Analysis of magnesium-ion battery electrode porosity and rate capability
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Electrode Porosity Background and Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of these batteries has gained significant momentum over the past decade, driven by the increasing demand for sustainable energy storage solutions and the limitations of current lithium-ion technology. Electrode porosity plays a crucial role in determining the performance characteristics of magnesium-ion batteries, particularly their rate capability and cycling stability.
The evolution of magnesium-ion battery technology can be traced back to the early 1990s, but significant breakthroughs have only been achieved in recent years. The technical trajectory has shifted from initial proof-of-concept demonstrations to more sophisticated electrode designs that optimize ion transport and electrochemical reactions. Understanding the relationship between electrode porosity and battery performance represents a critical frontier in advancing this technology toward commercial viability.
Current research indicates that electrode porosity affects multiple aspects of battery performance, including electrolyte penetration, ion diffusion kinetics, and active material utilization. The optimization of porosity parameters—such as pore size distribution, tortuosity, and connectivity—presents both challenges and opportunities for enhancing magnesium-ion battery performance, particularly at high charge/discharge rates.
The primary technical objectives of this investigation include: establishing quantitative relationships between electrode porosity characteristics and rate capability; identifying optimal porosity parameters for different electrode materials and electrolyte systems; developing predictive models that can guide electrode design; and exploring innovative manufacturing techniques to precisely control porosity during electrode fabrication.
This research aims to bridge fundamental understanding with practical applications by correlating microscopic porosity features with macroscopic battery performance metrics. By systematically analyzing how porosity affects the complex interplay of ion transport, electron conduction, and electrochemical reactions within magnesium-ion battery electrodes, we seek to establish design principles that can accelerate the development of high-performance magnesium-based energy storage systems.
The significance of this research extends beyond academic interest, as optimized electrode structures could potentially overcome key barriers to magnesium-ion battery commercialization, including limited rate capability and cycle life. Success in this domain could facilitate the transition toward more sustainable and resource-abundant battery technologies, addressing critical challenges in the global energy landscape.
The evolution of magnesium-ion battery technology can be traced back to the early 1990s, but significant breakthroughs have only been achieved in recent years. The technical trajectory has shifted from initial proof-of-concept demonstrations to more sophisticated electrode designs that optimize ion transport and electrochemical reactions. Understanding the relationship between electrode porosity and battery performance represents a critical frontier in advancing this technology toward commercial viability.
Current research indicates that electrode porosity affects multiple aspects of battery performance, including electrolyte penetration, ion diffusion kinetics, and active material utilization. The optimization of porosity parameters—such as pore size distribution, tortuosity, and connectivity—presents both challenges and opportunities for enhancing magnesium-ion battery performance, particularly at high charge/discharge rates.
The primary technical objectives of this investigation include: establishing quantitative relationships between electrode porosity characteristics and rate capability; identifying optimal porosity parameters for different electrode materials and electrolyte systems; developing predictive models that can guide electrode design; and exploring innovative manufacturing techniques to precisely control porosity during electrode fabrication.
This research aims to bridge fundamental understanding with practical applications by correlating microscopic porosity features with macroscopic battery performance metrics. By systematically analyzing how porosity affects the complex interplay of ion transport, electron conduction, and electrochemical reactions within magnesium-ion battery electrodes, we seek to establish design principles that can accelerate the development of high-performance magnesium-based energy storage systems.
The significance of this research extends beyond academic interest, as optimized electrode structures could potentially overcome key barriers to magnesium-ion battery commercialization, including limited rate capability and cycle life. Success in this domain could facilitate the transition toward more sustainable and resource-abundant battery technologies, addressing critical challenges in the global energy landscape.
Market Analysis for Next-Generation Mg-ion Battery Technologies
The global market for magnesium-ion batteries is experiencing significant growth potential as industries seek alternatives to lithium-ion technology. Current market valuations indicate that while lithium-ion batteries dominate with a market size exceeding $50 billion, magnesium-ion batteries are projected to capture substantial market share by 2030, driven by their theoretical advantages in energy density, safety, and resource availability.
Key market drivers include the increasing demand for electric vehicles (EVs), which is expected to grow at a CAGR of 21% through 2028. Magnesium-ion batteries with optimized electrode porosity could potentially offer faster charging capabilities—a critical factor for EV adoption. Additionally, the renewable energy storage sector presents substantial opportunities, with grid-scale storage needs projected to increase tenfold by 2030.
