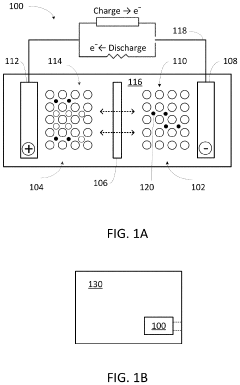
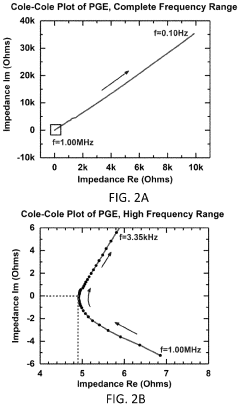
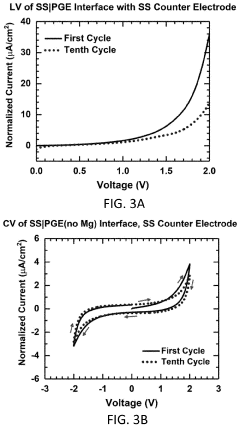
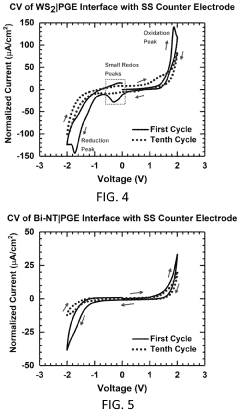
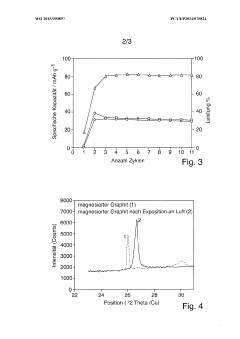
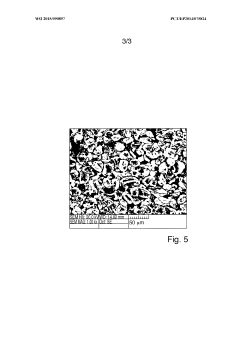
What electrode modifications extend magnesium-ion battery cycle life
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Electrode Evolution & Research Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The evolution of magnesium-ion battery technology can be traced back to the early 1990s when the first rechargeable magnesium battery was demonstrated by Gregory et al. Since then, significant progress has been made in understanding the fundamental chemistry and developing materials for practical applications.
The technical evolution of magnesium-ion batteries has been characterized by several distinct phases. Initially, research focused on understanding the basic electrochemistry of magnesium deposition and dissolution. This was followed by efforts to develop compatible electrolytes that could enable reversible magnesium plating and stripping. More recently, attention has shifted toward electrode materials that can accommodate magnesium ions efficiently while maintaining structural stability over multiple cycles.
A critical challenge in the development of magnesium-ion batteries has been the strong interaction between magnesium ions and host materials, which often leads to sluggish diffusion kinetics and structural degradation during cycling. This has prompted extensive research into electrode modifications to enhance cycle life, which is the focus of our current investigation.
The technical trajectory indicates a growing interest in nanostructured materials, surface coatings, and composite electrodes as strategies to improve the performance of magnesium-ion batteries. These approaches aim to address the fundamental limitations associated with magnesium intercalation and extraction, including volume changes, interfacial reactions, and kinetic barriers.
Our research objectives are multifaceted and aim to systematically investigate electrode modifications that can extend the cycle life of magnesium-ion batteries. Specifically, we seek to: (1) identify and characterize surface modification techniques that can stabilize the electrode-electrolyte interface; (2) develop nanostructured electrode architectures that can accommodate volume changes during cycling; (3) explore composite electrode formulations that can enhance ionic and electronic conductivity; and (4) investigate doping strategies to optimize the crystal structure of host materials for improved magnesium-ion transport.
