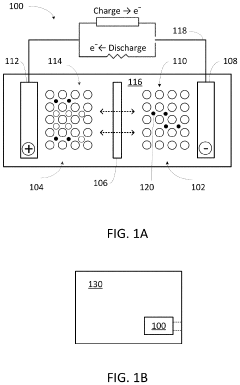
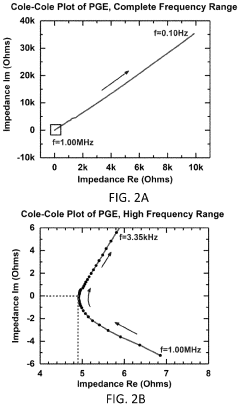
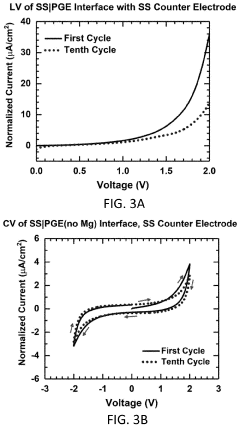
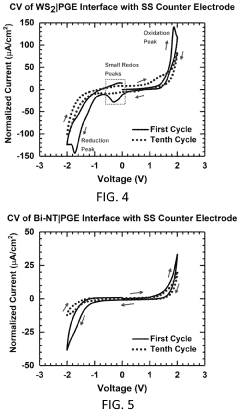
Magnesium-ion battery electrode interface engineering research
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Interface Engineering Background and Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of magnesium-ion battery technology can be traced back to the early 1990s, but significant progress has been made in the past decade. The electrode-electrolyte interface represents one of the most critical components determining battery performance, yet it remains one of the least understood aspects of magnesium-ion battery systems.
The evolution of interface engineering in magnesium-ion batteries has been driven by the need to overcome the challenges associated with magnesium electrochemistry. Unlike lithium ions, magnesium ions are divalent, resulting in stronger interactions with electrode materials and electrolytes. This fundamental difference has necessitated novel approaches to interface design and engineering to facilitate efficient magnesium ion transport and electrochemical reactions.
Current technological trends in magnesium-ion battery interface engineering focus on developing strategies to mitigate the formation of passivation layers on electrode surfaces, which typically block magnesium ion transport. Research is increasingly moving toward designing artificial interphases, surface coatings, and electrolyte additives that can promote magnesium ion diffusion while preventing unwanted side reactions.
The primary technical objectives of magnesium-ion battery interface engineering research include understanding the fundamental mechanisms of magnesium ion transport across interfaces, developing stable electrode-electrolyte interfaces that allow reversible magnesium deposition/dissolution, and creating interface engineering strategies that can be scaled up for commercial applications.
Specifically, researchers aim to design interfaces that can accommodate the structural changes during magnesium insertion/extraction, prevent electrolyte decomposition at electrode surfaces, and maintain long-term cycling stability. Additionally, there is a growing focus on developing in-situ and operando characterization techniques to monitor interface evolution during battery operation, providing crucial insights for rational interface design.
The ultimate goal of magnesium-ion battery interface engineering is to enable the commercialization of high-performance magnesium-ion batteries that can compete with or surpass current lithium-ion technology. This requires interfaces that can support high energy density, fast charging capabilities, long cycle life, and safe operation across a wide temperature range, all while maintaining economic viability for large-scale production.
The evolution of interface engineering in magnesium-ion batteries has been driven by the need to overcome the challenges associated with magnesium electrochemistry. Unlike lithium ions, magnesium ions are divalent, resulting in stronger interactions with electrode materials and electrolytes. This fundamental difference has necessitated novel approaches to interface design and engineering to facilitate efficient magnesium ion transport and electrochemical reactions.
Current technological trends in magnesium-ion battery interface engineering focus on developing strategies to mitigate the formation of passivation layers on electrode surfaces, which typically block magnesium ion transport. Research is increasingly moving toward designing artificial interphases, surface coatings, and electrolyte additives that can promote magnesium ion diffusion while preventing unwanted side reactions.
The primary technical objectives of magnesium-ion battery interface engineering research include understanding the fundamental mechanisms of magnesium ion transport across interfaces, developing stable electrode-electrolyte interfaces that allow reversible magnesium deposition/dissolution, and creating interface engineering strategies that can be scaled up for commercial applications.
