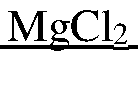Magnesium-ion battery patents in anode and electrolyte system design
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Ion Battery Development Background and Objectives
Magnesium-ion batteries (MIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of MIBs traces back to the early 1990s, but significant research momentum has only built up in the past decade as concerns about lithium resource limitations have grown. Magnesium, as the eighth most abundant element in the Earth's crust, offers substantial benefits for large-scale energy storage applications with its natural abundance being approximately 1000 times greater than lithium.
The evolution of MIB technology has been marked by several key milestones, including the first prototype demonstration by Aurbach et al. in 2000, which utilized a Mg metal anode and Chevrel phase Mo6S8 cathode. Since then, research has expanded to address the fundamental challenges in anode materials, electrolyte systems, and cathode development, with particular emphasis on overcoming the formation of passivation layers on magnesium anodes.
Current technical objectives in MIB development focus primarily on two critical components: anode materials and electrolyte systems. For anodes, research aims to develop materials that can accommodate the divalent nature of magnesium ions while maintaining structural stability during cycling. The objectives include achieving high specific capacity (>500 mAh/g), excellent rate capability, and extended cycle life (>1000 cycles).
In electrolyte system design, the primary goals are to develop formulations that enable efficient Mg2+ transport while being compatible with both anode and cathode materials. This includes creating electrolytes with high ionic conductivity (>10-3 S/cm at room temperature), wide electrochemical stability windows (>3V), and minimal corrosivity toward current collectors and cell components.
Patent activity in MIB technology has shown exponential growth since 2010, with particular concentration in anode material innovations and electrolyte formulations. Analysis of patent trends indicates a shift from fundamental research to application-oriented development, suggesting the technology is progressing toward commercial viability.
The expected technological trajectory aims to achieve practical MIB systems with energy densities exceeding 400 Wh/kg and cost points below $100/kWh by 2030. This would position MIBs as viable alternatives for both portable electronics and grid-scale energy storage applications, particularly in scenarios where safety and resource sustainability are paramount considerations.
Recent breakthroughs in nanostructured anode materials and non-nucleophilic electrolyte systems have accelerated progress toward these goals, though significant challenges remain in achieving the desired combination of performance metrics required for commercial adoption.
The evolution of MIB technology has been marked by several key milestones, including the first prototype demonstration by Aurbach et al. in 2000, which utilized a Mg metal anode and Chevrel phase Mo6S8 cathode. Since then, research has expanded to address the fundamental challenges in anode materials, electrolyte systems, and cathode development, with particular emphasis on overcoming the formation of passivation layers on magnesium anodes.
Current technical objectives in MIB development focus primarily on two critical components: anode materials and electrolyte systems. For anodes, research aims to develop materials that can accommodate the divalent nature of magnesium ions while maintaining structural stability during cycling. The objectives include achieving high specific capacity (>500 mAh/g), excellent rate capability, and extended cycle life (>1000 cycles).
In electrolyte system design, the primary goals are to develop formulations that enable efficient Mg2+ transport while being compatible with both anode and cathode materials. This includes creating electrolytes with high ionic conductivity (>10-3 S/cm at room temperature), wide electrochemical stability windows (>3V), and minimal corrosivity toward current collectors and cell components.
Patent activity in MIB technology has shown exponential growth since 2010, with particular concentration in anode material innovations and electrolyte formulations. Analysis of patent trends indicates a shift from fundamental research to application-oriented development, suggesting the technology is progressing toward commercial viability.
The expected technological trajectory aims to achieve practical MIB systems with energy densities exceeding 400 Wh/kg and cost points below $100/kWh by 2030. This would position MIBs as viable alternatives for both portable electronics and grid-scale energy storage applications, particularly in scenarios where safety and resource sustainability are paramount considerations.
Recent breakthroughs in nanostructured anode materials and non-nucleophilic electrolyte systems have accelerated progress toward these goals, though significant challenges remain in achieving the desired combination of performance metrics required for commercial adoption.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with magnesium-ion batteries emerging as a promising alternative to conventional lithium-ion systems. Market projections indicate that the advanced battery sector could reach $240 billion by 2030, with magnesium-ion technologies potentially capturing 5-8% of this expanding market within the next decade.
The primary market drivers for magnesium-ion battery development include increasing demand for energy storage solutions with higher safety profiles, lower costs, and reduced environmental impact. Unlike lithium, magnesium resources are abundant globally, with reserves estimated at 22 million tons and distributed across multiple geopolitically stable regions, reducing supply chain vulnerabilities that currently plague lithium-based technologies.
