How high-voltage cathodes influence magnesium-ion battery cycling stability
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Ion Battery Cathode Development Background & Objectives
Magnesium-ion batteries (MIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of MIBs traces back to the early 1990s, but significant research momentum has only been gained in the past decade. The evolution of this technology has been primarily driven by the increasing demand for safer, more sustainable, and higher energy density energy storage solutions beyond the limitations of lithium-ion systems.
High-voltage cathodes represent a critical component in advancing MIB technology, as they directly influence the energy density and cycling stability of these batteries. Historically, cathode materials for MIBs have evolved from simple conversion-type materials to more complex intercalation compounds capable of accommodating the divalent magnesium ions. This evolution reflects the growing understanding of the unique challenges posed by magnesium's divalent nature and strong coulombic interactions with host structures.
The technical objectives for high-voltage cathode development in MIBs center around addressing several key challenges. Primary among these is achieving stable cycling performance at high voltages (>3V vs. Mg/Mg²⁺), which has proven difficult due to electrolyte decomposition and cathode structural degradation. Researchers aim to develop cathode materials that can maintain structural integrity during repeated magnesium insertion/extraction cycles while operating at voltages that maximize energy density.
Another critical objective involves understanding and mitigating the kinetic limitations associated with magnesium-ion diffusion in cathode materials. The strong interaction between Mg²⁺ ions and host lattices often results in sluggish diffusion kinetics, which negatively impacts rate capability and practical energy density. Developing cathode architectures that facilitate faster magnesium-ion transport represents a key technical goal.
The field is also focused on elucidating the fundamental mechanisms of cathode degradation during high-voltage operation. This includes investigating interfacial phenomena, structural transformations, and chemical side reactions that occur during cycling. Understanding these mechanisms is essential for designing more stable cathode materials and compatible electrolyte systems.
Recent technical trends indicate growing interest in layered materials, spinel structures, and polyanionic compounds as potential high-voltage cathode candidates. Concurrently, there is increasing emphasis on developing multivalent-compatible electrolytes that remain stable at high operating voltages, as the electrolyte-cathode interface plays a crucial role in determining cycling stability.
The ultimate technical goal is to achieve a magnesium-ion battery system with high energy density (>400 Wh/kg), excellent cycling stability (>1000 cycles with minimal capacity fade), and rate capability comparable to current lithium-ion technologies, while maintaining the inherent safety and cost advantages of magnesium-based systems.
High-voltage cathodes represent a critical component in advancing MIB technology, as they directly influence the energy density and cycling stability of these batteries. Historically, cathode materials for MIBs have evolved from simple conversion-type materials to more complex intercalation compounds capable of accommodating the divalent magnesium ions. This evolution reflects the growing understanding of the unique challenges posed by magnesium's divalent nature and strong coulombic interactions with host structures.
The technical objectives for high-voltage cathode development in MIBs center around addressing several key challenges. Primary among these is achieving stable cycling performance at high voltages (>3V vs. Mg/Mg²⁺), which has proven difficult due to electrolyte decomposition and cathode structural degradation. Researchers aim to develop cathode materials that can maintain structural integrity during repeated magnesium insertion/extraction cycles while operating at voltages that maximize energy density.
Another critical objective involves understanding and mitigating the kinetic limitations associated with magnesium-ion diffusion in cathode materials. The strong interaction between Mg²⁺ ions and host lattices often results in sluggish diffusion kinetics, which negatively impacts rate capability and practical energy density. Developing cathode architectures that facilitate faster magnesium-ion transport represents a key technical goal.
The field is also focused on elucidating the fundamental mechanisms of cathode degradation during high-voltage operation. This includes investigating interfacial phenomena, structural transformations, and chemical side reactions that occur during cycling. Understanding these mechanisms is essential for designing more stable cathode materials and compatible electrolyte systems.
Recent technical trends indicate growing interest in layered materials, spinel structures, and polyanionic compounds as potential high-voltage cathode candidates. Concurrently, there is increasing emphasis on developing multivalent-compatible electrolytes that remain stable at high operating voltages, as the electrolyte-cathode interface plays a crucial role in determining cycling stability.
