels; proprietary crystallization technology achieves industry-leading purity levels. Weaknesses: Energy-intensive purification processes increase production costs; complex multi-stage system requires sophisticated maintenance and monitoring.
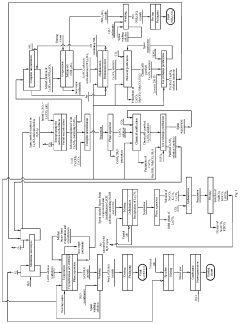
Chengdu Chemphys Chemical Industry Co. Ltd.
Technical Solution: Chengdu Chemphys has developed a specialized recrystallization technology for ultra-high purity lithium hydroxide production. Their process begins with conventional lithium hydroxide that undergoes a proprietary dissolution and recrystallization sequence under carefully controlled conditions. The company employs a gradient temperature crystallization approach where temperature is precisely manipulated to selectively crystallize lithium hydroxide while leaving impurities in solution. A key innovation is their use of seed crystal technology with precisely engineered crystal structures that promote epitaxial growth of high-purity lithium hydroxide monohydrate crystals. The process incorporates multiple crystallization stages with progressively stricter purity requirements, achieving 99.99% purity in the final product. Chemphys has also developed specialized analytical techniques for detecting trace impurities at sub-ppm levels, allowing for stringent quality control. Their system includes a closed-loop solvent recovery system that recycles over 95% of process solvents, minimizing environmental impact while maintaining economic viability.
Strengths: Recrystallization approach can achieve extremely high purity levels suitable for advanced battery applications; process can be applied to various lithium hydroxide feedstocks regardless of initial production method; highly efficient solvent recovery system reduces operational costs. Weaknesses: Multiple crystallization stages increase production time and energy consumption; requires highly skilled operators to maintain optimal crystallization conditions.
Critical Patents and Innovations in Purification Technology
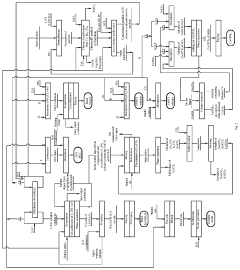
Method for producing high purity lithium hydroxide monohydrate
PatentPendingUS20240200206A1
Innovation
- The method involves membrane electrolysis of aqueous solutions of lithium sulfate or chloride, recycling sodium and potassium from the catholyte, using nickel-plated stainless steel cathodes, and employing specific cation-exchange membranes, along with chemical and ion exchange purification to produce high purity lithium hydroxide monohydrate, expanding the range of raw materials and coproducts while reducing waste and improving environmental performance.
Process for the purification of lithium salts
PatentWO2021099333A1
Innovation
- A process involving the dissolution of crude lithium hydroxide in a lower alcohol, followed by solid-liquid separation and additional purification steps, effectively removes fluoride impurities and achieves high purity lithium hydroxide production.
Environmental Impact and Sustainability Considerations
The purification of lithium hydroxide carries significant environmental implications that must be addressed for sustainable industry development. Traditional purification methods often involve extensive water usage, with some processes requiring up to 500,000 gallons of water per ton of lithium produced. This substantial water consumption poses serious challenges in regions where lithium extraction occurs, many of which are already water-stressed areas such as the lithium triangle in South America.
Chemical processes employed in conventional purification methods generate considerable waste streams containing contaminants like sodium, potassium, calcium, and magnesium salts. These waste products require proper management to prevent soil contamination and groundwater pollution. Additionally, the energy intensity of high-temperature calcination and crystallization processes contributes significantly to the carbon footprint of lithium hydroxide production, with estimates suggesting 5-15 tons of CO2 emissions per ton of battery-grade lithium hydroxide.
Recent advancements in purification technologies demonstrate promising sustainability improvements. Membrane-based separation techniques have shown potential to reduce water consumption by 30-40% compared to traditional methods. Similarly, solvent extraction processes utilizing bio-derived solvents present lower toxicity profiles while maintaining separation efficiency. These innovations align with circular economy principles by minimizing resource inputs and waste generation.
Life cycle assessments of newer purification methods indicate that implementing closed-loop water systems can recover up to 85% of process water, substantially reducing freshwater demand. Furthermore, integration of renewable energy sources for powering purification operations can decrease carbon emissions by 40-60%, depending on the specific technology and implementation context.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in critical mineral processing. The European Union's Battery Directive and similar regulations in North America and Asia are establishing stringent requirements for environmental impact reporting and sustainability metrics throughout the battery supply chain, including lithium hydroxide production.
Industry leaders are responding by developing proprietary purification technologies that not only achieve higher purity levels but also demonstrate improved environmental performance. Companies implementing these advanced purification methods report competitive advantages through reduced compliance costs, improved stakeholder relations, and access to premium markets where environmental credentials carry significant value.
Chemical processes employed in conventional purification methods generate considerable waste streams containing contaminants like sodium, potassium, calcium, and magnesium salts. These waste products require proper management to prevent soil contamination and groundwater pollution. Additionally, the energy intensity of high-temperature calcination and crystallization processes contributes significantly to the carbon footprint of lithium hydroxide production, with estimates suggesting 5-15 tons of CO2 emissions per ton of battery-grade lithium hydroxide.
Recent advancements in purification technologies demonstrate promising sustainability improvements. Membrane-based separation techniques have shown potential to reduce water consumption by 30-40% compared to traditional methods. Similarly, solvent extraction processes utilizing bio-derived solvents present lower toxicity profiles while maintaining separation efficiency. These innovations align with circular economy principles by minimizing resource inputs and waste generation.
