Lithium Hydroxide Vs Sodium Hydroxide: Thermal Reaction Analysis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium and Sodium Hydroxide Thermal Reaction Background
The thermal reactions of lithium hydroxide (LiOH) and sodium hydroxide (NaOH) have been subjects of scientific interest since the early 20th century, with significant advancements in understanding occurring during the 1950s and 1960s with the rise of nuclear and space technologies. These compounds, while sharing similar chemical properties as alkali metal hydroxides, exhibit distinct thermal behaviors that have profound implications for various industrial applications.
Lithium hydroxide's thermal decomposition pathway begins at approximately 450°C, where it decomposes to form lithium oxide (Li₂O) and water vapor. This reaction is particularly notable for its endothermic nature and the high purity of the resulting oxide. The thermal stability of LiOH has made it particularly valuable in applications requiring controlled heat absorption, such as in spacecraft carbon dioxide scrubbers and certain battery technologies.
Sodium hydroxide, by contrast, exhibits a more complex thermal profile. It melts at around 318°C without decomposition, and only begins significant decomposition at temperatures exceeding 800°C, producing sodium oxide (Na₂O) and water vapor. The higher thermal stability of NaOH compared to LiOH represents a fundamental difference that has shaped their respective industrial applications.
The evolution of thermal analysis techniques, particularly Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), has enabled more precise characterization of these thermal reactions since the 1980s. These advancements have revealed subtle aspects of the decomposition mechanisms, including intermediate phases and reaction kinetics that were previously undetectable.
Recent research trends since 2010 have focused on the nanoscale properties of these hydroxides and their thermal behaviors under various atmospheric conditions. The influence of particle size, crystallinity, and presence of impurities on thermal decomposition pathways has emerged as a critical area of study, particularly for applications in energy storage and conversion.
The technological goals in this field have evolved from basic understanding of thermal properties to engineered applications that leverage the specific thermal characteristics of each hydroxide. For LiOH, this includes optimization for CO₂ capture systems and high-temperature batteries, while NaOH research has focused on heat transfer applications and catalytic processes where its higher thermal stability provides advantages.
Environmental considerations have also become increasingly important in the study of these thermal reactions, with particular attention to the energy efficiency of production processes and the environmental impact of waste products. This represents a shift from purely technical performance metrics to a more holistic evaluation of these materials within sustainable industrial systems.
Lithium hydroxide's thermal decomposition pathway begins at approximately 450°C, where it decomposes to form lithium oxide (Li₂O) and water vapor. This reaction is particularly notable for its endothermic nature and the high purity of the resulting oxide. The thermal stability of LiOH has made it particularly valuable in applications requiring controlled heat absorption, such as in spacecraft carbon dioxide scrubbers and certain battery technologies.
Sodium hydroxide, by contrast, exhibits a more complex thermal profile. It melts at around 318°C without decomposition, and only begins significant decomposition at temperatures exceeding 800°C, producing sodium oxide (Na₂O) and water vapor. The higher thermal stability of NaOH compared to LiOH represents a fundamental difference that has shaped their respective industrial applications.
The evolution of thermal analysis techniques, particularly Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), has enabled more precise characterization of these thermal reactions since the 1980s. These advancements have revealed subtle aspects of the decomposition mechanisms, including intermediate phases and reaction kinetics that were previously undetectable.
Recent research trends since 2010 have focused on the nanoscale properties of these hydroxides and their thermal behaviors under various atmospheric conditions. The influence of particle size, crystallinity, and presence of impurities on thermal decomposition pathways has emerged as a critical area of study, particularly for applications in energy storage and conversion.
The technological goals in this field have evolved from basic understanding of thermal properties to engineered applications that leverage the specific thermal characteristics of each hydroxide. For LiOH, this includes optimization for CO₂ capture systems and high-temperature batteries, while NaOH research has focused on heat transfer applications and catalytic processes where its higher thermal stability provides advantages.
Environmental considerations have also become increasingly important in the study of these thermal reactions, with particular attention to the energy efficiency of production processes and the environmental impact of waste products. This represents a shift from purely technical performance metrics to a more holistic evaluation of these materials within sustainable industrial systems.