The ultimate technical goal is to achieve a magnesium-ion battery system with high energy density (>400 Wh/kg), excellent cycling stability (>1000 cycles with minimal capacity fade), and rate capability comparable to current lithium-ion technologies, while maintaining the inherent safety and cost advantages of magnesium-based systems.
Market Analysis for High-Voltage Mg-Ion Battery Applications
The global market for magnesium-ion batteries with high-voltage cathodes is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current projections indicate the energy storage market will reach $546 billion by 2035, with next-generation battery technologies like magnesium-ion systems poised to capture a growing segment as technical challenges are overcome.
High-voltage magnesium-ion batteries present compelling market advantages over traditional lithium-ion technologies, particularly in safety, resource abundance, and theoretical energy density. Magnesium is substantially more abundant in the Earth's crust than lithium (2.1% vs 0.006%), translating to potentially lower raw material costs and reduced supply chain vulnerabilities. This advantage becomes increasingly significant as global battery production scales to meet renewable energy and electric vehicle demands.
Market research indicates that sectors requiring high energy density, long cycle life, and enhanced safety profiles represent the primary target markets for high-voltage Mg-ion batteries. These include grid-scale energy storage, electric vehicles, aerospace applications, and military systems where safety considerations are paramount. The grid storage segment alone is projected to grow at a CAGR of 24% through 2030, creating substantial opportunities for advanced battery technologies.
Consumer electronics manufacturers have also expressed interest in magnesium-ion technology, particularly as concerns about lithium-ion battery safety incidents persist. The potential for higher energy density without corresponding safety risks presents a compelling value proposition for portable device manufacturers seeking differentiation in competitive markets.
Regional market analysis reveals varying levels of investment and research focus. Asia-Pacific leads in commercial development efforts, with significant research initiatives in Japan, China, and South Korea. North America and Europe maintain strong academic research programs but lag in commercialization pathways. Government funding for magnesium battery research has increased by approximately 35% over the past five years across major economies.
Market barriers remain significant, primarily centered on the technical challenges of cathode stability at high voltages. Industry surveys indicate that achieving 1000+ stable cycles at voltages above 3V would trigger substantial commercial interest from multiple sectors. Current performance limitations restrict market penetration to niche applications where specific advantages outweigh performance constraints.
Investment trends show growing venture capital interest, with funding for magnesium battery startups increasing from $87 million in 2018 to $215 million in 2022. Strategic partnerships between academic institutions, material science companies, and battery manufacturers have accelerated, indicating growing confidence in eventual commercial viability of high-voltage magnesium-ion systems.
High-voltage magnesium-ion batteries present compelling market advantages over traditional lithium-ion technologies, particularly in safety, resource abundance, and theoretical energy density. Magnesium is substantially more abundant in the Earth's crust than lithium (2.1% vs 0.006%), translating to potentially lower raw material costs and reduced supply chain vulnerabilities. This advantage becomes increasingly significant as global battery production scales to meet renewable energy and electric vehicle demands.
Market research indicates that sectors requiring high energy density, long cycle life, and enhanced safety profiles represent the primary target markets for high-voltage Mg-ion batteries. These include grid-scale energy storage, electric vehicles, aerospace applications, and military systems where safety considerations are paramount. The grid storage segment alone is projected to grow at a CAGR of 24% through 2030, creating substantial opportunities for advanced battery technologies.
Consumer electronics manufacturers have also expressed interest in magnesium-ion technology, particularly as concerns about lithium-ion battery safety incidents persist. The potential for higher energy density without corresponding safety risks presents a compelling value proposition for portable device manufacturers seeking differentiation in competitive markets.
Regional market analysis reveals varying levels of investment and research focus. Asia-Pacific leads in commercial development efforts, with significant research initiatives in Japan, China, and South Korea. North America and Europe maintain strong academic research programs but lag in commercialization pathways. Government funding for magnesium battery research has increased by approximately 35% over the past five years across major economies.
Market barriers remain significant, primarily centered on the technical challenges of cathode stability at high voltages. Industry surveys indicate that achieving 1000+ stable cycles at voltages above 3V would trigger substantial commercial interest from multiple sectors. Current performance limitations restrict market penetration to niche applications where specific advantages outweigh performance constraints.