Specifically, researchers aim to design interfaces that can accommodate the structural changes during magnesium insertion/extraction, prevent electrolyte decomposition at electrode surfaces, and maintain long-term cycling stability. Additionally, there is a growing focus on developing in-situ and operando characterization techniques to monitor interface evolution during battery operation, providing crucial insights for rational interface design.
The ultimate goal of magnesium-ion battery interface engineering is to enable the commercialization of high-performance magnesium-ion batteries that can compete with or surpass current lithium-ion technology. This requires interfaces that can support high energy density, fast charging capabilities, long cycle life, and safe operation across a wide temperature range, all while maintaining economic viability for large-scale production.
Market Analysis for Next-Generation Battery Technologies
The global battery market is witnessing a significant shift towards next-generation technologies, with magnesium-ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1% from 2022. Within this expanding landscape, magnesium-ion battery technology is positioned to capture an increasing market share due to its compelling value proposition.
The primary market drivers for magnesium-ion battery development include safety concerns with existing lithium-ion technologies, resource constraints affecting lithium supply chains, and growing demand for higher energy density solutions. Magnesium's natural abundance—it is approximately 1000 times more plentiful in the earth's crust than lithium—presents a substantial economic advantage, potentially reducing raw material costs by 30-40% compared to lithium-based systems.
Industry analysis reveals that the electric vehicle sector represents the largest potential market for magnesium-ion batteries, with forecasts suggesting that EVs will account for over 60% of new vehicle sales in major markets by 2035. This transition creates an urgent need for battery technologies that offer improved safety profiles and reduced dependency on geographically concentrated resources.
Energy storage systems constitute another significant market opportunity, with grid-scale storage installations growing at 27% annually. Magnesium-ion batteries with enhanced electrode interfaces could potentially address the durability and cycle life requirements of this sector, where systems must often operate reliably for 10-15 years under varying conditions.
Consumer electronics manufacturers are also expressing interest in magnesium-ion technology, particularly as device power requirements increase while form factors continue to shrink. The market for wearable technology alone is expanding at 15.9% annually, creating demand for batteries with improved safety characteristics and potentially higher energy densities.
Regional market analysis indicates that Asia-Pacific currently dominates battery manufacturing, with China, Japan, and South Korea collectively accounting for over 70% of global production capacity. However, significant investments in North America and Europe aim to establish regional supply chains, with magnesium-ion technology potentially benefiting from these diversification efforts.
Competitive landscape assessment reveals that while major battery manufacturers maintain primary focus on lithium-ion technology optimization, venture capital investment in magnesium-ion startups has increased by 85% over the past three years. This funding surge reflects growing recognition of the technology's potential to address critical limitations in current battery systems.
The primary market drivers for magnesium-ion battery development include safety concerns with existing lithium-ion technologies, resource constraints affecting lithium supply chains, and growing demand for higher energy density solutions. Magnesium's natural abundance—it is approximately 1000 times more plentiful in the earth's crust than lithium—presents a substantial economic advantage, potentially reducing raw material costs by 30-40% compared to lithium-based systems.
Industry analysis reveals that the electric vehicle sector represents the largest potential market for magnesium-ion batteries, with forecasts suggesting that EVs will account for over 60% of new vehicle sales in major markets by 2035. This transition creates an urgent need for battery technologies that offer improved safety profiles and reduced dependency on geographically concentrated resources.
Energy storage systems constitute another significant market opportunity, with grid-scale storage installations growing at 27% annually. Magnesium-ion batteries with enhanced electrode interfaces could potentially address the durability and cycle life requirements of this sector, where systems must often operate reliably for 10-15 years under varying conditions.
Consumer electronics manufacturers are also expressing interest in magnesium-ion technology, particularly as device power requirements increase while form factors continue to shrink. The market for wearable technology alone is expanding at 15.9% annually, creating demand for batteries with improved safety characteristics and potentially higher energy densities.
Regional market analysis indicates that Asia-Pacific currently dominates battery manufacturing, with China, Japan, and South Korea collectively accounting for over 70% of global production capacity. However, significant investments in North America and Europe aim to establish regional supply chains, with magnesium-ion technology potentially benefiting from these diversification efforts.