Market Applications and Demand Analysis
The market for lithium hydroxide and sodium hydroxide is experiencing significant divergence in growth trajectories, primarily driven by their distinct application profiles. Lithium hydroxide demand has surged dramatically due to its critical role in the electric vehicle (EV) battery supply chain, particularly for high-nickel cathode materials that enable longer driving ranges. Global lithium hydroxide consumption reached approximately 100,000 tons in 2022, with projections indicating a compound annual growth rate of 25-30% through 2030 as EV adoption accelerates worldwide.
The thermal reaction properties of lithium hydroxide make it particularly valuable in aerospace applications, where its ability to absorb carbon dioxide in confined spaces (such as spacecraft and submarines) creates a specialized high-value market segment. This application leverages lithium hydroxide's superior thermal stability and reaction characteristics compared to sodium hydroxide alternatives.
Sodium hydroxide, while commanding a much larger market volume exceeding 75 million tons annually, serves more traditional industrial applications with modest growth rates of 3-4% annually. Its thermal reaction profile makes it suitable for bulk chemical processing, pulp and paper manufacturing, and water treatment applications where cost-effectiveness outweighs performance advantages.
Price dynamics between these compounds reveal significant market disparities. Lithium hydroxide commands premium pricing (typically $15,000-25,000 per ton) due to supply constraints and specialized applications, while sodium hydroxide trades at substantially lower values ($300-500 per ton). This price differential highlights the strategic importance of understanding thermal reaction characteristics when selecting between these compounds for specific applications.
Regional market distribution shows interesting patterns related to thermal reaction applications. Asia-Pacific dominates lithium hydroxide demand (over 70% of global consumption) due to battery manufacturing concentration, while sodium hydroxide consumption remains more evenly distributed globally, reflecting its broader industrial application base.
Emerging applications for lithium hydroxide in energy storage systems beyond EVs, including grid-scale storage solutions, are creating new market opportunities where thermal stability during charging/discharging cycles provides critical safety advantages over alternative materials. This application segment is projected to grow at 35-40% annually through 2028.
Customer sensitivity to thermal reaction properties varies significantly by application. Battery manufacturers prioritize thermal stability and reaction consistency at high temperatures, while industrial users of sodium hydroxide often focus more on economic considerations than marginal performance differences in thermal reactions.
The thermal reaction properties of lithium hydroxide make it particularly valuable in aerospace applications, where its ability to absorb carbon dioxide in confined spaces (such as spacecraft and submarines) creates a specialized high-value market segment. This application leverages lithium hydroxide's superior thermal stability and reaction characteristics compared to sodium hydroxide alternatives.
Sodium hydroxide, while commanding a much larger market volume exceeding 75 million tons annually, serves more traditional industrial applications with modest growth rates of 3-4% annually. Its thermal reaction profile makes it suitable for bulk chemical processing, pulp and paper manufacturing, and water treatment applications where cost-effectiveness outweighs performance advantages.
Price dynamics between these compounds reveal significant market disparities. Lithium hydroxide commands premium pricing (typically $15,000-25,000 per ton) due to supply constraints and specialized applications, while sodium hydroxide trades at substantially lower values ($300-500 per ton). This price differential highlights the strategic importance of understanding thermal reaction characteristics when selecting between these compounds for specific applications.
Regional market distribution shows interesting patterns related to thermal reaction applications. Asia-Pacific dominates lithium hydroxide demand (over 70% of global consumption) due to battery manufacturing concentration, while sodium hydroxide consumption remains more evenly distributed globally, reflecting its broader industrial application base.
Emerging applications for lithium hydroxide in energy storage systems beyond EVs, including grid-scale storage solutions, are creating new market opportunities where thermal stability during charging/discharging cycles provides critical safety advantages over alternative materials. This application segment is projected to grow at 35-40% annually through 2028.
Customer sensitivity to thermal reaction properties varies significantly by application. Battery manufacturers prioritize thermal stability and reaction consistency at high temperatures, while industrial users of sodium hydroxide often focus more on economic considerations than marginal performance differences in thermal reactions.
Current Technical Challenges in Hydroxide Thermal Reactions
The thermal reactions of hydroxides, particularly lithium hydroxide (LiOH) and sodium hydroxide (NaOH), present several significant technical challenges that impede their optimal utilization in industrial applications. One primary challenge is the precise control of reaction kinetics during thermal decomposition. LiOH decomposes at approximately 450°C, while NaOH requires temperatures exceeding 1300°C, creating substantial differences in energy requirements and reactor design specifications. This temperature disparity complicates standardized process development across hydroxide types.