Consumer electronics represents another significant market segment, where devices requiring higher energy density and faster charging are becoming standard. The industrial sector, particularly in applications requiring robust battery performance in harsh conditions, is also showing interest in magnesium-ion technology.
Geographically, Asia-Pacific currently leads battery manufacturing, with China controlling approximately 75% of global battery production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian supply chains, creating regional market opportunities for new battery technologies including magnesium-ion.
Market barriers include the technological maturity gap compared to lithium-ion batteries, with challenges in electrode porosity optimization directly impacting commercial viability. Current magnesium-ion batteries face limitations in rate capability that restrict their market applications, particularly in fast-charging scenarios.
Price sensitivity remains a critical market factor. While raw magnesium is more abundant and potentially less expensive than lithium, the manufacturing processes for magnesium-ion batteries with optimized electrode structures currently carry higher costs. Market analysis suggests that achieving price parity with lithium-ion batteries will require significant advances in manufacturing efficiency and electrode design.
Regulatory trends favor battery technologies with improved safety profiles and reduced environmental impact. Magnesium-ion batteries potentially offer advantages in both areas, particularly if electrode porosity can be optimized to prevent dendrite formation while maintaining high rate capability.
Market forecasts suggest that magnesium-ion batteries could capture 5-10% of the total battery market by 2030, contingent upon resolving current technical challenges, particularly those related to electrode porosity and rate capability. This represents a substantial market opportunity worth pursuing through targeted research and development efforts.
Key market drivers include the increasing demand for electric vehicles (EVs), which is expected to grow at a CAGR of 21% through 2028. Magnesium-ion batteries with optimized electrode porosity could potentially offer faster charging capabilities—a critical factor for EV adoption. Additionally, the renewable energy storage sector presents substantial opportunities, with grid-scale storage needs projected to increase tenfold by 2030.
Consumer electronics represents another significant market segment, where devices requiring higher energy density and faster charging are becoming standard. The industrial sector, particularly in applications requiring robust battery performance in harsh conditions, is also showing interest in magnesium-ion technology.
Geographically, Asia-Pacific currently leads battery manufacturing, with China controlling approximately 75% of global battery production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian supply chains, creating regional market opportunities for new battery technologies including magnesium-ion.
Market barriers include the technological maturity gap compared to lithium-ion batteries, with challenges in electrode porosity optimization directly impacting commercial viability. Current magnesium-ion batteries face limitations in rate capability that restrict their market applications, particularly in fast-charging scenarios.
Price sensitivity remains a critical market factor. While raw magnesium is more abundant and potentially less expensive than lithium, the manufacturing processes for magnesium-ion batteries with optimized electrode structures currently carry higher costs. Market analysis suggests that achieving price parity with lithium-ion batteries will require significant advances in manufacturing efficiency and electrode design.
Regulatory trends favor battery technologies with improved safety profiles and reduced environmental impact. Magnesium-ion batteries potentially offer advantages in both areas, particularly if electrode porosity can be optimized to prevent dendrite formation while maintaining high rate capability.
Market forecasts suggest that magnesium-ion batteries could capture 5-10% of the total battery market by 2030, contingent upon resolving current technical challenges, particularly those related to electrode porosity and rate capability. This represents a substantial market opportunity worth pursuing through targeted research and development efforts.
Current Challenges in Mg-ion Electrode Porosity Engineering
Despite significant advancements in magnesium-ion battery technology, electrode porosity engineering remains a critical bottleneck limiting commercial viability. Current electrode designs struggle to balance the contradictory requirements of high energy density and fast charging capabilities. The fundamental challenge stems from magnesium's divalent nature, resulting in slower diffusion kinetics compared to lithium-ion systems, making porosity optimization even more crucial.
Conventional electrode manufacturing techniques developed for lithium-ion batteries prove inadequate when applied to magnesium systems. The standard slurry-based coating processes often create suboptimal pore structures that fail to accommodate the larger ionic radius and stronger electrostatic interactions of Mg2+ ions. This results in significant concentration polarization during cycling, particularly at higher current densities.
Material selection compounds these challenges, as many promising cathode materials for Mg-ion batteries exhibit substantial volume changes during cycling. These dimensional fluctuations can lead to pore collapse or electrode pulverization, compromising the carefully engineered porosity. Current binder systems lack the mechanical resilience to maintain structural integrity throughout extended cycling.