Investment trends show growing venture capital interest, with funding for magnesium battery startups increasing from $87 million in 2018 to $215 million in 2022. Strategic partnerships between academic institutions, material science companies, and battery manufacturers have accelerated, indicating growing confidence in eventual commercial viability of high-voltage magnesium-ion systems.
High-Voltage Cathode Technology Challenges & Limitations
High-voltage cathodes represent a critical frontier in magnesium-ion battery development, offering theoretical pathways to higher energy densities. However, these materials face substantial challenges that currently limit their practical implementation. The primary obstacle lies in the strong electrostatic interaction between divalent Mg2+ ions and host cathode structures, resulting in sluggish diffusion kinetics and significant structural degradation during cycling.
Conventional cathode materials experience severe voltage hysteresis when operating at high voltages (>3.0V), where the insertion/extraction potentials differ by more than 1V. This phenomenon drastically reduces energy efficiency and accelerates capacity fading. The root cause stems from the formation of strong Mg-O bonds within the cathode framework that require excessive energy to break during demagnesiation.
Structural stability presents another critical limitation. High-voltage operation induces substantial volume changes in cathode materials, leading to mechanical stress, particle fracturing, and eventual electrode pulverization. These structural changes create new surfaces that react with electrolytes, forming passivation layers that impede Mg2+ transport and increase cell impedance over multiple cycles.
Electrolyte compatibility issues further complicate high-voltage cathode development. Most Mg-ion electrolytes demonstrate narrow electrochemical stability windows, typically below 3.0V vs. Mg/Mg2+. When pushed beyond these limits, electrolytes undergo oxidative decomposition at the cathode surface, generating insulating layers that block ion transport pathways and contribute to rapid capacity decay.
The electronic conductivity of high-voltage cathode materials often deteriorates during cycling. The repeated insertion/extraction of Mg2+ ions can alter the electronic structure of transition metal centers, affecting charge transfer mechanisms and overall electrode kinetics. This phenomenon is particularly pronounced in materials containing redox-active transition metals like manganese, which may undergo dissolution at high potentials.
Surface chemistry challenges also emerge during high-voltage operation. Cathode surfaces frequently experience side reactions with electrolyte components, leading to the formation of resistive interphases. Unlike lithium-ion systems, these interphases in magnesium batteries are rarely conductive to Mg2+ ions, effectively blocking active sites and contributing to capacity loss.
Synthesis and processing limitations further restrict high-voltage cathode development. Achieving precise control over stoichiometry, crystallinity, and particle morphology remains challenging, yet these parameters significantly influence electrochemical performance. Additionally, many promising high-voltage cathode materials require complex synthesis routes that present scalability challenges for commercial production.
Conventional cathode materials experience severe voltage hysteresis when operating at high voltages (>3.0V), where the insertion/extraction potentials differ by more than 1V. This phenomenon drastically reduces energy efficiency and accelerates capacity fading. The root cause stems from the formation of strong Mg-O bonds within the cathode framework that require excessive energy to break during demagnesiation.
Structural stability presents another critical limitation. High-voltage operation induces substantial volume changes in cathode materials, leading to mechanical stress, particle fracturing, and eventual electrode pulverization. These structural changes create new surfaces that react with electrolytes, forming passivation layers that impede Mg2+ transport and increase cell impedance over multiple cycles.
Electrolyte compatibility issues further complicate high-voltage cathode development. Most Mg-ion electrolytes demonstrate narrow electrochemical stability windows, typically below 3.0V vs. Mg/Mg2+. When pushed beyond these limits, electrolytes undergo oxidative decomposition at the cathode surface, generating insulating layers that block ion transport pathways and contribute to rapid capacity decay.
The electronic conductivity of high-voltage cathode materials often deteriorates during cycling. The repeated insertion/extraction of Mg2+ ions can alter the electronic structure of transition metal centers, affecting charge transfer mechanisms and overall electrode kinetics. This phenomenon is particularly pronounced in materials containing redox-active transition metals like manganese, which may undergo dissolution at high potentials.