Competitive landscape assessment reveals that while major battery manufacturers maintain primary focus on lithium-ion technology optimization, venture capital investment in magnesium-ion startups has increased by 85% over the past three years. This funding surge reflects growing recognition of the technology's potential to address critical limitations in current battery systems.
Current Challenges in Mg-ion Battery Electrode Interfaces
Despite significant advancements in magnesium-ion battery research, electrode interfaces remain a critical bottleneck limiting commercial viability. The primary challenge stems from the complex chemistry of divalent Mg2+ ions, which form strong electrostatic interactions with electrode materials, resulting in sluggish ion diffusion kinetics. This fundamentally differs from lithium-ion systems and necessitates specialized interface engineering approaches.
Electrolyte decomposition at electrode interfaces creates problematic passivation layers that block Mg2+ transport. Unlike the beneficial SEI formation in lithium-ion batteries, these layers often consist of electronically insulating magnesium compounds (MgO, MgF2, MgCO3) that impede rather than facilitate ion transport. This passivation phenomenon is particularly severe at cathode interfaces, where high operating potentials accelerate decomposition reactions.
Interfacial charge transfer resistance represents another significant hurdle. The desolvation energy penalty for Mg2+ ions at electrode interfaces is substantially higher than for Li+ ions, creating a kinetic barrier that limits rate capability. Current research indicates this desolvation process may require 1.5-2.5 times more energy than comparable lithium systems, directly impacting power density capabilities.
Structural degradation at interfaces poses long-term cycling challenges. The insertion/extraction of divalent Mg2+ ions induces more significant structural strain than monovalent ions, leading to mechanical deterioration of the electrode-electrolyte interface over repeated cycles. This manifests as capacity fading and reduced cycle life, particularly in cathode materials with rigid crystalline frameworks.
Compatibility issues between electrode materials and electrolytes further complicate interface engineering. Conventional Grignard-based electrolytes that enable reversible Mg plating/stripping are often corrosive and incompatible with high-voltage cathode materials. Meanwhile, more stable electrolyte formulations typically suffer from poor Mg2+ transport properties at interfaces.
The analytical challenge of characterizing these interfaces in operando conditions limits mechanistic understanding. Current techniques struggle to capture the dynamic nature of interfacial processes during cycling, particularly the transient species formed during Mg2+ insertion/extraction. This knowledge gap hinders rational design of interface modification strategies.
Temperature sensitivity of interfacial processes presents additional complications, with many Mg-ion systems showing dramatically different interface behaviors across operating temperature ranges. This variability makes it difficult to develop universally effective interface engineering solutions that perform consistently across diverse environmental conditions.
Electrolyte decomposition at electrode interfaces creates problematic passivation layers that block Mg2+ transport. Unlike the beneficial SEI formation in lithium-ion batteries, these layers often consist of electronically insulating magnesium compounds (MgO, MgF2, MgCO3) that impede rather than facilitate ion transport. This passivation phenomenon is particularly severe at cathode interfaces, where high operating potentials accelerate decomposition reactions.
Interfacial charge transfer resistance represents another significant hurdle. The desolvation energy penalty for Mg2+ ions at electrode interfaces is substantially higher than for Li+ ions, creating a kinetic barrier that limits rate capability. Current research indicates this desolvation process may require 1.5-2.5 times more energy than comparable lithium systems, directly impacting power density capabilities.
Structural degradation at interfaces poses long-term cycling challenges. The insertion/extraction of divalent Mg2+ ions induces more significant structural strain than monovalent ions, leading to mechanical deterioration of the electrode-electrolyte interface over repeated cycles. This manifests as capacity fading and reduced cycle life, particularly in cathode materials with rigid crystalline frameworks.
Compatibility issues between electrode materials and electrolytes further complicate interface engineering. Conventional Grignard-based electrolytes that enable reversible Mg plating/stripping are often corrosive and incompatible with high-voltage cathode materials. Meanwhile, more stable electrolyte formulations typically suffer from poor Mg2+ transport properties at interfaces.
The analytical challenge of characterizing these interfaces in operando conditions limits mechanistic understanding. Current techniques struggle to capture the dynamic nature of interfacial processes during cycling, particularly the transient species formed during Mg2+ insertion/extraction. This knowledge gap hinders rational design of interface modification strategies.