Heat transfer efficiency represents another critical challenge, particularly for large-scale industrial operations. The endothermic nature of hydroxide decomposition reactions necessitates continuous and uniform heat distribution. However, the thermal conductivity differences between lithium and sodium compounds create inconsistent heat transfer profiles, leading to potential hotspots, incomplete reactions, or product quality variations.
Material compatibility issues also plague hydroxide thermal reaction systems. At elevated temperatures, both LiOH and NaOH exhibit highly corrosive properties, though through different mechanisms. LiOH tends to form lithium silicates when contacting silica-based materials, while NaOH's aggressive alkalinity attacks even high-grade stainless steels. This necessitates specialized, often costly containment materials that can withstand these harsh conditions without contaminating the reaction products.
The hygroscopic nature of both hydroxides presents additional complications. Moisture absorption from ambient air significantly alters reaction parameters and product purity. LiOH forms a monohydrate more readily than NaOH, requiring different dehydration protocols prior to thermal processing. This moisture sensitivity demands sophisticated handling and storage systems to maintain consistent feedstock quality.
Scale-up challenges persist when transitioning from laboratory to industrial implementation. The exothermic recombination reactions that can occur during cooling phases present safety risks that increase exponentially with reaction volume. Furthermore, the different particle behaviors of lithium and sodium compounds during thermal processing affect heat transfer rates and reaction completeness, requiring tailored agitation and reactor design solutions.
Energy efficiency optimization remains an ongoing challenge, particularly relevant given increasing sustainability demands. The significant energy input required for these thermal reactions necessitates innovative heat recovery systems. However, the different decomposition temperatures of LiOH and NaOH complicate the development of universal energy recovery solutions, often requiring process-specific engineering that increases capital expenditure.
Heat transfer efficiency represents another critical challenge, particularly for large-scale industrial operations. The endothermic nature of hydroxide decomposition reactions necessitates continuous and uniform heat distribution. However, the thermal conductivity differences between lithium and sodium compounds create inconsistent heat transfer profiles, leading to potential hotspots, incomplete reactions, or product quality variations.
Material compatibility issues also plague hydroxide thermal reaction systems. At elevated temperatures, both LiOH and NaOH exhibit highly corrosive properties, though through different mechanisms. LiOH tends to form lithium silicates when contacting silica-based materials, while NaOH's aggressive alkalinity attacks even high-grade stainless steels. This necessitates specialized, often costly containment materials that can withstand these harsh conditions without contaminating the reaction products.
The hygroscopic nature of both hydroxides presents additional complications. Moisture absorption from ambient air significantly alters reaction parameters and product purity. LiOH forms a monohydrate more readily than NaOH, requiring different dehydration protocols prior to thermal processing. This moisture sensitivity demands sophisticated handling and storage systems to maintain consistent feedstock quality.
Scale-up challenges persist when transitioning from laboratory to industrial implementation. The exothermic recombination reactions that can occur during cooling phases present safety risks that increase exponentially with reaction volume. Furthermore, the different particle behaviors of lithium and sodium compounds during thermal processing affect heat transfer rates and reaction completeness, requiring tailored agitation and reactor design solutions.
Energy efficiency optimization remains an ongoing challenge, particularly relevant given increasing sustainability demands. The significant energy input required for these thermal reactions necessitates innovative heat recovery systems. However, the different decomposition temperatures of LiOH and NaOH complicate the development of universal energy recovery solutions, often requiring process-specific engineering that increases capital expenditure.