Electrolyte compatibility presents another significant hurdle. The highly nucleophilic nature of magnesium electrolytes often leads to undesired side reactions with electrode components, resulting in surface film formation that can block pores and increase tortuosity. This phenomenon is particularly problematic in electrodes with smaller pore sizes, creating a difficult engineering tradeoff between surface area and electrolyte stability.
Analytical limitations further impede progress, as real-time characterization of pore evolution during cycling remains technically challenging. Current imaging techniques lack sufficient resolution to capture the dynamic changes in nanoscale porosity during operation, while computational models struggle to accurately predict ion transport through complex pore networks specific to magnesium systems.
The temperature sensitivity of magnesium-ion transport mechanisms introduces additional complexity. Porosity designs optimized for room temperature operation often perform poorly under extreme temperature conditions, limiting practical applications. Current thermal management strategies fail to address the unique heat generation patterns in magnesium electrodes with varying porosity profiles.
Manufacturing scalability represents perhaps the most immediate barrier to commercialization. Precise porosity control at industrial scales remains elusive, with significant batch-to-batch variations compromising performance consistency. Advanced techniques like freeze-casting and 3D printing show promise for creating tailored pore architectures but face significant hurdles in cost-effectiveness and production throughput for large-scale manufacturing.
Conventional electrode manufacturing techniques developed for lithium-ion batteries prove inadequate when applied to magnesium systems. The standard slurry-based coating processes often create suboptimal pore structures that fail to accommodate the larger ionic radius and stronger electrostatic interactions of Mg2+ ions. This results in significant concentration polarization during cycling, particularly at higher current densities.
Material selection compounds these challenges, as many promising cathode materials for Mg-ion batteries exhibit substantial volume changes during cycling. These dimensional fluctuations can lead to pore collapse or electrode pulverization, compromising the carefully engineered porosity. Current binder systems lack the mechanical resilience to maintain structural integrity throughout extended cycling.
Electrolyte compatibility presents another significant hurdle. The highly nucleophilic nature of magnesium electrolytes often leads to undesired side reactions with electrode components, resulting in surface film formation that can block pores and increase tortuosity. This phenomenon is particularly problematic in electrodes with smaller pore sizes, creating a difficult engineering tradeoff between surface area and electrolyte stability.
Analytical limitations further impede progress, as real-time characterization of pore evolution during cycling remains technically challenging. Current imaging techniques lack sufficient resolution to capture the dynamic changes in nanoscale porosity during operation, while computational models struggle to accurately predict ion transport through complex pore networks specific to magnesium systems.
The temperature sensitivity of magnesium-ion transport mechanisms introduces additional complexity. Porosity designs optimized for room temperature operation often perform poorly under extreme temperature conditions, limiting practical applications. Current thermal management strategies fail to address the unique heat generation patterns in magnesium electrodes with varying porosity profiles.
Manufacturing scalability represents perhaps the most immediate barrier to commercialization. Precise porosity control at industrial scales remains elusive, with significant batch-to-batch variations compromising performance consistency. Advanced techniques like freeze-casting and 3D printing show promise for creating tailored pore architectures but face significant hurdles in cost-effectiveness and production throughput for large-scale manufacturing.
Current Approaches to Optimize Electrode Porosity for Rate Capability
01 Electrode porosity optimization for magnesium-ion batteries
Optimizing electrode porosity is crucial for magnesium-ion battery performance. Controlled porosity enhances ion diffusion pathways and electrolyte penetration, leading to improved rate capability. The optimal porosity range typically falls between 30-50%, balancing ion transport with active material loading. Techniques such as using specific binders, controlled particle size distribution, and templating methods can be employed to achieve desired porosity levels in electrode structures.- Electrode porosity optimization for magnesium-ion batteries: Optimizing the porosity of electrodes in magnesium-ion batteries is crucial for enhancing their rate capability. Controlled porosity allows for better electrolyte penetration and ion diffusion throughout the electrode structure. By engineering the pore size distribution and overall porosity percentage, the effective surface area for electrochemical reactions increases, leading to improved charge/discharge rates. Various manufacturing techniques can be employed to achieve optimal porosity, including the use of specific binders, templating agents, and controlled drying processes.