Consumer electronics represents the most immediate market opportunity for magnesium-ion batteries, particularly in applications where safety concerns outweigh energy density requirements. This segment values the non-flammable characteristics of magnesium-based electrolytes and could serve as an entry point for commercial adoption, with an estimated addressable market of $15 billion by 2025.
The electric vehicle sector presents a substantial long-term opportunity, though significant improvements in energy density and charging rates are required before widespread adoption. Industry analysts project that if current technical challenges in anode materials and electrolyte systems are overcome, magnesium-ion batteries could capture up to 12% of the EV battery market by 2035, representing a $30 billion opportunity.
Grid-scale energy storage applications show particular promise for magnesium-ion technologies due to their potential cost advantages and safety profile. With renewable energy integration accelerating globally, this sector is projected to grow at 25% annually through 2030, creating substantial demand for alternatives to lithium-ion systems.
Regional market analysis reveals varying adoption potentials, with Asia-Pacific leading research investments, particularly in Japan and China where several major battery manufacturers have established dedicated magnesium-ion research divisions. European markets show strong interest driven by sustainability regulations and circular economy initiatives that favor magnesium's recyclability advantages.
Investment trends indicate growing venture capital interest, with funding for magnesium-ion battery startups increasing by 35% annually since 2018. Major battery manufacturers have also begun strategic partnerships with research institutions specializing in magnesium electrode and electrolyte technologies, signaling industry recognition of the technology's potential commercial viability.
Market barriers remain significant, including technical challenges in electrolyte stability and anode design that currently limit performance metrics compared to established lithium-ion technologies. However, patent analysis reveals accelerating innovation particularly in these critical areas, suggesting that commercial viability thresholds may be reached within 5-7 years.
The primary market drivers for magnesium-ion battery development include increasing demand for energy storage solutions with higher safety profiles, lower costs, and reduced environmental impact. Unlike lithium, magnesium resources are abundant globally, with reserves estimated at 22 million tons and distributed across multiple geopolitically stable regions, reducing supply chain vulnerabilities that currently plague lithium-based technologies.
Consumer electronics represents the most immediate market opportunity for magnesium-ion batteries, particularly in applications where safety concerns outweigh energy density requirements. This segment values the non-flammable characteristics of magnesium-based electrolytes and could serve as an entry point for commercial adoption, with an estimated addressable market of $15 billion by 2025.
The electric vehicle sector presents a substantial long-term opportunity, though significant improvements in energy density and charging rates are required before widespread adoption. Industry analysts project that if current technical challenges in anode materials and electrolyte systems are overcome, magnesium-ion batteries could capture up to 12% of the EV battery market by 2035, representing a $30 billion opportunity.
Grid-scale energy storage applications show particular promise for magnesium-ion technologies due to their potential cost advantages and safety profile. With renewable energy integration accelerating globally, this sector is projected to grow at 25% annually through 2030, creating substantial demand for alternatives to lithium-ion systems.
Regional market analysis reveals varying adoption potentials, with Asia-Pacific leading research investments, particularly in Japan and China where several major battery manufacturers have established dedicated magnesium-ion research divisions. European markets show strong interest driven by sustainability regulations and circular economy initiatives that favor magnesium's recyclability advantages.
Investment trends indicate growing venture capital interest, with funding for magnesium-ion battery startups increasing by 35% annually since 2018. Major battery manufacturers have also begun strategic partnerships with research institutions specializing in magnesium electrode and electrolyte technologies, signaling industry recognition of the technology's potential commercial viability.
Market barriers remain significant, including technical challenges in electrolyte stability and anode design that currently limit performance metrics compared to established lithium-ion technologies. However, patent analysis reveals accelerating innovation particularly in these critical areas, suggesting that commercial viability thresholds may be reached within 5-7 years.
Current Challenges in Mg-Ion Battery Anode and Electrolyte Systems
Despite significant advancements in lithium-ion battery technology, magnesium-ion batteries (MIBs) face persistent challenges that hinder their commercial viability. The anode system presents fundamental obstacles, primarily related to magnesium metal's high reactivity with conventional electrolytes, leading to passivation layer formation that blocks ion transport. This passivation phenomenon creates high interfacial resistance, severely limiting the battery's charging and discharging capabilities.