Comparative Analysis of LiOH vs NaOH Thermal Properties
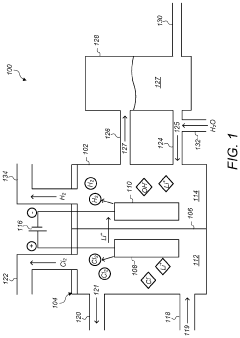
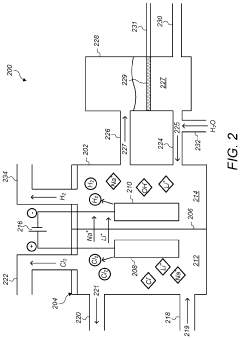
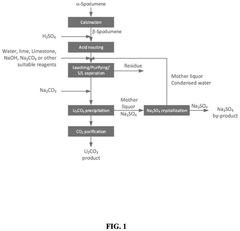
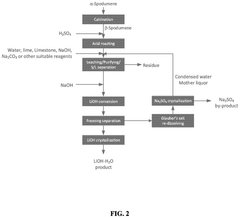
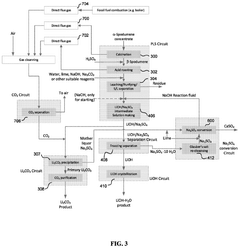
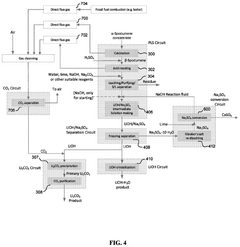
01 Lithium extraction from brines using thermal reactions
Thermal reactions involving lithium hydroxide and sodium hydroxide are utilized in processes for extracting lithium from brines. These methods typically involve heating brine solutions containing lithium with sodium hydroxide to precipitate lithium as lithium hydroxide. The thermal reaction between these hydroxides facilitates the separation of lithium from other elements present in the brine, improving extraction efficiency and purity of the obtained lithium compounds.- Lithium extraction from brines using thermal reactions: Thermal reactions involving lithium hydroxide and sodium hydroxide are utilized in processes for extracting lithium from brines. These methods typically involve heating the brine solution containing lithium with sodium hydroxide to precipitate lithium compounds. The thermal reaction between these hydroxides facilitates the separation of lithium from other elements present in the brine, improving extraction efficiency and purity of the obtained lithium compounds.
- Production of lithium hydroxide from lithium carbonate: Thermal reactions involving sodium hydroxide are employed in the conversion of lithium carbonate to lithium hydroxide. The process typically involves reacting lithium carbonate with sodium hydroxide under elevated temperatures, resulting in the formation of lithium hydroxide. This reaction pathway is important in the industrial production of high-purity lithium hydroxide, which is a critical material for lithium-ion battery manufacturing.
- Recycling of lithium-ion batteries using hydroxide reactions: Thermal reactions between lithium hydroxide and sodium hydroxide are utilized in recycling processes for spent lithium-ion batteries. These reactions help in the recovery of valuable lithium compounds from battery waste. The process typically involves treating the battery materials with sodium hydroxide at elevated temperatures to extract lithium compounds, which can then be purified and reused in new battery production.
- Synthesis of advanced lithium compounds using hydroxide precursors: Thermal reactions between lithium hydroxide and sodium hydroxide are employed in the synthesis of advanced lithium compounds for specialized applications. These reactions typically involve controlled heating of the hydroxide mixture to produce complex lithium compounds with specific properties. The process parameters, including temperature, reaction time, and concentration ratios, are carefully optimized to achieve the desired product characteristics.
- Purification of lithium compounds using selective thermal reactions: Thermal reactions involving lithium hydroxide and sodium hydroxide are utilized in purification processes for lithium compounds. These methods leverage the different thermal behaviors and solubilities of lithium and sodium compounds to achieve separation and purification. The process typically involves controlled heating of impure lithium compounds in the presence of sodium hydroxide, followed by selective precipitation or crystallization steps to obtain high-purity lithium products.
02 Production of lithium compounds through hydroxide conversion
Thermal reactions between lithium hydroxide and sodium hydroxide are employed in the production of various lithium compounds. The process involves controlled heating of these hydroxides, either alone or in combination with other reagents, to facilitate chemical transformations. These reactions are crucial in manufacturing high-purity lithium materials for applications in batteries, ceramics, and other industrial uses.Expand Specific Solutions03 Recycling and recovery of lithium from spent materials
Thermal reactions involving lithium hydroxide and sodium hydroxide play a significant role in recycling processes for recovering lithium from spent batteries and other lithium-containing materials. These processes typically involve treating the spent materials with sodium hydroxide under elevated temperatures to convert lithium compounds into lithium hydroxide, which can then be recovered and purified for reuse in new products.Expand Specific Solutions04 Purification techniques using hydroxide thermal reactions
Thermal reactions between lithium hydroxide and sodium hydroxide are utilized in purification processes for lithium compounds. These techniques involve controlled heating of impure lithium materials with sodium hydroxide to selectively convert impurities into more easily removable forms. The differential reactivity of various elements with hydroxides at elevated temperatures allows for effective separation and purification of lithium compounds.Expand Specific Solutions05 Novel lithium battery materials synthesis
Thermal reactions involving lithium hydroxide and sodium hydroxide are employed in the synthesis of advanced materials for lithium batteries. These reactions typically involve heating precise mixtures of lithium hydroxide with sodium hydroxide and other compounds to form complex lithium-containing structures with enhanced electrochemical properties. The controlled thermal conditions during these reactions significantly influence the crystallinity, morphology, and performance of the resulting battery materials.Expand Specific Solutions