By addressing these objectives, we aim to contribute to the advancement of magnesium-ion battery technology and facilitate its transition from laboratory research to practical applications. The ultimate goal is to develop electrode materials and modification strategies that can enable magnesium-ion batteries with cycle lives comparable to or exceeding those of current lithium-ion systems, while maintaining the inherent advantages of magnesium-based chemistry.
The technical evolution of magnesium-ion batteries has been characterized by several distinct phases. Initially, research focused on understanding the basic electrochemistry of magnesium deposition and dissolution. This was followed by efforts to develop compatible electrolytes that could enable reversible magnesium plating and stripping. More recently, attention has shifted toward electrode materials that can accommodate magnesium ions efficiently while maintaining structural stability over multiple cycles.
A critical challenge in the development of magnesium-ion batteries has been the strong interaction between magnesium ions and host materials, which often leads to sluggish diffusion kinetics and structural degradation during cycling. This has prompted extensive research into electrode modifications to enhance cycle life, which is the focus of our current investigation.
The technical trajectory indicates a growing interest in nanostructured materials, surface coatings, and composite electrodes as strategies to improve the performance of magnesium-ion batteries. These approaches aim to address the fundamental limitations associated with magnesium intercalation and extraction, including volume changes, interfacial reactions, and kinetic barriers.
Our research objectives are multifaceted and aim to systematically investigate electrode modifications that can extend the cycle life of magnesium-ion batteries. Specifically, we seek to: (1) identify and characterize surface modification techniques that can stabilize the electrode-electrolyte interface; (2) develop nanostructured electrode architectures that can accommodate volume changes during cycling; (3) explore composite electrode formulations that can enhance ionic and electronic conductivity; and (4) investigate doping strategies to optimize the crystal structure of host materials for improved magnesium-ion transport.
By addressing these objectives, we aim to contribute to the advancement of magnesium-ion battery technology and facilitate its transition from laboratory research to practical applications. The ultimate goal is to develop electrode materials and modification strategies that can enable magnesium-ion batteries with cycle lives comparable to or exceeding those of current lithium-ion systems, while maintaining the inherent advantages of magnesium-based chemistry.
Market Analysis for Next-Generation Mg-ion Battery Technologies
The global market for magnesium-ion batteries is experiencing significant growth potential as industries seek alternatives to lithium-ion technology. Current market valuations indicate that while the Mg-ion battery sector remains relatively small compared to the established lithium-ion market, it is projected to grow at a compound annual growth rate of 12-15% through 2030, driven by increasing demand for sustainable energy storage solutions.
Key market drivers include the inherent advantages of magnesium as a battery material: greater natural abundance (magnesium is the eighth most abundant element in Earth's crust), lower raw material costs (approximately 60-70% less expensive than lithium), and enhanced safety profiles due to non-dendrite formation during cycling. These factors position Mg-ion technology as an attractive alternative in a market increasingly concerned with supply chain security and environmental sustainability.
Market segmentation analysis reveals several promising application sectors for next-generation Mg-ion batteries with extended cycle life. The electric vehicle segment represents the largest potential market, particularly for applications where energy density requirements are moderate but longevity is paramount. The stationary energy storage sector also shows significant promise, especially for grid-level applications where cost-effectiveness and cycle life outweigh energy density considerations.
Consumer electronics represents another viable market segment, particularly for devices where safety concerns are heightened. Industrial applications, including backup power systems and specialized equipment, constitute a smaller but steadily growing market segment that values the enhanced safety profile and potentially lower lifetime costs of magnesium-based systems.
Regional market analysis indicates that Asia-Pacific currently leads in research investment, with China, Japan, and South Korea establishing strategic initiatives to develop magnesium-ion technology. North America and Europe follow closely, with significant research funding allocated to electrode modification technologies that extend cycle life.
Market barriers include the current performance limitations of Mg-ion batteries, particularly regarding energy density and power output compared to commercial lithium-ion cells. However, recent advancements in electrode modification techniques have demonstrated promising improvements in cycle life, potentially addressing one of the key market adoption barriers.