- Nanostructured electrode materials for enhanced rate performance: Nanostructured materials significantly improve the rate capability of magnesium-ion battery electrodes by shortening ion diffusion paths and increasing active surface area. These materials, including nanoparticles, nanowires, and nanosheets, provide abundant channels for magnesium ion transport while maintaining structural stability during cycling. The nanoscale architecture creates interconnected porous networks that facilitate electrolyte infiltration and ion movement, resulting in superior high-rate performance compared to conventional bulk materials. Additionally, nanostructuring helps accommodate the volume changes during magnesium insertion/extraction processes.
- Composite electrode structures with controlled porosity: Composite electrode structures combine multiple materials to create controlled porosity networks that enhance rate capability in magnesium-ion batteries. These composites often integrate conductive additives, active materials, and pore-forming agents to establish hierarchical porous structures. The synergistic effect of different components creates optimal ion transport pathways while maintaining mechanical integrity and electronic conductivity. By carefully designing the composition and fabrication process, these composite electrodes achieve balanced porosity that supports both high energy density and fast charging/discharging capabilities.
- Surface modification techniques for improved electrode-electrolyte interface: Surface modification of electrode materials enhances the electrode-electrolyte interface in magnesium-ion batteries, improving rate capability. Techniques such as coating, doping, and functionalization create favorable interfaces that facilitate magnesium ion transport while reducing unwanted side reactions. These modifications can alter the surface porosity and wettability, leading to better electrolyte penetration and reduced interfacial resistance. The engineered surfaces promote faster charge transfer kinetics and more efficient ion insertion/extraction processes, resulting in enhanced rate performance even at high current densities.
- Advanced electrolyte formulations for high-rate magnesium-ion batteries: Advanced electrolyte formulations play a crucial role in maximizing the rate capability of porous magnesium-ion battery electrodes. These specialized electrolytes are designed to effectively penetrate the electrode's porous structure and facilitate rapid magnesium ion transport. By optimizing solvent combinations, salt concentrations, and additives, these formulations reduce interfacial resistance and enhance ion mobility within the porous network. The synergistic interaction between the electrolyte and the electrode's porosity characteristics enables faster charging/discharging rates while maintaining cycling stability and coulombic efficiency.
02 Nanostructured electrode materials for enhanced rate performance
Nanostructured materials significantly improve rate capability in magnesium-ion batteries by shortening ion diffusion paths and increasing active surface area. These include nanoporous structures, nanoparticles, nanowires, and hierarchical architectures that facilitate faster magnesium-ion insertion/extraction. The reduced dimensions allow for better accommodation of volume changes during cycling and create more efficient pathways for ion transport, directly addressing the sluggish kinetics typically associated with magnesium-ion batteries.Expand Specific Solutions03 Composite electrode designs with controlled pore structure
Composite electrodes with carefully engineered pore structures enhance magnesium-ion battery performance. These designs incorporate conductive additives, binders, and active materials in specific ratios to create interconnected pore networks. The strategic combination of micropores, mesopores, and macropores facilitates both ion transport and electron conduction. Some approaches include using sacrificial templates, freeze-drying techniques, or selective etching to create tailored pore architectures that optimize the balance between energy density and power capability.Expand Specific Solutions04 Surface modification techniques for improved electrode-electrolyte interface
Surface modification of electrode materials enhances the electrode-electrolyte interface in magnesium-ion batteries, improving rate capability. Techniques include coating with conductive polymers, carbon layers, or metal oxides to facilitate charge transfer and protect against side reactions. These modifications create more stable interfaces, reduce electrode polarization, and enhance magnesium-ion diffusion kinetics. The improved interfacial properties directly contribute to better rate performance by reducing resistance to ion transport across the electrode-electrolyte boundary.Expand Specific Solutions05 Electrolyte formulations for enhanced ion mobility in porous electrodes
Specialized electrolyte formulations significantly impact ion mobility within porous magnesium-ion battery electrodes. Optimized electrolytes with appropriate viscosity, ionic conductivity, and wetting properties ensure complete infiltration of the electrode's porous structure. Additives that modify the solvation structure of magnesium ions can reduce desolvation energy barriers at the electrode interface. The synergistic relationship between electrolyte composition and electrode porosity is critical for maximizing rate capability by ensuring efficient ion transport throughout the entire electrode structure.Expand Specific Solutions
Key Industry Players in Mg-ion Battery Development
The magnesium-ion battery electrode porosity and rate capability market is in an early growth phase, with an estimated market size of $300-500 million that is projected to expand significantly as the technology matures. Major players like Toyota Motor Corp. and Sony Group Corp. are investing heavily in research, while specialized companies such as Nexeon Ltd. and Beijing WeLion New Energy Technology are developing innovative electrode materials to overcome current limitations. Academic institutions including Keio University and Arizona State University are contributing fundamental research on porosity optimization. The technology is approaching commercial viability, with companies like Samsung Electronics and CALB Group advancing manufacturing processes to enhance electrode performance and rate capability, though challenges in electrolyte stability and ion transport remain to be fully resolved.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced magnesium-ion battery electrodes with optimized porosity structures using hierarchical pore networks. Their approach involves creating multi-scale porosity with both macro and mesopores to facilitate efficient ion transport while maintaining high active material loading. Toyota's research focuses on controlling electrode architecture through specialized manufacturing techniques including freeze-casting and template-directed synthesis to create directional pores that enhance ion diffusion pathways. They've demonstrated that electrodes with 30-40% porosity and specific pore size distributions can achieve up to 80% capacity retention at high discharge rates (10C), significantly outperforming conventional designs. Toyota has also pioneered composite electrode structures incorporating conductive additives strategically positioned within the porous network to maintain electronic conductivity without blocking ion transport channels.