Surface chemistry challenges also emerge during high-voltage operation. Cathode surfaces frequently experience side reactions with electrolyte components, leading to the formation of resistive interphases. Unlike lithium-ion systems, these interphases in magnesium batteries are rarely conductive to Mg2+ ions, effectively blocking active sites and contributing to capacity loss.
Synthesis and processing limitations further restrict high-voltage cathode development. Achieving precise control over stoichiometry, crystallinity, and particle morphology remains challenging, yet these parameters significantly influence electrochemical performance. Additionally, many promising high-voltage cathode materials require complex synthesis routes that present scalability challenges for commercial production.
Current High-Voltage Cathode Design Strategies
01 Electrode materials for improved cycling stability
Various electrode materials can be used to enhance the cycling stability of magnesium-ion batteries. These include specially designed cathode materials, anode materials with optimized structures, and composite electrodes that can accommodate the volume changes during charge-discharge cycles. The selection of appropriate electrode materials is crucial for achieving long-term cycling performance and preventing capacity fading in magnesium-ion batteries.- Electrode materials for improved cycling stability: Various electrode materials can be used to enhance the cycling stability of magnesium-ion batteries. These include specially designed cathode materials, anode materials with optimized structures, and composite electrodes that can accommodate the volume changes during charge-discharge cycles. The incorporation of certain metal oxides, sulfides, and carbon-based materials can significantly improve the structural stability of electrodes during repeated magnesium ion insertion and extraction, leading to better cycling performance.
- Electrolyte formulations for enhanced stability: The composition and formulation of electrolytes play a crucial role in the cycling stability of magnesium-ion batteries. Non-corrosive electrolytes with high ionic conductivity, wide electrochemical windows, and compatibility with electrode materials can significantly improve battery performance. Electrolytes containing specific magnesium salts, solvents, and additives can reduce side reactions, prevent electrode degradation, and facilitate efficient magnesium ion transport, thereby enhancing the overall cycling stability of the battery system.
- Interface engineering and protective coatings: Engineering the electrode-electrolyte interface and applying protective coatings can significantly improve the cycling stability of magnesium-ion batteries. Surface modifications of electrode materials, application of thin protective layers, and interface stabilization techniques can prevent unwanted side reactions, reduce electrode dissolution, and maintain structural integrity during cycling. These approaches help to establish stable solid-electrolyte interfaces that are crucial for long-term battery performance.
- Novel battery architectures and designs: Innovative battery architectures and designs can address the cycling stability challenges in magnesium-ion batteries. These include dual-salt systems, hybrid battery configurations, three-dimensional electrode structures, and specially designed cell components. Advanced battery designs that accommodate the unique characteristics of magnesium ion transport and storage can minimize mechanical stress, improve ion diffusion kinetics, and enhance overall cycling performance.
- Additives and dopants for stability enhancement: Various additives and dopants can be incorporated into magnesium-ion battery components to enhance cycling stability. These include electrolyte additives that form protective films, electrode dopants that improve structural stability, and surface modifiers that facilitate ion transport. Strategic use of these additives can mitigate degradation mechanisms, suppress unwanted reactions, and maintain the electrochemical performance of the battery over extended cycling.