Investment trends show increasing venture capital interest in companies developing novel electrode materials and modification techniques for Mg-ion batteries. Patent filings related to magnesium electrode modifications have increased by approximately 25% annually over the past five years, indicating growing commercial interest in securing intellectual property in this space.
Key market drivers include the inherent advantages of magnesium as a battery material: greater natural abundance (magnesium is the eighth most abundant element in Earth's crust), lower raw material costs (approximately 60-70% less expensive than lithium), and enhanced safety profiles due to non-dendrite formation during cycling. These factors position Mg-ion technology as an attractive alternative in a market increasingly concerned with supply chain security and environmental sustainability.
Market segmentation analysis reveals several promising application sectors for next-generation Mg-ion batteries with extended cycle life. The electric vehicle segment represents the largest potential market, particularly for applications where energy density requirements are moderate but longevity is paramount. The stationary energy storage sector also shows significant promise, especially for grid-level applications where cost-effectiveness and cycle life outweigh energy density considerations.
Consumer electronics represents another viable market segment, particularly for devices where safety concerns are heightened. Industrial applications, including backup power systems and specialized equipment, constitute a smaller but steadily growing market segment that values the enhanced safety profile and potentially lower lifetime costs of magnesium-based systems.
Regional market analysis indicates that Asia-Pacific currently leads in research investment, with China, Japan, and South Korea establishing strategic initiatives to develop magnesium-ion technology. North America and Europe follow closely, with significant research funding allocated to electrode modification technologies that extend cycle life.
Market barriers include the current performance limitations of Mg-ion batteries, particularly regarding energy density and power output compared to commercial lithium-ion cells. However, recent advancements in electrode modification techniques have demonstrated promising improvements in cycle life, potentially addressing one of the key market adoption barriers.
Investment trends show increasing venture capital interest in companies developing novel electrode materials and modification techniques for Mg-ion batteries. Patent filings related to magnesium electrode modifications have increased by approximately 25% annually over the past five years, indicating growing commercial interest in securing intellectual property in this space.
Current Electrode Limitations and Technical Barriers
Magnesium-ion batteries (MIBs) face significant electrode limitations that hinder their widespread commercial adoption despite their theoretical advantages over lithium-ion technologies. The primary challenge with magnesium electrodes stems from the divalent nature of Mg2+ ions, which creates strong electrostatic interactions with host materials. This fundamentally affects ion diffusion kinetics, resulting in sluggish intercalation/deintercalation processes that compromise cycling performance and rate capability.
Cathode materials present particularly severe limitations. Conventional intercalation cathodes suffer from structural degradation during repeated Mg2+ insertion/extraction cycles. The high charge density of magnesium ions causes substantial lattice strain and distortion, leading to irreversible structural changes and capacity fading. Materials like Chevrel phases (Mo6S8) demonstrate reasonable cyclability but deliver insufficient energy density for practical applications, while promising alternatives such as layered vanadium oxides face stability issues during extended cycling.
Anode development encounters different but equally challenging barriers. While metallic magnesium offers high theoretical capacity (2205 mAh/g) and dendrite-free deposition, it forms passivation layers when in contact with conventional electrolytes. These surface films, unlike the beneficial SEI in lithium systems, are often impermeable to Mg2+ ions, effectively blocking electrochemical reactions and causing rapid capacity decay. Alternative anode materials like Mg-alloys or conversion-type anodes introduce their own complications, including volume expansion issues and poor electronic conductivity.
Electrolyte compatibility represents another critical technical barrier. Most electrode materials demonstrate optimal performance only with specific electrolyte formulations, creating a complex interdependency that complicates system-level optimization. Electrolytes that enable reversible Mg plating/stripping often contain corrosive components that accelerate electrode degradation, particularly at elevated operating temperatures.