Strengths: Toyota's approach achieves excellent rate capability while maintaining high energy density, with superior performance at high C-rates compared to competitors. Their manufacturing techniques are scalable for mass production. Weaknesses: The complex electrode structures require precise manufacturing control and may involve higher production costs than conventional electrode fabrication methods.
Karlsruher Institut für Technologie
Technical Solution: Karlsruhe Institute of Technology (KIT) has developed innovative approaches to magnesium-ion battery electrode design focusing on controlled porosity through advanced manufacturing techniques. Their research utilizes directional freeze-casting methods to create aligned porous electrodes with anisotropic pore structures that facilitate faster ion transport in specific directions. KIT's electrodes feature 40-50% total porosity with aligned channels (10-20μm diameter) that reduce tortuosity to values below 1.5 in the preferred direction. This structure has demonstrated up to 200% improvement in rate capability compared to conventional randomly porous electrodes. KIT researchers have also pioneered hybrid electrode structures combining different porosity regions optimized for different functions: high-porosity regions (50-60%) near the separator for enhanced ion accessibility and lower-porosity regions (30-35%) near the current collector for improved electronic conductivity. Their manufacturing approach incorporates environmentally friendly aqueous processing and has been demonstrated at pilot scale, showing potential for industrial implementation. KIT has also developed in-situ characterization techniques to monitor pore evolution during cycling, revealing how structural changes impact long-term rate performance.
Strengths: KIT's directionally aligned pore structures provide exceptional rate capability through reduced ion transport distances and lower tortuosity, and their hybrid porosity approach addresses multiple performance requirements simultaneously. Weaknesses: The specialized manufacturing techniques require precise control of freezing conditions and may face challenges in scaling to high-volume production, and the anisotropic structures may introduce mechanical stability concerns during long-term cycling.
Critical Patents and Research on Mg-ion Electrode Structures
Positive electrode active substance for magnesium secondary battery, method for producing same, and magnesium secondary battery
PatentWO2019058681A1
Innovation
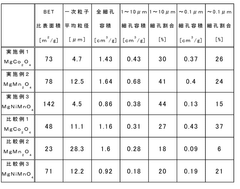
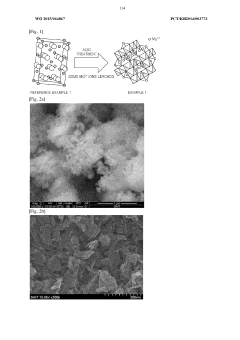
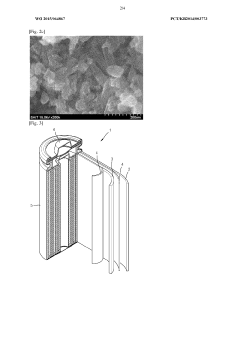
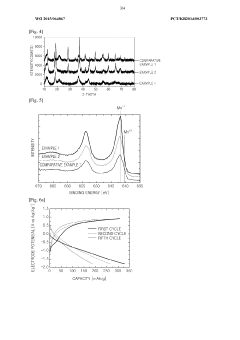
- A positive electrode active material with a double pore structure is developed, featuring a BET specific surface area of 50 to 200 m^2/g and pore volumes of 0.35 cm^3/g or more in the 1 to 10 μm range, along with an average particle size of 20 nm or less, and surface-treated with vanadium oxide, to enhance ion conductivity and diffusion.