02 Electrolyte formulations for enhanced stability
The composition and formulation of electrolytes significantly impact the cycling stability of magnesium-ion batteries. Non-corrosive electrolytes, electrolyte additives, and novel electrolyte systems can prevent electrode degradation and improve the reversibility of magnesium deposition/dissolution. Optimized electrolyte formulations help to form stable solid-electrolyte interfaces and reduce side reactions that would otherwise lead to capacity loss during cycling.Expand Specific Solutions03 Surface modification and coating technologies
Surface modification and coating of electrode materials can effectively improve the cycling stability of magnesium-ion batteries. Protective coatings can prevent direct contact between electrode materials and electrolytes, reducing unwanted side reactions. Surface treatments can also enhance the structural integrity of electrodes during repeated magnesium insertion/extraction, leading to improved cycling performance and extended battery life.Expand Specific Solutions04 Novel battery architectures and designs
Innovative battery architectures and cell designs can significantly enhance the cycling stability of magnesium-ion batteries. These include dual-salt systems, hybrid battery configurations, and specialized cell structures that accommodate the unique characteristics of magnesium-ion transport. Advanced battery designs can mitigate issues such as dendrite formation and volume expansion, which are common challenges affecting the cycling stability of magnesium-ion batteries.Expand Specific Solutions05 Advanced testing and characterization methods
Advanced testing protocols and characterization techniques are essential for evaluating and improving the cycling stability of magnesium-ion batteries. These include accelerated aging tests, in-situ monitoring of structural changes, and post-mortem analysis of cycled cells. By understanding the degradation mechanisms through sophisticated analytical methods, researchers can develop targeted strategies to enhance the long-term cycling performance of magnesium-ion battery systems.Expand Specific Solutions
Leading Research Groups & Industrial Players in Mg-Ion Technology
The magnesium-ion battery market is currently in an early development stage, with significant research focus on high-voltage cathodes to overcome cycling stability challenges. The global market remains relatively small but shows promising growth potential as an alternative to lithium-ion technology. Companies like Wildcat Discovery Technologies and BASF are leading research efforts in advanced cathode materials, while major battery manufacturers including Contemporary Amperex Technology, LG Chem, and Toyota are investing in magnesium-ion technology development. Academic institutions such as Central South University and Nankai University collaborate with industry players to address technical barriers. The technology remains at mid-maturity level, with commercial viability still requiring breakthroughs in cathode design to enhance cycling performance and energy density.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has developed a high-throughput screening platform specifically optimized for magnesium-ion battery cathode materials that addresses cycling stability at high voltages. Their approach combines computational modeling with rapid experimental validation to identify promising cathode compositions and structures. Wildcat has pioneered the development of spinel-type oxide cathodes with carefully engineered dopant combinations that stabilize the crystal structure during repeated magnesium insertion/extraction cycles. Their technology incorporates specialized surface coatings that prevent direct contact between the high-voltage cathode material and the electrolyte, significantly reducing parasitic reactions that lead to capacity fade. A key innovation in their approach is the simultaneous optimization of cathode materials and compatible electrolyte formulations, creating systems specifically designed to work together for enhanced stability. Their research has demonstrated that controlling the particle morphology and size distribution of cathode materials can significantly impact the kinetics of magnesium ion diffusion while maintaining structural integrity during high-voltage cycling.
Strengths: Industry-leading high-throughput screening capabilities allow rapid testing of numerous material combinations, accelerating discovery of optimal formulations with data-driven approach. Weaknesses: As a technology development company rather than a manufacturer, may face challenges in scaling promising materials to commercial production volumes without manufacturing partnerships.
Toyota Motor Corp.
Technical Solution: Toyota has developed proprietary high-voltage cathode materials for magnesium-ion batteries that significantly enhance cycling stability. Their approach centers on chevrel-phase materials (Mo6S8-based) with modified crystal structures that maintain stability during repeated magnesium insertion/extraction. Toyota's research teams have engineered specialized surface coatings for these cathodes that prevent detrimental side reactions with electrolytes at high voltages (>2.5V vs. Mg/Mg2+). A key innovation in their technology is the incorporation of specific transition metal dopants that stabilize the host structure while maintaining high electronic conductivity. Toyota has also developed advanced electrolyte formulations specifically designed to work synergistically with their cathode materials, preventing aluminum current collector corrosion and cathode dissolution at high voltages. Their integrated approach addresses the critical challenge of maintaining capacity retention over extended cycling, with demonstrated stability exceeding 500 cycles in prototype cells.
Strengths: Comprehensive approach that addresses both cathode material design and compatible electrolyte development, backed by extensive automotive battery expertise and manufacturing capabilities. Weaknesses: Their solutions may be optimized for automotive applications with specific performance requirements that might not translate to other applications requiring different power/energy balances.
Critical Patents & Research on Cycling Stability Enhancement
Composite cathode and methods for forming the same
PatentWO2025056687A1
Innovation
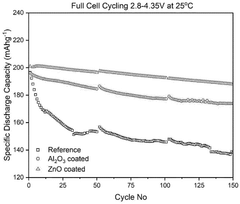
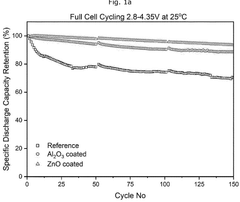
- A composite cathode is developed comprising a cathode active layer with composite particles having a core of cathode active material and a shell of stabilizing material, and a cathode coating with a passivating material like zinc oxide, applied using atomic layer deposition.
