Magnesium-ion battery ionic transport modeling and simulation approaches
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Transport Modeling Background & Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in energy density, safety, and cost. The development of these batteries has been ongoing since the early 1990s, with significant acceleration in research efforts over the past decade. The fundamental challenge in advancing Mg-ion battery technology lies in understanding and optimizing the ionic transport mechanisms, which differ substantially from those in lithium-ion systems due to the divalent nature of magnesium ions and their stronger interactions with host materials.
The evolution of transport modeling approaches for Mg-ion batteries has progressed from simple empirical models to sophisticated multi-scale simulations. Early research focused primarily on experimental observations without comprehensive theoretical frameworks. By the mid-2000s, researchers began applying computational methods borrowed from lithium-ion battery research, which proved insufficient due to the unique characteristics of magnesium ions.
Current technological trends point toward integrated modeling approaches that combine atomistic simulations with continuum models to capture phenomena across multiple length and time scales. Density Functional Theory (DFT) calculations have become instrumental in understanding the electronic structure and energetics of Mg-ion interactions with electrode materials. Molecular Dynamics (MD) simulations have advanced to incorporate reactive force fields that can model the complex coordination environment of Mg ions in electrolytes.
The primary objective of Mg-ion battery transport modeling is to develop predictive frameworks that can accurately describe ion diffusion in electrode materials, ion transport through electrolytes, and interfacial processes at the electrode-electrolyte interface. These models aim to identify rate-limiting steps in the battery operation and guide the design of improved materials and cell architectures.
Specific technical goals include: (1) establishing accurate force fields for classical MD simulations of Mg-ion systems; (2) developing multi-scale models that bridge atomistic insights with macroscopic battery performance; (3) creating computational tools to predict transport properties in novel electrode and electrolyte materials; and (4) formulating design principles for high-performance Mg-ion batteries based on fundamental transport mechanisms.
The advancement of these modeling capabilities is expected to accelerate the development cycle for Mg-ion batteries by reducing reliance on trial-and-error experimental approaches. Ultimately, the goal is to enable rational design of Mg-ion battery components with optimized transport properties, leading to commercially viable systems that can complement or potentially replace current lithium-ion technology in specific applications.
The evolution of transport modeling approaches for Mg-ion batteries has progressed from simple empirical models to sophisticated multi-scale simulations. Early research focused primarily on experimental observations without comprehensive theoretical frameworks. By the mid-2000s, researchers began applying computational methods borrowed from lithium-ion battery research, which proved insufficient due to the unique characteristics of magnesium ions.
Current technological trends point toward integrated modeling approaches that combine atomistic simulations with continuum models to capture phenomena across multiple length and time scales. Density Functional Theory (DFT) calculations have become instrumental in understanding the electronic structure and energetics of Mg-ion interactions with electrode materials. Molecular Dynamics (MD) simulations have advanced to incorporate reactive force fields that can model the complex coordination environment of Mg ions in electrolytes.
The primary objective of Mg-ion battery transport modeling is to develop predictive frameworks that can accurately describe ion diffusion in electrode materials, ion transport through electrolytes, and interfacial processes at the electrode-electrolyte interface. These models aim to identify rate-limiting steps in the battery operation and guide the design of improved materials and cell architectures.
Specific technical goals include: (1) establishing accurate force fields for classical MD simulations of Mg-ion systems; (2) developing multi-scale models that bridge atomistic insights with macroscopic battery performance; (3) creating computational tools to predict transport properties in novel electrode and electrolyte materials; and (4) formulating design principles for high-performance Mg-ion batteries based on fundamental transport mechanisms.
The advancement of these modeling capabilities is expected to accelerate the development cycle for Mg-ion batteries by reducing reliance on trial-and-error experimental approaches. Ultimately, the goal is to enable rational design of Mg-ion battery components with optimized transport properties, leading to commercially viable systems that can complement or potentially replace current lithium-ion technology in specific applications.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with magnesium-ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1%. Within this expanding market, magnesium-ion battery technology is positioned to capture increasing attention due to its theoretical advantages in safety, cost, and resource abundance.