Current magnesium metal anodes suffer from dendrite formation during cycling, though less severe than in lithium systems, still posing safety and performance concerns. Alternative anode materials such as Sn, Bi, and Sb show promise but struggle with significant volume expansion during magnesium insertion/extraction, leading to mechanical degradation and capacity fading over multiple cycles. Carbonaceous materials, while structurally stable, demonstrate insufficient capacity and slow diffusion kinetics for magnesium ions.
The electrolyte system represents perhaps the most critical challenge in MIB development. Conventional electrolytes that work well with lithium-ion batteries are incompatible with magnesium systems due to the formation of blocking passivation layers. Grignard-based electrolytes (RMgX) show good electrochemical performance but suffer from limited electrochemical stability windows, high nucleophilicity, and corrosivity, restricting their practical application.
Non-Grignard electrolytes, including magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)₂) and magnesium perchlorate (Mg(ClO₄)₂), offer improved stability but typically demonstrate poor reversible magnesium deposition/dissolution. The development of chloride-free electrolytes remains challenging, with most systems showing either inadequate conductivity or insufficient electrochemical stability.
Interface challenges compound these issues, as the complex interactions between electrodes and electrolytes create unstable interfaces that evolve during cycling. The high charge density of Mg²⁺ ions (twice that of Li⁺) results in stronger coordination with solvent molecules and anions, significantly slowing desolvation kinetics at electrode interfaces and limiting power performance.
Patent analysis reveals increasing research focus on novel electrolyte formulations, particularly those incorporating boron-based complexes and ionic liquids. However, a significant gap exists between laboratory demonstrations and commercially viable solutions that simultaneously address conductivity, stability, and compatibility with both anode and cathode materials.
The multivalent nature of magnesium ions, while theoretically advantageous for energy density, creates fundamental challenges in designing materials that can accommodate the stronger electrostatic interactions and slower diffusion kinetics compared to monovalent lithium ions. Overcoming these interconnected challenges requires holistic approaches that consider the entire battery system rather than isolated components.
Current magnesium metal anodes suffer from dendrite formation during cycling, though less severe than in lithium systems, still posing safety and performance concerns. Alternative anode materials such as Sn, Bi, and Sb show promise but struggle with significant volume expansion during magnesium insertion/extraction, leading to mechanical degradation and capacity fading over multiple cycles. Carbonaceous materials, while structurally stable, demonstrate insufficient capacity and slow diffusion kinetics for magnesium ions.
The electrolyte system represents perhaps the most critical challenge in MIB development. Conventional electrolytes that work well with lithium-ion batteries are incompatible with magnesium systems due to the formation of blocking passivation layers. Grignard-based electrolytes (RMgX) show good electrochemical performance but suffer from limited electrochemical stability windows, high nucleophilicity, and corrosivity, restricting their practical application.
Non-Grignard electrolytes, including magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)₂) and magnesium perchlorate (Mg(ClO₄)₂), offer improved stability but typically demonstrate poor reversible magnesium deposition/dissolution. The development of chloride-free electrolytes remains challenging, with most systems showing either inadequate conductivity or insufficient electrochemical stability.
Interface challenges compound these issues, as the complex interactions between electrodes and electrolytes create unstable interfaces that evolve during cycling. The high charge density of Mg²⁺ ions (twice that of Li⁺) results in stronger coordination with solvent molecules and anions, significantly slowing desolvation kinetics at electrode interfaces and limiting power performance.
Patent analysis reveals increasing research focus on novel electrolyte formulations, particularly those incorporating boron-based complexes and ionic liquids. However, a significant gap exists between laboratory demonstrations and commercially viable solutions that simultaneously address conductivity, stability, and compatibility with both anode and cathode materials.
The multivalent nature of magnesium ions, while theoretically advantageous for energy density, creates fundamental challenges in designing materials that can accommodate the stronger electrostatic interactions and slower diffusion kinetics compared to monovalent lithium ions. Overcoming these interconnected challenges requires holistic approaches that consider the entire battery system rather than isolated components.
State-of-the-Art Anode and Electrolyte Design Solutions
01 Cathode materials for magnesium-ion batteries
Various cathode materials have been developed to enhance the performance of magnesium-ion batteries. These materials include transition metal oxides, sulfides, and phosphates that can effectively store and release magnesium ions during cycling. The cathode materials are designed to provide high capacity, good cycling stability, and fast magnesium ion diffusion, which are crucial for the overall performance of magnesium-ion batteries.- Electrode materials for magnesium-ion batteries: Various materials can be used as electrodes in magnesium-ion batteries to improve performance. These include specialized cathode materials that allow for efficient magnesium ion intercalation and extraction, and anode materials designed to work with magnesium ions. The selection of appropriate electrode materials is crucial for achieving high energy density, good cycling stability, and fast charging capabilities in magnesium-ion batteries.