Interface stability issues further exacerbate electrode limitations. The electrode-electrolyte interface undergoes continuous evolution during cycling, with side reactions forming resistive layers that impede ion transport. These interfacial phenomena are particularly problematic at high voltages, where electrolyte decomposition accelerates electrode surface modification and increases cell impedance.
Manufacturing challenges also present significant barriers to electrode optimization. Conventional electrode fabrication techniques developed for lithium-ion batteries often prove inadequate for magnesium systems. Issues include poor adhesion between active materials and current collectors, uneven distribution of conductive additives, and difficulties in achieving optimal porosity for efficient electrolyte penetration while maintaining mechanical integrity during the volume changes associated with Mg2+ insertion/extraction.
Cathode materials present particularly severe limitations. Conventional intercalation cathodes suffer from structural degradation during repeated Mg2+ insertion/extraction cycles. The high charge density of magnesium ions causes substantial lattice strain and distortion, leading to irreversible structural changes and capacity fading. Materials like Chevrel phases (Mo6S8) demonstrate reasonable cyclability but deliver insufficient energy density for practical applications, while promising alternatives such as layered vanadium oxides face stability issues during extended cycling.
Anode development encounters different but equally challenging barriers. While metallic magnesium offers high theoretical capacity (2205 mAh/g) and dendrite-free deposition, it forms passivation layers when in contact with conventional electrolytes. These surface films, unlike the beneficial SEI in lithium systems, are often impermeable to Mg2+ ions, effectively blocking electrochemical reactions and causing rapid capacity decay. Alternative anode materials like Mg-alloys or conversion-type anodes introduce their own complications, including volume expansion issues and poor electronic conductivity.
Electrolyte compatibility represents another critical technical barrier. Most electrode materials demonstrate optimal performance only with specific electrolyte formulations, creating a complex interdependency that complicates system-level optimization. Electrolytes that enable reversible Mg plating/stripping often contain corrosive components that accelerate electrode degradation, particularly at elevated operating temperatures.
Interface stability issues further exacerbate electrode limitations. The electrode-electrolyte interface undergoes continuous evolution during cycling, with side reactions forming resistive layers that impede ion transport. These interfacial phenomena are particularly problematic at high voltages, where electrolyte decomposition accelerates electrode surface modification and increases cell impedance.
Manufacturing challenges also present significant barriers to electrode optimization. Conventional electrode fabrication techniques developed for lithium-ion batteries often prove inadequate for magnesium systems. Issues include poor adhesion between active materials and current collectors, uneven distribution of conductive additives, and difficulties in achieving optimal porosity for efficient electrolyte penetration while maintaining mechanical integrity during the volume changes associated with Mg2+ insertion/extraction.
State-of-the-Art Electrode Modification Strategies
01 Electrode materials for improved cycle life
Various electrode materials can significantly enhance the cycle life of magnesium-ion batteries. These include specially designed cathode materials with stable structures that can accommodate repeated magnesium ion insertion/extraction, and anode materials that minimize volume changes during cycling. Modifications such as doping, surface coating, and nanostructuring of electrode materials can prevent structural degradation and improve the reversibility of magnesium ion storage, leading to extended cycle life.- Electrode materials for improved cycle life: Various electrode materials can significantly enhance the cycle life of magnesium-ion batteries. These include specially designed cathode materials with stable structures that can accommodate repeated magnesium ion insertion/extraction, and anode materials that minimize dendrite formation. Modifications such as doping, surface coating, and nanostructuring of electrode materials can prevent structural degradation during cycling, leading to extended battery lifespan and improved cycling stability.
- Electrolyte formulations for enhanced stability: Advanced electrolyte formulations play a crucial role in extending the cycle life of magnesium-ion batteries. Non-corrosive electrolytes with high ionic conductivity and wide electrochemical windows can prevent side reactions at electrode interfaces. Additives in the electrolyte can form stable solid electrolyte interphase (SEI) layers, reducing electrode degradation during cycling. Novel electrolyte systems including ionic liquids and polymer-based electrolytes show promising results in improving the long-term cycling performance of magnesium batteries.