Electrolyte-Cathode Interface Phenomena Analysis
The interface between high-voltage cathodes and electrolytes represents a critical frontier in magnesium-ion battery (MIB) research, significantly impacting cycling stability. At this dynamic boundary, complex electrochemical reactions occur that can either facilitate or hinder magnesium ion transport, directly affecting battery performance and longevity.
High-voltage cathode materials, while offering enhanced energy density, create particularly challenging interface conditions. The elevated operating potentials accelerate electrolyte decomposition, forming surface films that fundamentally differ from those in conventional lithium-ion systems. These cathode-electrolyte interphase (CEI) layers exhibit unique composition and morphology characteristics that determine magnesium ion diffusion kinetics.
Spectroscopic analyses reveal that high-voltage operation triggers the formation of magnesium-containing compounds at the interface, including MgF₂, MgCO₃, and various magnesium alkoxides. These compounds result from nucleophilic attacks by electrolyte components on the cathode surface, particularly when operating beyond 3.0V vs. Mg/Mg²⁺. The chemical nature of these interface species directly correlates with capacity retention during extended cycling.
Interface resistance measurements demonstrate a progressive increase during cycling, with impedance spectroscopy confirming the growth of resistive layers that impede magnesium ion insertion/extraction. This resistance growth accelerates with higher voltage cutoffs, establishing a clear relationship between operating voltage and interface degradation rates.
Recent in-situ characterization techniques have identified voltage-dependent phase transformations at cathode surfaces that significantly alter interface properties. These transformations often lead to structural reorganization that can either enhance or obstruct magnesium ion diffusion pathways, depending on the specific cathode chemistry and electrolyte composition.
Computational studies suggest that solvent co-intercalation phenomena become more pronounced at high voltages, where electrolyte molecules insert alongside magnesium ions into cathode structures. This co-intercalation expands interlayer spacing but simultaneously introduces additional interface complexity that can compromise long-term cycling stability.
The development of protective coatings and interface engineering strategies has emerged as a promising approach to mitigate these challenges. Atomic layer deposition of Al₂O₃, TiO₂, or specialized polymer coatings has demonstrated significant improvements in interface stability, reducing parasitic reactions while maintaining efficient magnesium ion transport across the critical cathode-electrolyte boundary.
High-voltage cathode materials, while offering enhanced energy density, create particularly challenging interface conditions. The elevated operating potentials accelerate electrolyte decomposition, forming surface films that fundamentally differ from those in conventional lithium-ion systems. These cathode-electrolyte interphase (CEI) layers exhibit unique composition and morphology characteristics that determine magnesium ion diffusion kinetics.
Spectroscopic analyses reveal that high-voltage operation triggers the formation of magnesium-containing compounds at the interface, including MgF₂, MgCO₃, and various magnesium alkoxides. These compounds result from nucleophilic attacks by electrolyte components on the cathode surface, particularly when operating beyond 3.0V vs. Mg/Mg²⁺. The chemical nature of these interface species directly correlates with capacity retention during extended cycling.
Interface resistance measurements demonstrate a progressive increase during cycling, with impedance spectroscopy confirming the growth of resistive layers that impede magnesium ion insertion/extraction. This resistance growth accelerates with higher voltage cutoffs, establishing a clear relationship between operating voltage and interface degradation rates.
Recent in-situ characterization techniques have identified voltage-dependent phase transformations at cathode surfaces that significantly alter interface properties. These transformations often lead to structural reorganization that can either enhance or obstruct magnesium ion diffusion pathways, depending on the specific cathode chemistry and electrolyte composition.
Computational studies suggest that solvent co-intercalation phenomena become more pronounced at high voltages, where electrolyte molecules insert alongside magnesium ions into cathode structures. This co-intercalation expands interlayer spacing but simultaneously introduces additional interface complexity that can compromise long-term cycling stability.