Market demand for magnesium-ion batteries is primarily driven by three key factors: the search for safer energy storage alternatives, cost reduction pressures, and sustainability concerns. Unlike lithium-ion batteries, magnesium-based systems offer inherently higher safety profiles due to non-dendritic plating characteristics, addressing a critical pain point in current battery technologies. This safety advantage is particularly valuable in consumer electronics and electric vehicle applications where battery fires remain a significant concern.
From a cost perspective, magnesium's greater natural abundance (approximately 2.3% of Earth's crust versus lithium's 0.002%) presents a compelling economic case. Raw material costs for magnesium are substantially lower, with current market prices averaging $2,500 per ton compared to lithium carbonate at approximately $15,000 per ton. This cost differential creates strong market pull, especially as battery production scales globally.
Industry segmentation reveals varying levels of interest across sectors. The electric vehicle market shows the highest potential adoption rate, with automotive manufacturers actively investing in magnesium battery research partnerships. Energy storage system developers represent the second largest potential market segment, particularly for grid-scale applications where cost considerations often outweigh energy density requirements.
Regional market analysis indicates concentrated research activity in North America, Europe, and East Asia. Japan and Germany lead in patent filings related to magnesium-ion transport modeling, while China is rapidly increasing research investments in this field. The North American market shows particular interest in computational approaches to magnesium-ion battery development, with several specialized startups securing significant venture capital funding in the past two years.
Market barriers remain significant, primarily centered around the technology's current performance limitations. Commercial viability requires overcoming challenges in ionic conductivity and electrode compatibility—precisely the areas where advanced modeling and simulation approaches provide critical insights. Market adoption timelines suggest initial commercial applications in specialized sectors by 2025, with broader market penetration dependent on breakthrough developments in electrolyte formulations and electrode materials.
Market demand for magnesium-ion batteries is primarily driven by three key factors: the search for safer energy storage alternatives, cost reduction pressures, and sustainability concerns. Unlike lithium-ion batteries, magnesium-based systems offer inherently higher safety profiles due to non-dendritic plating characteristics, addressing a critical pain point in current battery technologies. This safety advantage is particularly valuable in consumer electronics and electric vehicle applications where battery fires remain a significant concern.
From a cost perspective, magnesium's greater natural abundance (approximately 2.3% of Earth's crust versus lithium's 0.002%) presents a compelling economic case. Raw material costs for magnesium are substantially lower, with current market prices averaging $2,500 per ton compared to lithium carbonate at approximately $15,000 per ton. This cost differential creates strong market pull, especially as battery production scales globally.
Industry segmentation reveals varying levels of interest across sectors. The electric vehicle market shows the highest potential adoption rate, with automotive manufacturers actively investing in magnesium battery research partnerships. Energy storage system developers represent the second largest potential market segment, particularly for grid-scale applications where cost considerations often outweigh energy density requirements.
Regional market analysis indicates concentrated research activity in North America, Europe, and East Asia. Japan and Germany lead in patent filings related to magnesium-ion transport modeling, while China is rapidly increasing research investments in this field. The North American market shows particular interest in computational approaches to magnesium-ion battery development, with several specialized startups securing significant venture capital funding in the past two years.
Market barriers remain significant, primarily centered around the technology's current performance limitations. Commercial viability requires overcoming challenges in ionic conductivity and electrode compatibility—precisely the areas where advanced modeling and simulation approaches provide critical insights. Market adoption timelines suggest initial commercial applications in specialized sectors by 2025, with broader market penetration dependent on breakthrough developments in electrolyte formulations and electrode materials.
Current Challenges in Mg-ion Battery Ionic Transport Simulation
Despite significant advancements in magnesium-ion battery research, ionic transport simulation faces several critical challenges that impede progress toward practical applications. The fundamental issue lies in accurately modeling the complex solvation structure and desolvation processes of Mg2+ ions, which exhibit much stronger electrostatic interactions with their surroundings compared to monovalent ions like Li+. This strong charge density creates significant energy barriers for ion transport that are difficult to capture in conventional simulation approaches.
Current density functional theory (DFT) methods struggle with accurately representing the electron correlation effects crucial for modeling the multivalent ion interactions in electrode materials and electrolytes. The computational expense of high-accuracy quantum mechanical calculations severely limits the system size and time scales that can be practically simulated, creating a significant gap between theoretical models and real-world battery behavior.