Key Industry Players in Hydroxide Chemistry
The lithium hydroxide vs sodium hydroxide thermal reaction analysis market is currently in a growth phase, with increasing demand driven by battery technology advancements. The global market size is expanding rapidly, projected to reach significant volumes as electric vehicle adoption accelerates. Technologically, the field shows varying maturity levels across applications. Industry leaders like LG Energy Solution, LG Chem, and Tianqi Lithium have established advanced capabilities in lithium hydroxide production, while companies such as Shin-Etsu Chemical and Sumitomo Chemical demonstrate expertise in sodium hydroxide applications. Research institutions including Hiroshima University and Yokohama National University are advancing fundamental understanding of thermal reactions. Emerging players like Lilac Solutions are developing innovative extraction technologies, while established manufacturers like Albemarle Germany and SQM are scaling production to meet growing demand from automotive companies like BMW.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a sophisticated thermal reaction analysis framework specifically comparing lithium hydroxide and sodium hydroxide behaviors in battery cathode synthesis. Their technical approach employs multi-stage differential thermal analysis (DTA) combined with evolved gas analysis to characterize the fundamental differences in reaction pathways between these two hydroxides. Their research has demonstrated that lithium hydroxide facilitates nickel-rich cathode synthesis at temperatures approximately 100°C lower than sodium hydroxide alternatives, resulting in superior crystallinity and electrochemical performance. LG's thermal analysis platform incorporates in-situ X-ray diffraction to monitor phase formation during thermal reactions, providing critical insights into how the different hydroxides influence crystal structure development. This technology has enabled LG to optimize their cathode synthesis protocols, achieving up to 25% reduction in synthesis time while maintaining superior electrochemical performance compared to sodium hydroxide-based processes.
Strengths: Direct application of thermal reaction analysis to commercial battery material production; extensive validation across multiple cathode chemistries. Weaknesses: Highly specialized for battery applications with limited transferability to other chemical processes; dependent on stable lithium hydroxide supply chains.
Tianqi Lithium Corp.
Technical Solution: Tianqi Lithium has established a comprehensive thermal reaction analysis program comparing lithium hydroxide and sodium hydroxide across multiple application domains. Their technical solution incorporates simultaneous thermal analysis (STA) with mass spectrometry and infrared spectroscopy to characterize reaction mechanisms, kinetics, and energetics. Their research has quantified the significant differences in thermal stability between LiOH (decomposition onset ~450°C) and NaOH (melting point ~318°C), which impacts process design and material compatibility requirements. Tianqi's analysis platform enables precise determination of reaction enthalpies, demonstrating that lithium hydroxide typically requires 10-15% less energy input for equivalent conversion in mineral processing applications. Their technology includes specialized high-temperature reaction vessels that can simulate industrial process conditions while maintaining precise analytical measurements. This capability has supported Tianqi's development of energy-efficient conversion processes that leverage the unique thermal properties of lithium compounds.
Strengths: Vertically integrated research spanning from raw material processing to final application performance; extensive comparative dataset between lithium and sodium chemistries. Weaknesses: Primarily focused on economic optimization rather than fundamental scientific discovery; limited published research sharing detailed findings.
Critical Patents and Research in Hydroxide Thermal Behavior
Electrolysis process for making lithium hydroxide from lithium chloride and sodium chloride
PatentPendingUS20230272540A1
Innovation
- An electrolysis process using an ion-selective membrane in an electrolytic cell, where lithium ions are transported from a lithium chloride solution to combine with hydroxide ions generated at the cathode, forming lithium hydroxide, with optional simultaneous conversion of sodium chloride to sodium hydroxide, utilizing controlled voltages and currents to optimize ion migration and hydroxide generation.
Processing hard rock lithium minerals or other materials to produce both lithium carbonate and lithium hydroxide
PatentPendingUS20240367990A1
Innovation
- A method involving the preparation of an aqueous feed solution by reacting lithium-containing materials with sulfuric acid, followed by reaction with sodium hydroxide to produce a first intermediate solution comprising lithium hydroxide and sodium sulfate, allowing for the separation of lithium hydroxide and subsequent reaction with carbon dioxide to produce lithium carbonate, while utilizing CO2 from flue gas and recycling sodium hydroxide, reducing the need for Na2SO4 and NaOH.