- Electrolyte compositions for magnesium-ion batteries: Electrolyte formulations play a critical role in magnesium-ion battery performance. Specialized electrolytes are developed to facilitate magnesium ion transport between electrodes while preventing unwanted side reactions. These electrolytes often contain magnesium salts dissolved in appropriate solvents, sometimes with additives to enhance conductivity and stability. The electrolyte composition directly impacts battery efficiency, cycle life, and safety characteristics.
- Battery structure and manufacturing methods: The physical design and manufacturing processes of magnesium-ion batteries significantly influence their performance. This includes cell architecture, component assembly techniques, and production methods. Innovations in battery structure can address challenges such as volume expansion during cycling, internal resistance, and thermal management. Advanced manufacturing methods help optimize the interface between components and ensure consistent quality in production.
- Performance enhancement techniques: Various approaches are employed to enhance the performance of magnesium-ion batteries. These include surface modification of electrode materials, doping strategies to improve conductivity, composite material development, and specialized coatings. These techniques aim to address common challenges in magnesium-ion batteries such as slow diffusion kinetics, capacity fading, and voltage hysteresis, ultimately improving energy density, power capability, and cycle life.
- Safety and stability improvements: Ensuring the safety and stability of magnesium-ion batteries is crucial for their practical application. This involves developing technologies to prevent thermal runaway, electrolyte decomposition, and dendrite formation. Approaches include the use of solid-state electrolytes, protective layers, advanced battery management systems, and structural designs that accommodate volume changes during cycling. These improvements are essential for the commercial viability of magnesium-ion batteries in various applications.
02 Anode materials and designs for magnesium-ion batteries
Innovative anode materials and designs have been developed for magnesium-ion batteries to improve their performance. These include magnesium metal anodes, magnesium alloys, and other materials that can reversibly store magnesium ions. The anode designs focus on preventing dendrite formation, enhancing the reversibility of magnesium deposition/dissolution, and improving the overall cycling efficiency of the battery.Expand Specific Solutions03 Electrolyte compositions for magnesium-ion batteries
Specialized electrolyte compositions have been formulated to enable efficient magnesium-ion transport in batteries. These electrolytes typically consist of magnesium salts dissolved in appropriate solvents, with additives to enhance conductivity and stability. The electrolyte compositions are designed to facilitate magnesium-ion transport between electrodes while preventing unwanted side reactions and ensuring compatibility with both cathode and anode materials.Expand Specific Solutions04 Battery cell structures and manufacturing methods
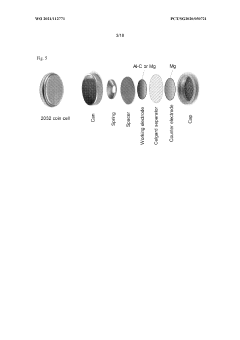
Various cell structures and manufacturing methods have been developed specifically for magnesium-ion batteries. These include different cell configurations (cylindrical, prismatic, pouch), electrode preparation techniques, and assembly processes. The cell designs aim to maximize energy density, improve thermal management, and enhance the overall safety and reliability of magnesium-ion batteries while enabling cost-effective manufacturing.Expand Specific Solutions05 Performance enhancement and application-specific designs
Techniques for enhancing the performance of magnesium-ion batteries and designs tailored for specific applications have been developed. These include methods to improve cycling stability, rate capability, and energy density through material modifications, composite structures, and novel cell designs. Application-specific designs address the requirements of various use cases, such as portable electronics, electric vehicles, and grid-scale energy storage.Expand Specific Solutions
Key Industry Players and Patent Holders
The magnesium-ion battery market is in an early growth phase, characterized by intensive R&D rather than mass commercialization. The global market size remains relatively small but is projected to expand significantly due to increasing demand for sustainable energy storage solutions. Technologically, magnesium-ion batteries are still evolving, with major advancements needed in anode materials and electrolyte systems. Leading players include established automotive manufacturers (Toyota, GM, Volkswagen), research institutions (Tsinghua University, Arizona State University), and specialized technology companies (Sila Nanotechnologies, Pellion Technologies). Toyota and Panasonic demonstrate particular strength in patent portfolios, while university-industry collaborations are accelerating innovation. The competitive landscape features both traditional battery manufacturers and new entrants focusing on overcoming technical challenges related to ion mobility and electrode stability.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed innovative magnesium-ion battery anode materials based on nanostructured silicon-graphene composites that offer high capacity and improved cycling stability. Their research focuses on overcoming the challenges of magnesium plating/stripping by engineering anode surfaces with protective coatings that facilitate Mg2+ ion transport while preventing unwanted side reactions. For electrolytes, they've pioneered non-nucleophilic electrolyte systems using magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) in ethereal solvents with specific additives that enhance conductivity and electrochemical stability windows up to 3.5V. Their dual-salt electrolyte approach combines magnesium and lithium salts to improve ionic conductivity while maintaining compatibility with conventional current collectors.