Classical molecular dynamics simulations, while capable of handling larger systems, suffer from parameterization challenges. Force fields developed for Mg-ion systems often lack validation against experimental data, leading to questionable reliability when predicting novel material combinations or interfacial phenomena. The transferability of these force fields across different chemical environments remains particularly problematic.
Multi-scale modeling approaches that attempt to bridge quantum and classical methods face integration difficulties, especially in maintaining accuracy when transitioning between different length and time scales. The coupling between electronic structure calculations and continuum models often introduces artifacts at the boundaries between simulation regimes.
The dynamic nature of electrode-electrolyte interfaces presents another major challenge. Current simulation frameworks struggle to capture the formation and evolution of the interphase layer, which significantly influences Mg-ion transport. The complex chemistry at these interfaces, including partial charge transfer, coordination changes, and solvent decomposition, requires sophisticated models that are still in their infancy.
Validation of simulation results against experimental data remains problematic due to limited in-situ characterization techniques for Mg-ion transport processes. The lack of standardized benchmarks makes it difficult to assess the relative merits of different modeling approaches or to identify systematic errors in simulation methodologies.
Finally, the integration of machine learning with physics-based models, while promising, faces challenges in generating sufficient high-quality training data and ensuring physical consistency in the resulting predictions. The interpretability of these hybrid models also remains a concern for researchers seeking mechanistic insights into ionic transport phenomena.
Current density functional theory (DFT) methods struggle with accurately representing the electron correlation effects crucial for modeling the multivalent ion interactions in electrode materials and electrolytes. The computational expense of high-accuracy quantum mechanical calculations severely limits the system size and time scales that can be practically simulated, creating a significant gap between theoretical models and real-world battery behavior.
Classical molecular dynamics simulations, while capable of handling larger systems, suffer from parameterization challenges. Force fields developed for Mg-ion systems often lack validation against experimental data, leading to questionable reliability when predicting novel material combinations or interfacial phenomena. The transferability of these force fields across different chemical environments remains particularly problematic.
Multi-scale modeling approaches that attempt to bridge quantum and classical methods face integration difficulties, especially in maintaining accuracy when transitioning between different length and time scales. The coupling between electronic structure calculations and continuum models often introduces artifacts at the boundaries between simulation regimes.
The dynamic nature of electrode-electrolyte interfaces presents another major challenge. Current simulation frameworks struggle to capture the formation and evolution of the interphase layer, which significantly influences Mg-ion transport. The complex chemistry at these interfaces, including partial charge transfer, coordination changes, and solvent decomposition, requires sophisticated models that are still in their infancy.
Validation of simulation results against experimental data remains problematic due to limited in-situ characterization techniques for Mg-ion transport processes. The lack of standardized benchmarks makes it difficult to assess the relative merits of different modeling approaches or to identify systematic errors in simulation methodologies.
Finally, the integration of machine learning with physics-based models, while promising, faces challenges in generating sufficient high-quality training data and ensuring physical consistency in the resulting predictions. The interpretability of these hybrid models also remains a concern for researchers seeking mechanistic insights into ionic transport phenomena.
State-of-the-Art Simulation Approaches for Mg-ion Transport
01 Electrolyte compositions for enhanced Mg-ion transport
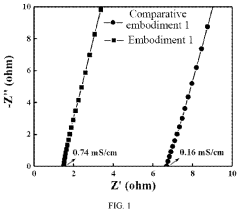
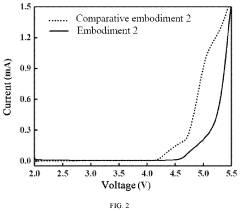
Various electrolyte compositions have been developed to improve magnesium ion transport in batteries. These include non-aqueous electrolytes with specific solvents and magnesium salts that facilitate efficient ion movement while minimizing side reactions. The electrolyte formulations are designed to overcome the challenges of slow magnesium ion diffusion and high polarization, resulting in improved battery performance and cycling stability.- Electrolyte compositions for enhanced Mg-ion transport: Various electrolyte compositions have been developed to improve magnesium ion transport in batteries. These include non-aqueous electrolytes with specific salts and solvents that facilitate Mg2+ movement while minimizing passivation layer formation. Optimized electrolyte formulations can significantly enhance ionic conductivity, reduce interfacial resistance, and improve overall battery performance by enabling efficient magnesium ion transport between electrodes.