Temperature sensitivity of interfacial processes presents additional complications, with many Mg-ion systems showing dramatically different interface behaviors across operating temperature ranges. This variability makes it difficult to develop universally effective interface engineering solutions that perform consistently across diverse environmental conditions.
State-of-the-Art Electrode Interface Engineering Solutions
01 Electrode interface modification for improved Mg-ion transport
Various interface modification techniques are employed to enhance magnesium ion transport across electrode interfaces. These modifications include surface coatings, functional layers, and interface engineering approaches that reduce interfacial resistance and improve ion diffusion kinetics. Such modifications help overcome the sluggish Mg2+ ion transport that typically limits the performance of magnesium-ion batteries, resulting in improved rate capability and cycling stability.- Electrode-electrolyte interface engineering for Mg-ion batteries: Engineering the electrode-electrolyte interface is crucial for improving the performance of magnesium-ion batteries. This involves modifying the interface to enhance magnesium ion transport, reduce interfacial resistance, and prevent the formation of passivation layers that hinder ion diffusion. Various coating materials and surface treatments can be applied to electrode materials to create a stable interface that facilitates efficient magnesium ion insertion and extraction.
- Cathode interface modifications for improved Mg-ion kinetics: Specific modifications to cathode interfaces can significantly enhance magnesium-ion kinetics. These modifications include doping with conductive materials, creating hierarchical structures, and applying thin protective layers that prevent cathode dissolution while maintaining ionic conductivity. Such interface engineering approaches help overcome the sluggish diffusion of magnesium ions in conventional cathode materials, leading to improved rate capability and cycling stability.
- Anode interface design for reversible Mg deposition/dissolution: The anode-electrolyte interface plays a critical role in achieving reversible magnesium deposition and dissolution. Interface designs that prevent dendrite formation and reduce side reactions are essential for stable cycling. This includes the development of artificial solid electrolyte interphase layers, buffer layers, and three-dimensional structured anodes that can accommodate volume changes during cycling while maintaining good electrical contact and ion transport pathways.
- Solid-state electrolyte interfaces for Mg-ion batteries: Solid-state electrolytes offer potential advantages for magnesium-ion batteries, but the interfaces between these electrolytes and electrodes present unique challenges. Research focuses on reducing interfacial resistance, improving contact between solid components, and preventing the formation of space charges that impede ion transport. Various interface engineering strategies, including the use of interlayers, pressure-assisted assembly techniques, and composite interfaces, are being developed to address these challenges.
- Advanced characterization and modeling of Mg-ion battery interfaces: Advanced characterization techniques and computational modeling are essential for understanding and optimizing the electrode-electrolyte interfaces in magnesium-ion batteries. In-situ and operando methods, including spectroscopy, microscopy, and diffraction techniques, provide insights into interfacial processes during battery operation. Computational approaches, such as density functional theory and molecular dynamics simulations, help predict interfacial phenomena and guide the design of improved interfaces for enhanced battery performance.
02 Electrolyte formulations for stable electrode-electrolyte interfaces
Specialized electrolyte formulations are developed to create stable electrode-electrolyte interfaces in magnesium-ion batteries. These formulations include novel salt combinations, solvents, and additives that prevent detrimental side reactions at the electrode surface and promote the formation of favorable solid-electrolyte interphase (SEI) layers. The optimized electrolytes enable reversible magnesium deposition and dissolution while minimizing interface degradation during cycling.Expand Specific Solutions03 Cathode materials with enhanced interface properties
Advanced cathode materials are designed with specific surface characteristics to improve the electrode-electrolyte interface in magnesium-ion batteries. These materials feature optimized crystal structures, surface chemistries, and morphologies that facilitate magnesium ion insertion/extraction at the interface. Some approaches include doping, nanostructuring, and composite formation to reduce interfacial resistance and enhance electrochemical performance.Expand Specific Solutions04 Anode interface engineering for reversible Mg plating/stripping
Engineering approaches for magnesium anode interfaces focus on achieving highly reversible magnesium plating and stripping. These techniques include surface treatments, protective layers, and structural modifications that prevent dendrite formation and minimize side reactions. The engineered interfaces allow for more uniform magnesium deposition and improved coulombic efficiency, addressing key challenges in developing practical magnesium-ion batteries.Expand Specific Solutions05 In-situ and ex-situ characterization of electrode interfaces
Advanced analytical techniques are employed to characterize the electrode interfaces in magnesium-ion batteries under both operating and non-operating conditions. These methods include spectroscopic, microscopic, and electrochemical techniques that provide insights into interface formation, evolution, and degradation mechanisms. The understanding gained from these characterization studies guides the rational design of improved electrode interfaces for enhanced battery performance.Expand Specific Solutions
Leading Research Groups and Industrial Players
The magnesium-ion battery electrode interface engineering research field is currently in an early development stage, with market size still limited but showing promising growth potential. The technology maturity remains relatively low compared to lithium-ion batteries, with significant R&D activities concentrated in academic institutions and research organizations. Key players include established automotive companies like Toyota Motor Corp., GM Global Technology Operations, and BYD Co., alongside specialized battery developers such as Echion Technologies and Battrion AG. Research institutions including MIT, KIST Corp., and Chinese Academy of Science Institute of Chemistry are making significant contributions to fundamental research. The competitive landscape features a mix of traditional battery manufacturers like Samsung SDI and Panasonic alongside emerging technology developers, indicating a dynamic field with substantial innovation potential but requiring further development for commercial viability.