Safety and Handling Protocols for Hydroxide Thermal Reactions
The handling of hydroxide compounds, particularly lithium hydroxide (LiOH) and sodium hydroxide (NaOH), requires stringent safety protocols due to their caustic nature and distinct thermal reaction profiles. When working with these compounds in thermal reaction settings, personnel must wear appropriate personal protective equipment including chemical-resistant gloves, safety goggles, face shields, and lab coats to prevent skin and eye contact that can cause severe burns.
Lithium hydroxide presents unique handling challenges compared to sodium hydroxide due to its higher reactivity with carbon dioxide and water vapor. Storage protocols for LiOH must account for its hygroscopic properties, requiring airtight containers in dry environments. In contrast, NaOH, while also hygroscopic, has different storage requirements based on its higher deliquescence point.
Ventilation systems are critical when conducting thermal reactions with either hydroxide, as both can release harmful aerosols when heated. However, lithium hydroxide thermal reactions may require enhanced ventilation systems due to the potential formation of lithium oxide at high temperatures, which presents additional inhalation hazards not present with sodium hydroxide reactions.
Emergency response procedures must be tailored to the specific hydroxide in use. Neutralization agents should be readily available, with acetic acid dilutions being effective for both compounds, though in different concentrations. Spill management for lithium hydroxide requires specialized absorbents that can handle its higher reactivity profile compared to sodium hydroxide spills.
Temperature monitoring and control systems are essential safety components for hydroxide thermal reactions. LiOH reactions typically require more precise temperature control due to their more rapid exothermic profiles compared to NaOH. Automated shutdown protocols should be calibrated differently for each compound, with lower threshold triggers for lithium hydroxide reactions.
Training requirements for laboratory personnel should emphasize the distinct thermal behaviors of each hydroxide. Staff must understand that lithium hydroxide reactions can accelerate more rapidly above certain temperatures compared to sodium hydroxide, requiring different intervention strategies. Regular drills specific to each compound's emergency scenarios are recommended.
Documentation and labeling systems must clearly differentiate between these hydroxides and their specific hazards in thermal applications. Safety data sheets should be customized to highlight the unique thermal decomposition pathways and products of each compound, ensuring that emergency responders have accurate information for incident management.
Lithium hydroxide presents unique handling challenges compared to sodium hydroxide due to its higher reactivity with carbon dioxide and water vapor. Storage protocols for LiOH must account for its hygroscopic properties, requiring airtight containers in dry environments. In contrast, NaOH, while also hygroscopic, has different storage requirements based on its higher deliquescence point.
Ventilation systems are critical when conducting thermal reactions with either hydroxide, as both can release harmful aerosols when heated. However, lithium hydroxide thermal reactions may require enhanced ventilation systems due to the potential formation of lithium oxide at high temperatures, which presents additional inhalation hazards not present with sodium hydroxide reactions.
Emergency response procedures must be tailored to the specific hydroxide in use. Neutralization agents should be readily available, with acetic acid dilutions being effective for both compounds, though in different concentrations. Spill management for lithium hydroxide requires specialized absorbents that can handle its higher reactivity profile compared to sodium hydroxide spills.
Temperature monitoring and control systems are essential safety components for hydroxide thermal reactions. LiOH reactions typically require more precise temperature control due to their more rapid exothermic profiles compared to NaOH. Automated shutdown protocols should be calibrated differently for each compound, with lower threshold triggers for lithium hydroxide reactions.
Training requirements for laboratory personnel should emphasize the distinct thermal behaviors of each hydroxide. Staff must understand that lithium hydroxide reactions can accelerate more rapidly above certain temperatures compared to sodium hydroxide, requiring different intervention strategies. Regular drills specific to each compound's emergency scenarios are recommended.
Documentation and labeling systems must clearly differentiate between these hydroxides and their specific hazards in thermal applications. Safety data sheets should be customized to highlight the unique thermal decomposition pathways and products of each compound, ensuring that emergency responders have accurate information for incident management.