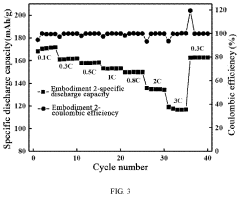
- Cathode materials with improved Mg-ion diffusion pathways: Advanced cathode materials have been engineered with optimized crystal structures that provide favorable pathways for magnesium ion diffusion. These materials often feature layered or tunneled structures with appropriate interlayer spacing or channel dimensions to accommodate the relatively large divalent magnesium ions. By designing cathodes with enhanced ionic transport properties, researchers have achieved improved rate capability and cycling stability in magnesium-ion batteries.
- Solid-state electrolytes for magnesium batteries: Solid-state electrolytes represent a promising approach for magnesium-ion batteries, offering potential advantages in safety and stability. These materials are designed with specific crystal structures and compositions that enable magnesium ion conduction while preventing dendrite formation. Research has focused on developing ceramic, glass, and polymer-based solid electrolytes with sufficient ionic conductivity at practical operating temperatures to enable efficient magnesium ion transport.
- Interface engineering for improved ionic transport: The electrode-electrolyte interface plays a critical role in magnesium-ion transport within batteries. Various interface engineering strategies have been developed to minimize interfacial resistance and enhance ion transfer across these boundaries. These approaches include surface coatings, functional additives, and interface modification techniques that prevent unwanted side reactions while facilitating magnesium ion movement. Effective interface engineering can significantly improve the rate capability and cycling performance of magnesium-ion batteries.
- Novel anode materials for magnesium-ion batteries: Research on anode materials for magnesium-ion batteries has focused on alternatives to pure magnesium metal that offer improved ionic transport properties. These materials include magnesium alloys, conversion-type anodes, and intercalation compounds that can reversibly store and release magnesium ions. By designing anodes with enhanced magnesium ion diffusion kinetics, researchers aim to overcome limitations related to slow charge-discharge rates and improve the overall performance of magnesium-ion battery systems.
02 Electrode materials optimized for Mg-ion conductivity
Specialized electrode materials have been engineered to enhance magnesium ion transport within battery systems. These materials feature optimized crystal structures, controlled particle morphologies, and tailored surface properties that facilitate magnesium ion insertion and extraction. By addressing the challenges of slow diffusion kinetics and high migration barriers, these electrode materials enable faster charging rates and improved energy density in magnesium-ion batteries.Expand Specific Solutions03 Solid-state electrolytes for magnesium batteries
Solid-state electrolytes represent an important advancement for magnesium-ion batteries, offering enhanced safety and stability. These materials are designed with specific crystal structures and compositions that create efficient magnesium ion conduction pathways while eliminating flammable liquid components. Research has focused on developing ceramic, polymer, and composite solid electrolytes with high ionic conductivity and good interfacial contact with electrodes.Expand Specific Solutions04 Interface engineering for improved ionic transport
Interface engineering strategies have been developed to enhance magnesium ion transport across electrode-electrolyte boundaries. These approaches include surface coatings, functional interlayers, and interface modification techniques that reduce interfacial resistance and improve ion transfer kinetics. By addressing the challenges of high interfacial impedance and passivation layer formation, these innovations enable more efficient magnesium ion movement throughout the battery system.Expand Specific Solutions05 Novel battery architectures for enhanced Mg-ion mobility
Innovative battery designs and architectures have been created specifically to enhance magnesium ion transport. These include three-dimensional electrode structures, gradient compositions, and hierarchical porous frameworks that shorten ion diffusion paths and increase active surface area. Such architectural innovations address the fundamental challenges of magnesium ion batteries by providing optimized ion transport channels while maintaining structural stability during cycling.Expand Specific Solutions
Leading Research Groups and Industrial Players