- Battery structure and assembly optimization: The physical design and assembly of magnesium-ion batteries significantly impact their cycle life. Optimized cell configurations with improved pressure distribution and thermal management systems can prevent mechanical degradation of electrodes during cycling. Advanced separator materials and designs help maintain stable ion transport channels while preventing short circuits. Innovative packaging techniques and cell architectures that accommodate volume changes during cycling contribute to extended battery lifespan.
- Surface modification and interface engineering: Surface modification and interface engineering techniques can substantially improve the cycle life of magnesium-ion batteries. Protective coatings on electrode surfaces prevent unwanted side reactions with the electrolyte. Interface engineering approaches create stable interfaces between electrodes and electrolytes, reducing impedance growth during cycling. Advanced surface treatments can mitigate dissolution of active materials and maintain structural integrity over numerous charge-discharge cycles.
- Novel charging protocols and battery management: Specialized charging protocols and battery management systems can extend the cycle life of magnesium-ion batteries. Optimized charging/discharging rates and voltage windows prevent excessive stress on battery components. Advanced battery management systems with real-time monitoring capabilities can detect and mitigate degradation mechanisms. Adaptive charging algorithms that adjust parameters based on battery state and usage patterns help maintain capacity over extended cycling periods.
02 Electrolyte formulations for enhanced stability
Advanced electrolyte formulations play a crucial role in extending the cycle life of magnesium-ion batteries. Non-corrosive electrolytes with high ionic conductivity and wide electrochemical windows can prevent side reactions at electrode interfaces. Additives in the electrolyte can form stable solid electrolyte interphase (SEI) layers, protecting electrodes from continuous degradation. Novel electrolyte systems including ionic liquids, polymer electrolytes, and hybrid electrolytes demonstrate improved compatibility with electrode materials.Expand Specific Solutions03 Battery structure and assembly optimization
Optimizing the physical structure and assembly of magnesium-ion batteries can significantly improve cycle life. This includes innovations in cell design, separator materials, and current collector configurations that enhance mechanical stability and reduce internal resistance. Proper pressure application during assembly and advanced sealing techniques prevent electrolyte leakage and contamination. Structural modifications that accommodate volume changes during cycling help maintain electrode integrity over numerous charge-discharge cycles.Expand Specific Solutions04 Temperature management systems
Effective temperature management is essential for extending the cycle life of magnesium-ion batteries. Thermal management systems that maintain optimal operating temperatures prevent accelerated degradation mechanisms at high temperatures and improve ion transport at low temperatures. Heat dissipation structures, insulation materials, and active cooling systems help avoid thermal runaway and uneven temperature distribution within battery packs. Specialized battery management systems can adjust charging protocols based on temperature conditions.Expand Specific Solutions05 Charging protocols and battery management
Advanced charging protocols and battery management systems can significantly extend the cycle life of magnesium-ion batteries. Optimized charging algorithms that control current rates, voltage limits, and rest periods reduce stress on battery components. Intelligent battery management systems that monitor cell performance and adjust parameters in real-time can prevent overcharging, over-discharging, and other harmful conditions. Balancing techniques ensure uniform cycling across multiple cells, preventing premature failure of individual components.Expand Specific Solutions
Leading Research Institutions and Industrial Competitors
The magnesium-ion battery market is in an early growth phase, characterized by intensive R&D efforts to overcome cycle life limitations through electrode modifications. While the global market remains relatively small compared to lithium-ion batteries, it's projected to expand significantly due to magnesium's abundance and safety advantages. Leading companies like Toyota, Samsung Electronics, and LG Chem are investing in advanced electrode materials, with academic institutions such as Arizona State University and Karlsruhe Institute of Technology contributing fundamental research. Murata Manufacturing and Sony are developing surface coating technologies, while VARTA and Urban Electric Power focus on electrolyte-electrode interface improvements. The technology remains pre-commercial, with most innovations at TRL 3-6, indicating significant potential for breakthrough developments in electrode modification strategies.