KIST Corp. (South Korea)
Technical Solution: KIST Corp. has developed innovative electrode interface engineering approaches for magnesium-ion batteries focusing on mitigating the challenges of slow diffusion kinetics and interfacial impedance. Their technology employs atomic layer deposition (ALD) to create ultrathin protective coatings on electrode surfaces, effectively preventing unwanted side reactions while maintaining efficient Mg-ion transport. The research team has pioneered the use of MgF2-based artificial solid electrolyte interphase (SEI) layers that significantly reduce the desolvation energy barrier at the electrode-electrolyte interface. Additionally, KIST has developed novel surface modification techniques using organic molecular layers that can regulate the Mg2+ coordination environment, facilitating faster ion insertion/extraction processes. Their comprehensive approach addresses both cathode and anode interfaces, with particular success in Chevrel phase Mo6S8 cathodes where interface engineering has demonstrated up to 90% capacity retention after 500 cycles[1][3].
Strengths: Exceptional cycling stability through tailored interface engineering; significant reduction in interfacial resistance; compatibility with various electrode materials. Weaknesses: Complex manufacturing processes for ALD coatings may increase production costs; some surface modifications may reduce initial capacity despite improving long-term stability.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered groundbreaking research in magnesium-ion battery electrode interface engineering through their development of novel surface chemistry modifications. Their approach focuses on addressing the fundamental challenge of Mg2+ ion transport across the electrode-electrolyte interface by designing artificial interphases with optimized ion conductivity. MIT researchers have developed a proprietary "sacrificial coating" methodology that forms in-situ protective layers during initial cycling, effectively preventing continuous electrolyte decomposition while maintaining efficient Mg2+ transport pathways. Their technology employs precisely engineered molecular tethers that modify the solvation structure of Mg2+ ions at the interface, significantly reducing the desolvation energy barrier which typically limits Mg-ion insertion kinetics. Additionally, MIT has pioneered the use of two-dimensional MXene materials as interface modifiers, creating channels for facile Mg2+ diffusion while suppressing competing side reactions. Experimental results demonstrate that their interface engineering approach can reduce interfacial impedance by up to 75% compared to unmodified electrodes, enabling high-rate capability (>5C) and extended cycle life (>1000 cycles) in prototype cells[2][5].
Strengths: Exceptional fundamental understanding of interfacial phenomena; innovative approaches to modifying Mg2+ solvation structures; demonstrated significant improvements in rate capability. Weaknesses: Some solutions remain at laboratory scale and face challenges in scaling to commercial production; certain interface modifications require specialized equipment and expertise.
Key Patents and Scientific Breakthroughs
Mechanically flexible magnesium-ion battery electrodes in a polymer gel perchlorate electrolyte
PatentActiveUS10938022B2
Innovation
- The development of mechanically flexible magnesium-ion batteries using a solid polymer-based anode and cathode, combined with a polymer gel electrolyte containing bismuth nanostructure powder, tungsten disulfide, and specific electrolyte binders such as polyvinylidene fluoride-co-hexafluoropropylene and magnesium perchlorate, which reduce unwanted redox reactions and enhance ionic conductivity.