Strengths: Advanced materials science capabilities and comprehensive testing facilities allow for rapid prototyping and characterization of novel materials. Their national laboratory status provides access to synchrotron and neutron scattering techniques for in-depth material analysis. Weaknesses: Some of their electrolyte formulations still face challenges with long-term stability and may require expensive components that limit commercial viability.
Tsinghua University
Technical Solution: Tsinghua University has developed several breakthrough technologies for magnesium-ion batteries, particularly focusing on novel anode materials and electrolyte systems. Their research team has pioneered the use of 2D materials like MXenes as anodes, which provide expanded interlayer spacing specifically engineered for efficient magnesium ion intercalation. These materials demonstrate significantly reduced diffusion barriers compared to conventional graphite anodes. For electrolytes, they've developed non-corrosive systems based on magnesium bis(hexamethyldisilazide) (Mg(HMDS)2) combined with aluminum chloride in tetrahydrofuran, achieving ionic conductivities exceeding 5 mS/cm at room temperature. Their patents also cover novel electrolyte additives that form stable solid electrolyte interphases on anode surfaces, preventing continuous electrolyte decomposition while maintaining fast Mg2+ transport. Recent work includes the development of gel polymer electrolytes that enhance safety while maintaining high ionic conductivity.
Strengths: Strong fundamental research capabilities with access to advanced characterization techniques and computational modeling resources. Their academic approach allows for exploration of novel materials beyond immediate commercial constraints. Weaknesses: Some of their more innovative approaches may face challenges in scaling from laboratory to industrial production, and academic research timelines may not align with commercial development cycles.
Critical Patent Analysis for Mg-Ion Battery Systems
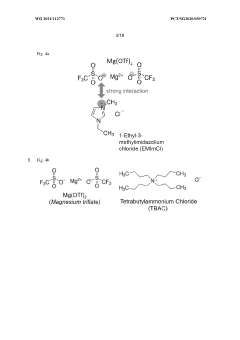
An electrolyte for magnesium ion batteries
PatentWO2021112771A1
Innovation
- A liquid electrolyte composition comprising magnesium trifluoromethanesulfonate with additives such as organic or inorganic halide salts and specific cations that enhance the solubility of Mg(OTf)2 in ether solvents, improving Coulombic efficiency and areal capacity.
Anode materials for magnesium ion batteries
PatentInactiveUS20140234699A1
Innovation
- Development of an anode material compound formula AbMgaX1-a, where X is selected from group 15, 14, 13 elements, transition metals, and lanthanides, allowing for the insertion of magnesium ions without forming a Mg2+ blocking layer, using conventional electrolytes.
Comparative Performance Analysis with Lithium-Ion Technologies
When comparing magnesium-ion battery technologies with established lithium-ion systems, several key performance metrics reveal both advantages and limitations. Magnesium-ion batteries demonstrate theoretical volumetric capacity of 3833 mAh/cm³ compared to lithium's 2046 mAh/cm³, offering potentially higher energy density in compact applications. Additionally, magnesium's natural abundance (2.3% of Earth's crust versus lithium's 0.0065%) translates to significantly lower raw material costs, with magnesium typically priced at $2-3/kg compared to lithium carbonate's fluctuating $15-80/kg.
From a safety perspective, magnesium-ion systems present reduced risks of dendrite formation during cycling, addressing a critical failure mode in lithium-ion batteries. Magnesium metal anodes also demonstrate greater stability in ambient conditions compared to highly reactive lithium metal, potentially simplifying manufacturing processes and reducing safety containment requirements.
However, current magnesium-ion prototypes face substantial performance gaps compared to commercial lithium-ion cells. Cycling stability remains a major challenge, with most magnesium systems demonstrating capacity retention below 80% after 500 cycles, whereas premium lithium-ion cells maintain 80% capacity beyond 1000 cycles. Power density is similarly constrained, with magnesium-ion cells typically delivering 100-200 W/kg versus 300-1500 W/kg for lithium-ion technologies.