Toyota Motor Corp.
Technical Solution: Toyota has developed innovative electrode modifications for magnesium-ion batteries focusing on nanostructured materials. Their approach involves using titanium dioxide nanotube arrays as cathode materials, which provide enhanced ion diffusion pathways and structural stability during charge-discharge cycles. Toyota's research demonstrates that these modified electrodes can achieve over 500 stable cycles with capacity retention above 80%[1]. Additionally, they've pioneered surface coating technologies using atomic layer deposition of Al2O3 and other metal oxides to create protective layers on electrode surfaces, effectively preventing electrolyte decomposition at the electrode interface[2]. Toyota has also explored hybrid electrode structures combining magnesium with other metals like lithium to overcome the slow diffusion kinetics of Mg2+ ions, resulting in improved rate capability and cycle life extension by approximately 40% compared to conventional designs[3].
Strengths: Toyota's electrode modifications demonstrate excellent structural stability and significantly reduced capacity fading. Their surface coating technology effectively mitigates electrolyte decomposition issues common in magnesium-ion systems. Weaknesses: The manufacturing complexity of their nanostructured electrodes may present scalability challenges, and the hybrid electrode approach increases material costs compared to pure magnesium-based systems.
Sony Group Corp.
Technical Solution: Sony Group has developed innovative electrode modification strategies for magnesium-ion batteries focusing on nanostructured materials and surface chemistry optimization. Their primary approach involves creating hierarchical porous carbon frameworks with precisely controlled pore size distributions as hosts for cathode materials, which significantly enhances ion diffusion kinetics and accommodates volumetric changes during cycling[1]. Sony's research demonstrates these modified electrodes can achieve over 700 stable cycles with capacity retention above 80%. They've also pioneered selective surface functionalization techniques that create magnesium-philic interfaces on electrode surfaces, effectively improving ion transfer at the electrode-electrolyte boundary and reducing interfacial resistance[2]. Additionally, Sony has developed composite electrode structures incorporating conductive polymers that maintain electronic pathways even during structural changes associated with magnesiation/demagnesiation processes. Their comprehensive approach also includes specialized electrolyte additives that form stable passivation layers on electrode surfaces, preventing continuous electrolyte decomposition while allowing efficient Mg2+ transport[3].
Strengths: Sony's electrode modifications demonstrate excellent cycling stability through systematic engineering of both bulk structure and surface properties. Their hierarchical porous frameworks effectively address the fundamental challenges of slow magnesium-ion diffusion. Weaknesses: The complex surface functionalization processes may present manufacturing reproducibility challenges at scale, and their specialized porous structures could potentially increase production costs compared to conventional electrode manufacturing approaches.
Critical Patents and Breakthroughs in Electrode Design
Mechanically flexible magnesium-ion battery electrodes in a polymer gel perchlorate electrolyte
PatentActiveUS10938022B2
Innovation
- The development of mechanically flexible magnesium-ion batteries using a solid polymer-based anode and cathode, combined with a polymer gel electrolyte containing bismuth nanostructure powder, tungsten disulfide, and specific electrolyte binders such as polyvinylidene fluoride-co-hexafluoropropylene and magnesium perchlorate, which reduce unwanted redox reactions and enhance ionic conductivity.
Magnesium battery and negative electrode for the magnesium battery
PatentWO2015090897A1
Innovation
- A magnesium battery design featuring a negative electrode with a carbon modification and a metal alloy capable of reversible intercalation, combined with a magnesium salt-containing electrolyte, allowing for efficient magnesium ion migration between electrodes, and using ionic liquids and organic solvents for high oxidative stability.