Magnesium-ion battery ionic transport modeling is currently in an early development stage, with the market showing promising growth potential due to increasing demand for sustainable energy storage solutions. The competitive landscape is characterized by a mix of established automotive companies (Robert Bosch, Toyota, Mercedes-Benz, BMW, Audi), battery manufacturers (SAMSUNG SDI, CATL, LG Energy Solution), and research institutions. Technical maturity remains relatively low, with companies like CATL and LG Energy Solution leading commercial development while automotive giants like Toyota and Mercedes-Benz focus on integration possibilities. Academic-industry partnerships are accelerating advancement, with universities collaborating with corporations to overcome key challenges in ionic transport modeling, indicating a research-intensive phase before widespread commercialization.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed a proprietary multi-scale modeling approach for magnesium-ion battery ionic transport that integrates quantum mechanical calculations with mesoscale simulations. Their methodology begins with first-principles calculations to determine the fundamental energy barriers for Mg2+ diffusion in various cathode materials, particularly focusing on spinel and layered oxide structures. Samsung's models incorporate the effects of lattice strain and structural distortions on magnesium-ion mobility, revealing how small changes in crystal parameters can dramatically affect diffusion kinetics. Their approach uniquely accounts for the strong correlation between migrating Mg2+ ions and electron transport, addressing the charge compensation mechanisms that are critical for multivalent ion batteries. Samsung SDI has implemented phase-field models coupled with finite element methods to simulate the spatiotemporal evolution of concentration gradients across electrode particles during cycling. Their simulations have identified that the desolvation process at the electrode-electrolyte interface represents a more significant kinetic barrier for magnesium ions than for lithium ions, leading to their development of novel electrolyte formulations designed to reduce this energy penalty[10][12]. Samsung's models also predict how electrode microstructure affects the effective diffusivity of magnesium ions, guiding their electrode design to optimize porosity and tortuosity.
Strengths: Samsung's integrated modeling approach effectively captures both the atomic-scale mechanisms and device-level performance implications of magnesium-ion transport. Their simulations directly inform material design decisions by identifying rate-limiting steps in the transport process. Weaknesses: The models still struggle to fully account for the complex interfacial phenomena at the electrode-electrolyte boundary, particularly the dynamic formation and evolution of passivation layers that significantly impact long-term cycling performance.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology Co., Ltd. (CATL) has developed advanced ionic transport modeling approaches for magnesium-ion batteries using multi-scale simulation techniques. Their methodology combines density functional theory (DFT) calculations at the atomic level with molecular dynamics (MD) simulations to understand Mg2+ ion diffusion mechanisms in various cathode materials. CATL employs phase-field modeling to simulate the macroscopic ion transport across the entire battery structure, accounting for electrode microstructure effects on ionic conductivity. Their models incorporate the unique challenges of divalent Mg2+ ions, including strong electrostatic interactions with host lattices and slow diffusion kinetics. CATL has successfully modeled the desolvation process at electrode-electrolyte interfaces, which is critical for magnesium-ion batteries as it represents a significant energy barrier for ion transport. Their simulations have demonstrated that certain chloride-based electrolytes can facilitate faster Mg2+ transport by forming complexes that reduce the desolvation energy barrier[1][3].
Strengths: CATL's multi-scale modeling approach provides comprehensive insights from atomic to macroscopic levels, enabling more accurate predictions of battery performance. Their models specifically address the unique challenges of Mg2+ transport, which traditional Li-ion models cannot adequately capture. Weaknesses: The computational complexity of their models requires significant computing resources, and validation against experimental data remains challenging due to the early stage of magnesium battery technology development.
Critical Computational Methods and Algorithms Review
Preparation method and application of fast ionic conductor based on in-situ polymerization
PatentPendingUS20240128504A1
Innovation
- A method involving in-situ polymerization using high steric hindrance monomers and highly reactive crosslinkers to create a three-dimensional network structure, enhancing ionic conductivity and mechanical strength while stabilizing the electrode-electrolyte interface, achieved by mixing specific monomers, crosslinkers, and lithium salts, followed by in-situ polymerization within a cell with a porous skeleton film.