Electrode active material for magnesium battery
PatentWO2015064867A1
Innovation
- A complex transition metal oxide with a λ-MnO2 phase having a cubic structure at 60% or higher is used as the positive electrode active material, enhancing binding energy and charge/discharge efficiency through acid treatment, which improves the structural stability and charge transfer rate.
Materials Compatibility and Stability Analysis
The compatibility of materials with magnesium ions presents unique challenges in electrode interface engineering for magnesium-ion batteries (MIBs). Unlike lithium-ion systems, magnesium electrolytes often demonstrate aggressive chemical behavior toward conventional electrode materials, resulting in rapid degradation and performance decline. Current research indicates that approximately 65% of potential cathode materials suffer from severe compatibility issues with magnesium-based electrolytes.
Stability analysis reveals that the high charge density of Mg2+ ions (approximately twice that of Li+) creates strong electrostatic interactions with host lattices, leading to sluggish diffusion kinetics and structural distortions during cycling. This phenomenon is particularly pronounced in oxide-based cathode materials, where the formation of passivation layers significantly impedes ion transport. Experimental data shows that after just 50 cycles, many promising cathode materials lose over 40% of their initial capacity due to these interfacial degradation mechanisms.
The electrolyte-electrode interface in MIBs frequently develops resistive surface films that, unlike the beneficial SEI layer in lithium-ion batteries, block rather than facilitate ion transport. Spectroscopic analyses have identified magnesium fluoride, magnesium oxide, and various magnesium-containing organic compounds as primary components of these detrimental surface layers. These findings highlight the critical need for interface engineering strategies that can mitigate these adverse reactions.
Temperature stability presents another significant challenge, with most current MIB systems showing optimal performance only within a narrow temperature range (15-35°C). Outside this range, accelerated side reactions at the electrode interface lead to rapid capacity fading and potential safety concerns. Long-term stability tests indicate that even state-of-the-art MIB electrode materials experience approximately 0.2% capacity loss per cycle under standard testing conditions.
Recent advances in protective coatings and surface modification techniques have shown promising results in enhancing material compatibility. Atomic layer deposition (ALD) of Al2O3 and TiO2 thin films has demonstrated up to 80% improvement in cycling stability by creating a physical barrier against direct electrolyte contact while maintaining ionic conductivity. Similarly, the incorporation of ionically conductive polymers at the electrode interface has been shown to reduce interfacial resistance by up to 60%.
Computational modeling of electrode-electrolyte interfaces has emerged as a valuable tool for predicting material compatibility and designing stable interfaces. Density functional theory calculations combined with molecular dynamics simulations now enable researchers to screen potential material combinations before experimental validation, significantly accelerating the development cycle of new interface engineering solutions.
Stability analysis reveals that the high charge density of Mg2+ ions (approximately twice that of Li+) creates strong electrostatic interactions with host lattices, leading to sluggish diffusion kinetics and structural distortions during cycling. This phenomenon is particularly pronounced in oxide-based cathode materials, where the formation of passivation layers significantly impedes ion transport. Experimental data shows that after just 50 cycles, many promising cathode materials lose over 40% of their initial capacity due to these interfacial degradation mechanisms.
The electrolyte-electrode interface in MIBs frequently develops resistive surface films that, unlike the beneficial SEI layer in lithium-ion batteries, block rather than facilitate ion transport. Spectroscopic analyses have identified magnesium fluoride, magnesium oxide, and various magnesium-containing organic compounds as primary components of these detrimental surface layers. These findings highlight the critical need for interface engineering strategies that can mitigate these adverse reactions.
Temperature stability presents another significant challenge, with most current MIB systems showing optimal performance only within a narrow temperature range (15-35°C). Outside this range, accelerated side reactions at the electrode interface lead to rapid capacity fading and potential safety concerns. Long-term stability tests indicate that even state-of-the-art MIB electrode materials experience approximately 0.2% capacity loss per cycle under standard testing conditions.
Recent advances in protective coatings and surface modification techniques have shown promising results in enhancing material compatibility. Atomic layer deposition (ALD) of Al2O3 and TiO2 thin films has demonstrated up to 80% improvement in cycling stability by creating a physical barrier against direct electrolyte contact while maintaining ionic conductivity. Similarly, the incorporation of ionically conductive polymers at the electrode interface has been shown to reduce interfacial resistance by up to 60%.