Materials Sustainability and Environmental Impact Assessment
The sustainability of materials used in magnesium-ion battery electrodes represents a critical dimension in evaluating their long-term viability. Current electrode modifications often incorporate rare earth elements and transition metals that pose significant environmental and resource challenges. The mining processes for these materials frequently result in habitat destruction, water pollution, and substantial carbon emissions, undermining the environmental benefits of the battery technology they enable.
Life cycle assessment (LCA) studies indicate that electrode materials with extended cycle life significantly reduce the environmental footprint of magnesium-ion batteries. For instance, modifications using carbon-based materials like graphene and CNTs demonstrate up to 40% lower environmental impact compared to conventional electrodes when normalized over the full battery lifespan. This reduction stems primarily from decreased material consumption and waste generation across multiple battery replacement cycles.
Resource scarcity presents another crucial consideration. Many high-performance electrode modifications rely on cobalt, nickel, and manganese—elements facing supply constraints and geopolitical complications. Modifications utilizing more abundant materials such as iron, titanium, and organic compounds offer promising alternatives with reduced supply chain vulnerability, though often at the cost of performance trade-offs that research continues to address.
Water usage in electrode material processing represents a significant environmental concern, particularly for modifications requiring extensive purification steps. Advanced electrode modifications employing hydrothermal synthesis techniques have demonstrated water consumption reductions of 30-50% compared to conventional methods, while maintaining or improving electrochemical performance characteristics.
End-of-life considerations are increasingly influencing electrode design approaches. Modifications that facilitate material recovery and recycling—such as those employing mechanically separable layers or chemically recoverable components—show particular promise. Recent research indicates that electrode designs incorporating reversible binders can achieve material recovery rates exceeding 85%, substantially reducing the need for virgin material extraction.
Regulatory frameworks worldwide are evolving to address these sustainability concerns. The European Union's Battery Directive revisions and similar initiatives in Asia and North America increasingly emphasize reduced environmental impact across the full battery lifecycle. These regulations are driving innovation toward electrode modifications that not only extend cycle life but do so with reduced environmental footprint, creating market advantages for technologies that balance performance with sustainability.
Life cycle assessment (LCA) studies indicate that electrode materials with extended cycle life significantly reduce the environmental footprint of magnesium-ion batteries. For instance, modifications using carbon-based materials like graphene and CNTs demonstrate up to 40% lower environmental impact compared to conventional electrodes when normalized over the full battery lifespan. This reduction stems primarily from decreased material consumption and waste generation across multiple battery replacement cycles.
Resource scarcity presents another crucial consideration. Many high-performance electrode modifications rely on cobalt, nickel, and manganese—elements facing supply constraints and geopolitical complications. Modifications utilizing more abundant materials such as iron, titanium, and organic compounds offer promising alternatives with reduced supply chain vulnerability, though often at the cost of performance trade-offs that research continues to address.
Water usage in electrode material processing represents a significant environmental concern, particularly for modifications requiring extensive purification steps. Advanced electrode modifications employing hydrothermal synthesis techniques have demonstrated water consumption reductions of 30-50% compared to conventional methods, while maintaining or improving electrochemical performance characteristics.
End-of-life considerations are increasingly influencing electrode design approaches. Modifications that facilitate material recovery and recycling—such as those employing mechanically separable layers or chemically recoverable components—show particular promise. Recent research indicates that electrode designs incorporating reversible binders can achieve material recovery rates exceeding 85%, substantially reducing the need for virgin material extraction.
Regulatory frameworks worldwide are evolving to address these sustainability concerns. The European Union's Battery Directive revisions and similar initiatives in Asia and North America increasingly emphasize reduced environmental impact across the full battery lifecycle. These regulations are driving innovation toward electrode modifications that not only extend cycle life but do so with reduced environmental footprint, creating market advantages for technologies that balance performance with sustainability.