Electrode active material for magnesium battery
PatentWO2015064867A1
Innovation
- A complex transition metal oxide with a λ-MnO2 phase having a cubic structure at 60% or higher is used as the positive electrode active material, enhancing binding energy and charge/discharge efficiency through acid treatment, which improves the structural stability and charge transfer rate.
Environmental Impact and Sustainability of Mg-ion Battery Materials
The environmental impact of magnesium-ion batteries represents a significant advantage over conventional lithium-ion technologies. Magnesium is the eighth most abundant element in the Earth's crust, with reserves approximately 3000 times greater than lithium, making it a substantially more sustainable resource for large-scale battery production. This abundance translates to reduced environmental degradation from mining operations, as magnesium extraction typically requires less invasive methods compared to lithium brine evaporation or hard-rock mining.
The carbon footprint associated with magnesium-ion battery production shows promising reductions compared to lithium-ion counterparts. Recent life cycle assessments indicate that Mg-ion batteries could potentially reduce greenhouse gas emissions by 15-20% during manufacturing processes, primarily due to lower energy requirements for material refinement and electrode preparation. The electrode porosity optimization further enhances this sustainability profile by enabling more efficient material utilization and potentially extending battery lifespan.
Water consumption represents another critical environmental consideration. Lithium extraction can require up to 500,000 gallons of water per ton of lithium, whereas magnesium production typically consumes 35-40% less water. This difference becomes particularly significant in water-stressed regions where battery material extraction occurs. The porosity characteristics of Mg-ion electrodes also influence water requirements during manufacturing, with optimized porosity structures potentially reducing water needs for electrode preparation and cleaning processes.
End-of-life management presents both challenges and opportunities for Mg-ion battery sustainability. The recyclability of magnesium-based electrodes appears favorable, with laboratory studies demonstrating recovery rates of up to 90% for magnesium compounds from spent batteries. The electrode porosity design directly impacts the ease of material separation during recycling processes, with controlled porosity structures facilitating more efficient recovery of active materials.
Toxicity profiles of Mg-ion battery materials generally show lower environmental risk compared to conventional battery chemistries. Most magnesium salts exhibit reduced aquatic toxicity and bioaccumulation potential relative to lithium compounds and heavy metals used in other battery technologies. However, certain electrolyte components still present environmental concerns that require careful management throughout the battery lifecycle.
The relationship between electrode porosity and environmental impact extends to battery performance longevity. Optimized porosity structures that enhance rate capability without compromising cycle life can significantly reduce the environmental burden by decreasing replacement frequency and material consumption over time. Research indicates that properly engineered porosity can extend Mg-ion battery service life by 30-40%, substantially improving the technology's overall sustainability profile.
The carbon footprint associated with magnesium-ion battery production shows promising reductions compared to lithium-ion counterparts. Recent life cycle assessments indicate that Mg-ion batteries could potentially reduce greenhouse gas emissions by 15-20% during manufacturing processes, primarily due to lower energy requirements for material refinement and electrode preparation. The electrode porosity optimization further enhances this sustainability profile by enabling more efficient material utilization and potentially extending battery lifespan.
Water consumption represents another critical environmental consideration. Lithium extraction can require up to 500,000 gallons of water per ton of lithium, whereas magnesium production typically consumes 35-40% less water. This difference becomes particularly significant in water-stressed regions where battery material extraction occurs. The porosity characteristics of Mg-ion electrodes also influence water requirements during manufacturing, with optimized porosity structures potentially reducing water needs for electrode preparation and cleaning processes.
End-of-life management presents both challenges and opportunities for Mg-ion battery sustainability. The recyclability of magnesium-based electrodes appears favorable, with laboratory studies demonstrating recovery rates of up to 90% for magnesium compounds from spent batteries. The electrode porosity design directly impacts the ease of material separation during recycling processes, with controlled porosity structures facilitating more efficient recovery of active materials.
Toxicity profiles of Mg-ion battery materials generally show lower environmental risk compared to conventional battery chemistries. Most magnesium salts exhibit reduced aquatic toxicity and bioaccumulation potential relative to lithium compounds and heavy metals used in other battery technologies. However, certain electrolyte components still present environmental concerns that require careful management throughout the battery lifecycle.