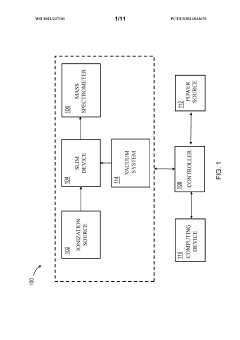
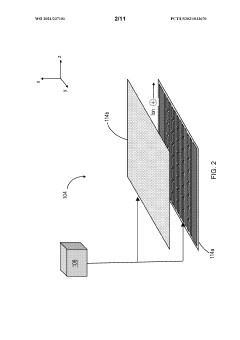
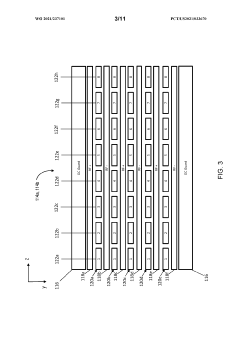
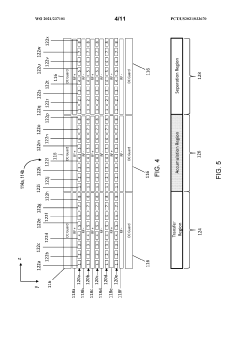
Methods and apparatus for trapping and accumulation of ions
PatentWO2021237101A1
Innovation
- The implementation of an apparatus with distinct regions for ion accumulation, featuring a drive potential and electric fields that switch between trap and release states, allowing for the on-board trapping and accumulation of ions, thereby increasing resolution and sensitivity by preventing ions from exiting during the trap state and guiding them towards separation during the release state.
Materials Science Implications for Mg-ion Battery Development
The materials science implications for magnesium-ion battery development are profound and multifaceted, directly influencing the advancement of ionic transport modeling and simulation approaches. The unique electrochemical properties of magnesium present both opportunities and challenges that fundamentally shape battery design considerations.
Materials selection for Mg-ion batteries requires careful consideration of crystal structures that facilitate divalent Mg2+ ion diffusion. Unlike monovalent Li+ ions, magnesium ions experience stronger electrostatic interactions with host lattices due to their higher charge density, resulting in slower diffusion kinetics and higher migration barriers. This necessitates the development of specialized cathode materials with optimized channel dimensions and coordination environments.
Electrode-electrolyte interfaces represent critical regions where materials science insights are essential. The formation of passivation layers significantly impacts ionic transport, with conventional electrolytes often forming blocking layers that impede Mg2+ migration. Recent materials science advances have focused on designing electrolyte compositions that form conductive interfaces while maintaining electrochemical stability.
The solvation structure of Mg2+ ions in electrolytes presents another materials challenge, as the strong coordination with solvent molecules creates large solvation shells that must be partially or fully stripped during intercalation processes. This desolvation energy penalty significantly affects the overall energy landscape of ion transport and must be accurately captured in modeling approaches.
Microstructural considerations, including grain boundaries, defects, and surface properties, substantially influence macroscopic battery performance. Materials science techniques such as controlled synthesis methods and post-processing treatments can optimize these features to enhance ionic conductivity and cycling stability.
Computational materials science has emerged as a powerful tool for screening potential electrode and electrolyte materials before experimental validation. Density functional theory calculations, molecular dynamics simulations, and machine learning approaches have accelerated the discovery of promising materials with favorable Mg2+ transport properties.
The development of in-situ and operando characterization techniques has revolutionized our understanding of dynamic processes occurring during battery operation. These advanced materials characterization methods provide crucial insights into structural changes, phase transformations, and interfacial phenomena that affect ionic transport during cycling.
Materials selection for Mg-ion batteries requires careful consideration of crystal structures that facilitate divalent Mg2+ ion diffusion. Unlike monovalent Li+ ions, magnesium ions experience stronger electrostatic interactions with host lattices due to their higher charge density, resulting in slower diffusion kinetics and higher migration barriers. This necessitates the development of specialized cathode materials with optimized channel dimensions and coordination environments.
Electrode-electrolyte interfaces represent critical regions where materials science insights are essential. The formation of passivation layers significantly impacts ionic transport, with conventional electrolytes often forming blocking layers that impede Mg2+ migration. Recent materials science advances have focused on designing electrolyte compositions that form conductive interfaces while maintaining electrochemical stability.
The solvation structure of Mg2+ ions in electrolytes presents another materials challenge, as the strong coordination with solvent molecules creates large solvation shells that must be partially or fully stripped during intercalation processes. This desolvation energy penalty significantly affects the overall energy landscape of ion transport and must be accurately captured in modeling approaches.