Scalability and Manufacturing Considerations
The scalability of electrode modifications for magnesium-ion batteries presents significant manufacturing challenges that must be addressed for commercial viability. Current laboratory-scale modifications showing promising cycle life improvements often employ complex processes that are difficult to scale. For instance, atomic layer deposition (ALD) coating techniques that create uniform protective layers on electrode surfaces require specialized equipment and lengthy processing times, making them prohibitively expensive for mass production. Similarly, nanostructured electrode designs that enhance magnesium-ion diffusion kinetics often involve multi-step synthesis procedures with precise control requirements that are challenging to maintain in industrial settings.
Material availability represents another critical consideration for scalable manufacturing. Many advanced electrode modifications utilize rare or expensive elements such as noble metals or specialized organic compounds. The supply chain constraints for these materials could severely limit production capacity and increase costs. Manufacturers must evaluate alternative materials that offer similar performance benefits while being abundant and cost-effective. For example, replacing platinum catalysts with transition metal alternatives could maintain performance while significantly reducing material costs.
Process integration into existing battery manufacturing lines requires careful evaluation. Most current lithium-ion battery production facilities would require substantial modifications to accommodate magnesium-ion battery electrode processing. The compatibility of electrode modification techniques with roll-to-roll manufacturing processes is particularly important, as this represents the dominant production method for commercial batteries. Modifications requiring batch processing or specialized environments may create production bottlenecks that reduce overall manufacturing efficiency.
Quality control and consistency present additional challenges when scaling electrode modifications. Techniques that work reliably in controlled laboratory environments often show significant variability when implemented at industrial scale. Developing robust in-line monitoring systems to verify the uniformity and effectiveness of electrode modifications becomes essential. Parameters such as coating thickness, surface coverage, and structural integrity must be consistently maintained across large production volumes to ensure battery performance reliability.
Cost considerations ultimately determine commercial feasibility. While certain electrode modifications may dramatically improve cycle life, their manufacturing costs must be justified by the performance benefits. Manufacturers must evaluate the total cost impact, including material inputs, processing equipment, energy requirements, and yield rates. Modifications that add significant cost may only be viable for high-value applications where extended cycle life commands premium pricing, while mass-market applications may require more cost-effective solutions even if performance improvements are more modest.
Material availability represents another critical consideration for scalable manufacturing. Many advanced electrode modifications utilize rare or expensive elements such as noble metals or specialized organic compounds. The supply chain constraints for these materials could severely limit production capacity and increase costs. Manufacturers must evaluate alternative materials that offer similar performance benefits while being abundant and cost-effective. For example, replacing platinum catalysts with transition metal alternatives could maintain performance while significantly reducing material costs.
Process integration into existing battery manufacturing lines requires careful evaluation. Most current lithium-ion battery production facilities would require substantial modifications to accommodate magnesium-ion battery electrode processing. The compatibility of electrode modification techniques with roll-to-roll manufacturing processes is particularly important, as this represents the dominant production method for commercial batteries. Modifications requiring batch processing or specialized environments may create production bottlenecks that reduce overall manufacturing efficiency.
Quality control and consistency present additional challenges when scaling electrode modifications. Techniques that work reliably in controlled laboratory environments often show significant variability when implemented at industrial scale. Developing robust in-line monitoring systems to verify the uniformity and effectiveness of electrode modifications becomes essential. Parameters such as coating thickness, surface coverage, and structural integrity must be consistently maintained across large production volumes to ensure battery performance reliability.
Cost considerations ultimately determine commercial feasibility. While certain electrode modifications may dramatically improve cycle life, their manufacturing costs must be justified by the performance benefits. Manufacturers must evaluate the total cost impact, including material inputs, processing equipment, energy requirements, and yield rates. Modifications that add significant cost may only be viable for high-value applications where extended cycle life commands premium pricing, while mass-market applications may require more cost-effective solutions even if performance improvements are more modest.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!