The development of protective coatings and interface engineering strategies has emerged as a promising approach to mitigate these challenges. Atomic layer deposition of Al₂O₃, TiO₂, or specialized polymer coatings has demonstrated significant improvements in interface stability, reducing parasitic reactions while maintaining efficient magnesium ion transport across the critical cathode-electrolyte boundary.
Environmental & Economic Impact Assessment
The environmental and economic impacts of high-voltage cathodes in magnesium-ion batteries represent critical considerations for their commercial viability and sustainability. From an environmental perspective, magnesium-ion batteries offer significant advantages over conventional lithium-ion technologies due to the greater natural abundance of magnesium resources. Magnesium is the eighth most abundant element in Earth's crust, approximately 1000 times more plentiful than lithium, reducing extraction-related environmental degradation.
High-voltage cathodes specifically contribute to environmental sustainability by enabling higher energy densities, which translates to fewer raw materials required per kilowatt-hour of storage capacity. This reduction in material intensity decreases the overall environmental footprint associated with battery manufacturing. Additionally, the enhanced cycling stability provided by optimized high-voltage cathodes extends battery lifespan, reducing waste generation and resource consumption associated with battery replacement.
From an economic standpoint, the development of stable high-voltage cathodes presents a compelling value proposition. The improved energy density directly correlates with reduced cost per unit of energy stored, potentially decreasing battery pack costs by 20-30% compared to current commercial alternatives. This cost reduction is particularly significant for grid-scale energy storage applications, where price sensitivity remains a primary barrier to widespread adoption.
The manufacturing processes for high-voltage cathode materials currently represent a cost challenge, requiring precise control of synthesis conditions and often expensive precursors. However, economic modeling suggests that at scale, these production costs could decrease by up to 40% through process optimization and economies of scale, making magnesium-ion batteries increasingly competitive with established technologies.
Supply chain considerations also favor magnesium-based systems. Unlike lithium, which faces geopolitical supply constraints due to concentrated reserves in specific regions, magnesium resources are more evenly distributed globally. This distribution pattern reduces supply chain vulnerabilities and price volatility, offering more stable long-term economics for battery manufacturers and end-users.
Life-cycle assessment studies indicate that high-voltage cathode magnesium-ion batteries could achieve a carbon footprint approximately 15-25% lower than equivalent lithium-ion systems when accounting for production, use, and end-of-life management. This environmental advantage strengthens as renewable energy increasingly powers manufacturing facilities, creating a virtuous cycle of clean energy production and storage.
High-voltage cathodes specifically contribute to environmental sustainability by enabling higher energy densities, which translates to fewer raw materials required per kilowatt-hour of storage capacity. This reduction in material intensity decreases the overall environmental footprint associated with battery manufacturing. Additionally, the enhanced cycling stability provided by optimized high-voltage cathodes extends battery lifespan, reducing waste generation and resource consumption associated with battery replacement.
From an economic standpoint, the development of stable high-voltage cathodes presents a compelling value proposition. The improved energy density directly correlates with reduced cost per unit of energy stored, potentially decreasing battery pack costs by 20-30% compared to current commercial alternatives. This cost reduction is particularly significant for grid-scale energy storage applications, where price sensitivity remains a primary barrier to widespread adoption.
The manufacturing processes for high-voltage cathode materials currently represent a cost challenge, requiring precise control of synthesis conditions and often expensive precursors. However, economic modeling suggests that at scale, these production costs could decrease by up to 40% through process optimization and economies of scale, making magnesium-ion batteries increasingly competitive with established technologies.
Supply chain considerations also favor magnesium-based systems. Unlike lithium, which faces geopolitical supply constraints due to concentrated reserves in specific regions, magnesium resources are more evenly distributed globally. This distribution pattern reduces supply chain vulnerabilities and price volatility, offering more stable long-term economics for battery manufacturers and end-users.
Life-cycle assessment studies indicate that high-voltage cathode magnesium-ion batteries could achieve a carbon footprint approximately 15-25% lower than equivalent lithium-ion systems when accounting for production, use, and end-of-life management. This environmental advantage strengthens as renewable energy increasingly powers manufacturing facilities, creating a virtuous cycle of clean energy production and storage.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!