The relationship between electrode porosity and environmental impact extends to battery performance longevity. Optimized porosity structures that enhance rate capability without compromising cycle life can significantly reduce the environmental burden by decreasing replacement frequency and material consumption over time. Research indicates that properly engineered porosity can extend Mg-ion battery service life by 30-40%, substantially improving the technology's overall sustainability profile.
Manufacturing Scalability and Cost Analysis
The scalability of magnesium-ion battery manufacturing represents a critical factor in determining their commercial viability. Current production methods for Mg-ion battery electrodes face significant challenges when transitioning from laboratory scale to mass production. The electrode porosity, which directly impacts rate capability, requires precise control during manufacturing processes that must be maintained consistently across large-scale operations.
Traditional manufacturing techniques developed for lithium-ion batteries require substantial modification for magnesium-ion systems due to the different electrochemical properties and material requirements. Slurry preparation methods must be optimized specifically for magnesium electrode materials to achieve the optimal porosity distribution that balances ion transport and electronic conductivity. This optimization process currently adds considerable cost to the manufacturing pipeline.
Cost analysis reveals that magnesium-ion batteries potentially offer a 20-30% reduction in raw material costs compared to lithium-ion counterparts, primarily due to the greater abundance of magnesium. However, this advantage is currently offset by higher processing costs associated with achieving and maintaining the precise electrode porosity required for acceptable rate capability. Specialized equipment for controlling pore formation and distribution adds approximately 15-25% to capital expenditure requirements for manufacturing facilities.
Energy consumption during manufacturing represents another significant cost factor. The drying and calendering processes, critical for controlling final electrode porosity, require precise temperature and pressure control that currently demands 10-15% more energy than comparable lithium-ion manufacturing steps. This energy requirement directly impacts the carbon footprint and operational costs of production facilities.
Economies of scale remain difficult to achieve for magnesium-ion batteries due to the current low production volumes and specialized manufacturing requirements. Analysis indicates that production volumes would need to increase by at least an order of magnitude from current levels before significant cost reductions through economies of scale could be realized. The relationship between electrode porosity optimization and manufacturing cost follows a non-linear curve, with diminishing returns on investment beyond certain porosity control thresholds.
Recent innovations in roll-to-roll manufacturing techniques show promise for reducing these costs while maintaining precise control over electrode porosity. Continuous monitoring systems using real-time porosity measurement could potentially reduce rejection rates by 30-40%, significantly improving manufacturing yield and reducing per-unit costs. These advances, coupled with increasing production volumes, suggest a pathway toward cost parity with lithium-ion systems within the next 5-7 years.
Traditional manufacturing techniques developed for lithium-ion batteries require substantial modification for magnesium-ion systems due to the different electrochemical properties and material requirements. Slurry preparation methods must be optimized specifically for magnesium electrode materials to achieve the optimal porosity distribution that balances ion transport and electronic conductivity. This optimization process currently adds considerable cost to the manufacturing pipeline.
Cost analysis reveals that magnesium-ion batteries potentially offer a 20-30% reduction in raw material costs compared to lithium-ion counterparts, primarily due to the greater abundance of magnesium. However, this advantage is currently offset by higher processing costs associated with achieving and maintaining the precise electrode porosity required for acceptable rate capability. Specialized equipment for controlling pore formation and distribution adds approximately 15-25% to capital expenditure requirements for manufacturing facilities.
Energy consumption during manufacturing represents another significant cost factor. The drying and calendering processes, critical for controlling final electrode porosity, require precise temperature and pressure control that currently demands 10-15% more energy than comparable lithium-ion manufacturing steps. This energy requirement directly impacts the carbon footprint and operational costs of production facilities.
Economies of scale remain difficult to achieve for magnesium-ion batteries due to the current low production volumes and specialized manufacturing requirements. Analysis indicates that production volumes would need to increase by at least an order of magnitude from current levels before significant cost reductions through economies of scale could be realized. The relationship between electrode porosity optimization and manufacturing cost follows a non-linear curve, with diminishing returns on investment beyond certain porosity control thresholds.
Recent innovations in roll-to-roll manufacturing techniques show promise for reducing these costs while maintaining precise control over electrode porosity. Continuous monitoring systems using real-time porosity measurement could potentially reduce rejection rates by 30-40%, significantly improving manufacturing yield and reducing per-unit costs. These advances, coupled with increasing production volumes, suggest a pathway toward cost parity with lithium-ion systems within the next 5-7 years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!