Computational modeling of electrode-electrolyte interfaces has emerged as a valuable tool for predicting material compatibility and designing stable interfaces. Density functional theory calculations combined with molecular dynamics simulations now enable researchers to screen potential material combinations before experimental validation, significantly accelerating the development cycle of new interface engineering solutions.
Scalability and Manufacturing Considerations
The scalability of magnesium-ion battery electrode interface engineering represents a critical challenge in transitioning from laboratory-scale research to commercial production. Current manufacturing processes for conventional lithium-ion batteries cannot be directly applied to magnesium-ion systems due to fundamental differences in electrode-electrolyte interactions and interface chemistry. The highly reactive nature of magnesium metal anodes and the complex interface phenomena require specialized manufacturing environments and techniques that can maintain consistent interface properties at scale.
Material selection for large-scale production presents significant hurdles, as many promising electrode materials that perform well in laboratory settings face stability and durability issues when scaled up. The synthesis of interface modification layers, particularly protective coatings for magnesium anodes, demands precise control over thickness and composition uniformity across large surface areas. Current deposition techniques such as atomic layer deposition (ALD) and physical vapor deposition (PVD) face throughput limitations when applied to high-volume production scenarios.
Cost considerations further complicate the manufacturing landscape. Many advanced interface engineering approaches rely on expensive precursors or energy-intensive processes that become economically prohibitive at scale. The trade-off between interface performance and manufacturing cost requires careful optimization, potentially necessitating the development of alternative, more cost-effective interface engineering strategies specifically designed for mass production environments.
Quality control and characterization present additional challenges in scaled manufacturing. The analytical techniques commonly used in research settings, such as transmission electron microscopy and X-ray photoelectron spectroscopy, are not suitable for in-line production monitoring. New rapid characterization methods must be developed to ensure interface quality and consistency during high-volume manufacturing without creating production bottlenecks.
Environmental and safety considerations also impact manufacturing scalability. Many electrolyte formulations that enable effective magnesium-ion transport contain components that pose handling challenges in industrial settings. Engineering safer production processes while maintaining interface performance represents a key area requiring innovation before widespread commercialization becomes viable.
Recent advances in roll-to-roll processing techniques show promise for scaling certain interface modification approaches. Continuous deposition methods adapted from other industries could potentially address some throughput limitations, though significant process optimization remains necessary. Collaborative efforts between battery researchers and manufacturing engineers will be essential to bridge the gap between laboratory discoveries and commercially viable production methods for next-generation magnesium-ion battery systems.
Material selection for large-scale production presents significant hurdles, as many promising electrode materials that perform well in laboratory settings face stability and durability issues when scaled up. The synthesis of interface modification layers, particularly protective coatings for magnesium anodes, demands precise control over thickness and composition uniformity across large surface areas. Current deposition techniques such as atomic layer deposition (ALD) and physical vapor deposition (PVD) face throughput limitations when applied to high-volume production scenarios.
Cost considerations further complicate the manufacturing landscape. Many advanced interface engineering approaches rely on expensive precursors or energy-intensive processes that become economically prohibitive at scale. The trade-off between interface performance and manufacturing cost requires careful optimization, potentially necessitating the development of alternative, more cost-effective interface engineering strategies specifically designed for mass production environments.
Quality control and characterization present additional challenges in scaled manufacturing. The analytical techniques commonly used in research settings, such as transmission electron microscopy and X-ray photoelectron spectroscopy, are not suitable for in-line production monitoring. New rapid characterization methods must be developed to ensure interface quality and consistency during high-volume manufacturing without creating production bottlenecks.
Environmental and safety considerations also impact manufacturing scalability. Many electrolyte formulations that enable effective magnesium-ion transport contain components that pose handling challenges in industrial settings. Engineering safer production processes while maintaining interface performance represents a key area requiring innovation before widespread commercialization becomes viable.
Recent advances in roll-to-roll processing techniques show promise for scaling certain interface modification approaches. Continuous deposition methods adapted from other industries could potentially address some throughput limitations, though significant process optimization remains necessary. Collaborative efforts between battery researchers and manufacturing engineers will be essential to bridge the gap between laboratory discoveries and commercially viable production methods for next-generation magnesium-ion battery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!