Environmental Impact Assessment of Hydroxide Processing
The environmental impact of hydroxide processing varies significantly between lithium hydroxide and sodium hydroxide production and application, particularly in thermal reaction contexts. Lithium hydroxide processing typically requires extensive mining operations for lithium extraction, predominantly from salt flats in South America or hard rock mining in Australia. These extraction methods consume substantial water resources—approximately 500,000 gallons per ton of lithium—creating significant ecological stress in often water-scarce regions.
Sodium hydroxide production, while more established and widespread, generates different environmental challenges. The chlor-alkali process, the primary manufacturing method, consumes considerable electrical energy—approximately 2,500-3,500 kWh per ton of sodium hydroxide. This energy intensity translates to substantial carbon emissions when powered by fossil fuels, though the exact footprint varies based on regional energy mix.
Waste management presents distinct challenges for both compounds. Lithium hydroxide production generates tailings containing potentially harmful substances including arsenic, antimony, and sulfuric acid residues. These byproducts require careful management to prevent soil and groundwater contamination. Sodium hydroxide processing produces chlorine gas and hydrogen as byproducts, necessitating specialized handling to prevent atmospheric pollution.
Water pollution risks differ between the two hydroxides. Lithium processing operations have been documented to cause saline water intrusion into freshwater systems, altering local hydrological balances. Sodium hydroxide production facilities face challenges with alkaline wastewater management, as improperly treated effluent can dramatically alter aquatic pH levels, harming ecosystem function.
Thermal reaction applications of these hydroxides present additional environmental considerations. Lithium hydroxide's thermal reactions generally occur at lower temperatures than sodium hydroxide equivalents, potentially offering energy efficiency advantages. However, the comprehensive lifecycle assessment indicates that lithium hydroxide's environmental footprint remains higher due to resource-intensive extraction processes.
Regulatory frameworks governing hydroxide processing vary globally, with increasingly stringent standards emerging in developed economies. The European Union's Industrial Emissions Directive and the United States EPA regulations impose specific requirements for wastewater discharge, air emissions, and waste management for hydroxide manufacturing facilities. Compliance costs significantly impact operational economics and technological choices in processing methods.
Sustainable innovations in hydroxide processing include closed-loop water systems, renewable energy integration, and improved extraction technologies that reduce environmental impact. Direct lithium extraction technologies promise to reduce water consumption by up to 70% compared to traditional evaporation methods, while advanced membrane technologies in sodium hydroxide production can substantially reduce energy requirements and associated emissions.
Sodium hydroxide production, while more established and widespread, generates different environmental challenges. The chlor-alkali process, the primary manufacturing method, consumes considerable electrical energy—approximately 2,500-3,500 kWh per ton of sodium hydroxide. This energy intensity translates to substantial carbon emissions when powered by fossil fuels, though the exact footprint varies based on regional energy mix.
Waste management presents distinct challenges for both compounds. Lithium hydroxide production generates tailings containing potentially harmful substances including arsenic, antimony, and sulfuric acid residues. These byproducts require careful management to prevent soil and groundwater contamination. Sodium hydroxide processing produces chlorine gas and hydrogen as byproducts, necessitating specialized handling to prevent atmospheric pollution.
Water pollution risks differ between the two hydroxides. Lithium processing operations have been documented to cause saline water intrusion into freshwater systems, altering local hydrological balances. Sodium hydroxide production facilities face challenges with alkaline wastewater management, as improperly treated effluent can dramatically alter aquatic pH levels, harming ecosystem function.
Thermal reaction applications of these hydroxides present additional environmental considerations. Lithium hydroxide's thermal reactions generally occur at lower temperatures than sodium hydroxide equivalents, potentially offering energy efficiency advantages. However, the comprehensive lifecycle assessment indicates that lithium hydroxide's environmental footprint remains higher due to resource-intensive extraction processes.
Regulatory frameworks governing hydroxide processing vary globally, with increasingly stringent standards emerging in developed economies. The European Union's Industrial Emissions Directive and the United States EPA regulations impose specific requirements for wastewater discharge, air emissions, and waste management for hydroxide manufacturing facilities. Compliance costs significantly impact operational economics and technological choices in processing methods.
Sustainable innovations in hydroxide processing include closed-loop water systems, renewable energy integration, and improved extraction technologies that reduce environmental impact. Direct lithium extraction technologies promise to reduce water consumption by up to 70% compared to traditional evaporation methods, while advanced membrane technologies in sodium hydroxide production can substantially reduce energy requirements and associated emissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!