Life cycle assessments of newer purification methods indicate that implementing closed-loop water systems can recover up to 85% of process water, substantially reducing freshwater demand. Furthermore, integration of renewable energy sources for powering purification operations can decrease carbon emissions by 40-60%, depending on the specific technology and implementation context.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in critical mineral processing. The European Union's Battery Directive and similar regulations in North America and Asia are establishing stringent requirements for environmental impact reporting and sustainability metrics throughout the battery supply chain, including lithium hydroxide production.
Industry leaders are responding by developing proprietary purification technologies that not only achieve higher purity levels but also demonstrate improved environmental performance. Companies implementing these advanced purification methods report competitive advantages through reduced compliance costs, improved stakeholder relations, and access to premium markets where environmental credentials carry significant value.
Quality Control and Testing Standards for High-Purity Products
Quality control and testing standards are paramount in the production of high-purity lithium hydroxide, as even minor impurities can significantly impact performance in battery applications. The industry has established rigorous protocols that combine multiple analytical techniques to ensure product consistency and reliability.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) serve as primary methods for detecting metallic impurities at parts-per-billion levels. These techniques can identify trace elements such as sodium, potassium, calcium, and transition metals that may compromise battery performance.
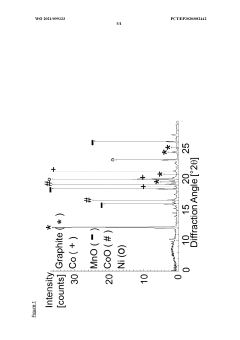
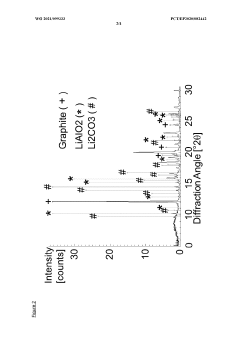
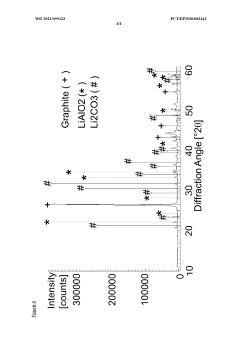
X-Ray Diffraction (XRD) analysis provides critical information about the crystalline structure of lithium hydroxide, helping manufacturers verify phase purity and detect unwanted crystalline impurities. This technique is particularly valuable for identifying structural anomalies that might affect electrochemical performance.
Particle size distribution analysis using laser diffraction techniques has become standard practice, as particle morphology directly impacts dissolution rates and reactivity in battery manufacturing processes. The industry typically requires D50 values below 10 microns for high-grade material.
Moisture content determination through Karl Fischer titration represents another critical quality parameter, with specifications typically requiring less than 0.5% water content for battery-grade lithium hydroxide. Excessive moisture can lead to handling difficulties and reduced shelf life.
Certification standards have evolved significantly, with organizations like IATF 16949 for automotive supply chains and ISO 9001:2015 providing frameworks for quality management systems. Battery manufacturers increasingly require suppliers to maintain these certifications as minimum entry requirements.
Statistical Process Control (SPC) methodologies have been widely adopted to monitor production consistency, with control charts tracking key parameters to detect process drift before specifications are violated. This proactive approach has substantially reduced batch-to-batch variability.
Real-time monitoring technologies are gaining traction, with in-line spectroscopic methods allowing continuous quality verification rather than relying solely on batch sampling. These systems can trigger automatic process adjustments when parameters begin to drift outside optimal ranges.
Third-party verification through independent laboratories has become standard practice, providing customers with additional confidence in product quality claims. Leading producers now routinely submit samples to multiple laboratories to verify consistency of analytical results.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) serve as primary methods for detecting metallic impurities at parts-per-billion levels. These techniques can identify trace elements such as sodium, potassium, calcium, and transition metals that may compromise battery performance.
X-Ray Diffraction (XRD) analysis provides critical information about the crystalline structure of lithium hydroxide, helping manufacturers verify phase purity and detect unwanted crystalline impurities. This technique is particularly valuable for identifying structural anomalies that might affect electrochemical performance.
Particle size distribution analysis using laser diffraction techniques has become standard practice, as particle morphology directly impacts dissolution rates and reactivity in battery manufacturing processes. The industry typically requires D50 values below 10 microns for high-grade material.
Moisture content determination through Karl Fischer titration represents another critical quality parameter, with specifications typically requiring less than 0.5% water content for battery-grade lithium hydroxide. Excessive moisture can lead to handling difficulties and reduced shelf life.
Certification standards have evolved significantly, with organizations like IATF 16949 for automotive supply chains and ISO 9001:2015 providing frameworks for quality management systems. Battery manufacturers increasingly require suppliers to maintain these certifications as minimum entry requirements.
Statistical Process Control (SPC) methodologies have been widely adopted to monitor production consistency, with control charts tracking key parameters to detect process drift before specifications are violated. This proactive approach has substantially reduced batch-to-batch variability.
Real-time monitoring technologies are gaining traction, with in-line spectroscopic methods allowing continuous quality verification rather than relying solely on batch sampling. These systems can trigger automatic process adjustments when parameters begin to drift outside optimal ranges.
Third-party verification through independent laboratories has become standard practice, providing customers with additional confidence in product quality claims. Leading producers now routinely submit samples to multiple laboratories to verify consistency of analytical results.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!