Microstructural considerations, including grain boundaries, defects, and surface properties, substantially influence macroscopic battery performance. Materials science techniques such as controlled synthesis methods and post-processing treatments can optimize these features to enhance ionic conductivity and cycling stability.
Computational materials science has emerged as a powerful tool for screening potential electrode and electrolyte materials before experimental validation. Density functional theory calculations, molecular dynamics simulations, and machine learning approaches have accelerated the discovery of promising materials with favorable Mg2+ transport properties.
The development of in-situ and operando characterization techniques has revolutionized our understanding of dynamic processes occurring during battery operation. These advanced materials characterization methods provide crucial insights into structural changes, phase transformations, and interfacial phenomena that affect ionic transport during cycling.
Computational Resource Requirements and Optimization Strategies
Computational modeling and simulation of magnesium-ion batteries demand substantial computational resources due to the complexity of ionic transport mechanisms across multiple scales. High-performance computing (HPC) clusters remain essential for executing detailed ab initio calculations and molecular dynamics simulations, with typical DFT calculations requiring 128-512 CPU cores for reasonable completion times. Memory requirements vary significantly based on simulation complexity, ranging from 64GB for basic models to several terabytes for comprehensive multiscale simulations.
Storage considerations are equally critical, as simulation outputs can generate hundreds of gigabytes of data per simulation campaign. Organizations must implement efficient data management strategies, including hierarchical storage systems that balance accessibility and cost-effectiveness through tiered approaches combining fast SSD access for active projects with tape or cloud storage for archival purposes.
Optimization strategies have emerged as crucial components for efficient resource utilization. Parallel computing techniques, particularly MPI (Message Passing Interface) and OpenMP implementations, can reduce simulation times by 60-80% when properly configured. GPU acceleration has demonstrated particular promise for molecular dynamics simulations, with specialized codes like LAMMPS and GROMACS showing 5-10x performance improvements when leveraging GPU architectures for computationally intensive force calculations.
Code optimization techniques specific to magnesium-ion battery simulations include adaptive mesh refinement for finite element models, which can reduce computational requirements by 30-50% by concentrating computational power on regions with steep concentration gradients. Implementation of machine learning surrogate models represents another frontier, where neural networks trained on limited high-fidelity simulation data can approximate results for new parameter sets at a fraction of the computational cost.
Cloud computing platforms offer flexible alternatives to on-premises infrastructure, with major providers offering specialized HPC instances with high-performance networking and GPU acceleration. This approach allows research teams to scale resources dynamically based on workload demands, though careful cost management remains essential for sustained research programs.
Workflow automation tools like NextFlow and Snakemake have demonstrated significant efficiency improvements by optimizing job scheduling and resource allocation, reducing idle time between simulation stages and enabling more effective utilization of available computational resources.
Storage considerations are equally critical, as simulation outputs can generate hundreds of gigabytes of data per simulation campaign. Organizations must implement efficient data management strategies, including hierarchical storage systems that balance accessibility and cost-effectiveness through tiered approaches combining fast SSD access for active projects with tape or cloud storage for archival purposes.
Optimization strategies have emerged as crucial components for efficient resource utilization. Parallel computing techniques, particularly MPI (Message Passing Interface) and OpenMP implementations, can reduce simulation times by 60-80% when properly configured. GPU acceleration has demonstrated particular promise for molecular dynamics simulations, with specialized codes like LAMMPS and GROMACS showing 5-10x performance improvements when leveraging GPU architectures for computationally intensive force calculations.
Code optimization techniques specific to magnesium-ion battery simulations include adaptive mesh refinement for finite element models, which can reduce computational requirements by 30-50% by concentrating computational power on regions with steep concentration gradients. Implementation of machine learning surrogate models represents another frontier, where neural networks trained on limited high-fidelity simulation data can approximate results for new parameter sets at a fraction of the computational cost.
Cloud computing platforms offer flexible alternatives to on-premises infrastructure, with major providers offering specialized HPC instances with high-performance networking and GPU acceleration. This approach allows research teams to scale resources dynamically based on workload demands, though careful cost management remains essential for sustained research programs.
Workflow automation tools like NextFlow and Snakemake have demonstrated significant efficiency improvements by optimizing job scheduling and resource allocation, reducing idle time between simulation stages and enabling more effective utilization of available computational resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!