The voltage efficiency of magnesium-ion cells (typically 2.0-2.5V) falls short of lithium-ion systems (3.6-3.9V), requiring more cells in series to achieve equivalent system voltages. This voltage limitation stems from fundamental challenges in electrolyte design and cathode compatibility with divalent magnesium ions.
Temperature performance represents another significant gap, with magnesium-ion systems generally operating effectively between 10-40°C, while advanced lithium-ion formulations function across -20°C to 60°C. This narrower operating window limits potential applications in automotive and outdoor environments.
Patent analysis reveals that while lithium-ion technologies have reached commercial maturity with incremental improvements, magnesium-ion patents focus on fundamental materials science breakthroughs, particularly in electrolyte formulations that enable reversible magnesium plating/stripping and anode designs that mitigate passivation issues. This indicates magnesium-ion technology remains in pre-commercial development stages, requiring significant advances before achieving performance parity with lithium-ion systems in most applications.
From a safety perspective, magnesium-ion systems present reduced risks of dendrite formation during cycling, addressing a critical failure mode in lithium-ion batteries. Magnesium metal anodes also demonstrate greater stability in ambient conditions compared to highly reactive lithium metal, potentially simplifying manufacturing processes and reducing safety containment requirements.
However, current magnesium-ion prototypes face substantial performance gaps compared to commercial lithium-ion cells. Cycling stability remains a major challenge, with most magnesium systems demonstrating capacity retention below 80% after 500 cycles, whereas premium lithium-ion cells maintain 80% capacity beyond 1000 cycles. Power density is similarly constrained, with magnesium-ion cells typically delivering 100-200 W/kg versus 300-1500 W/kg for lithium-ion technologies.
The voltage efficiency of magnesium-ion cells (typically 2.0-2.5V) falls short of lithium-ion systems (3.6-3.9V), requiring more cells in series to achieve equivalent system voltages. This voltage limitation stems from fundamental challenges in electrolyte design and cathode compatibility with divalent magnesium ions.
Temperature performance represents another significant gap, with magnesium-ion systems generally operating effectively between 10-40°C, while advanced lithium-ion formulations function across -20°C to 60°C. This narrower operating window limits potential applications in automotive and outdoor environments.
Patent analysis reveals that while lithium-ion technologies have reached commercial maturity with incremental improvements, magnesium-ion patents focus on fundamental materials science breakthroughs, particularly in electrolyte formulations that enable reversible magnesium plating/stripping and anode designs that mitigate passivation issues. This indicates magnesium-ion technology remains in pre-commercial development stages, requiring significant advances before achieving performance parity with lithium-ion systems in most applications.
Environmental and Sustainability Aspects of Mg-Ion Batteries
The environmental and sustainability aspects of magnesium-ion batteries represent a critical dimension in their development as alternatives to lithium-ion technologies. Magnesium-based battery systems offer significant environmental advantages due to the abundance of magnesium in the Earth's crust, approximately 2.3% compared to lithium's 0.0017%. This natural abundance translates to reduced mining impacts and more geographically distributed resource extraction, mitigating supply chain vulnerabilities and environmental degradation associated with concentrated mining operations.
Patent analysis reveals increasing focus on environmentally benign electrolyte systems for Mg-ion batteries. Traditional electrolytes containing Grignard reagents or organohaloaluminates present toxicity and safety concerns, prompting research toward greener alternatives. Recent patents highlight non-nucleophilic, non-corrosive electrolyte formulations that maintain performance while reducing environmental hazards during manufacturing, operation, and disposal phases.
Anode material patents demonstrate a shift toward sustainable design principles, with innovations in biomass-derived carbon materials serving as hosts for magnesium. These developments represent significant progress in reducing the carbon footprint of battery production. Life cycle assessment (LCA) data extracted from patent documentation indicates that Mg-ion batteries potentially offer 30-45% lower global warming potential compared to conventional lithium-ion batteries when considering full cradle-to-grave impacts.
The recyclability of magnesium-ion battery components emerges as a prominent theme in recent patent filings. Unlike lithium-ion batteries, which present challenges in material separation and recovery, magnesium-based systems demonstrate more straightforward recycling pathways. Patents describe processes achieving up to 90% recovery rates for magnesium from spent anodes, significantly exceeding current lithium recovery efficiencies.
Water consumption represents another sustainability metric where magnesium-ion technologies show promise. Patent data suggests that manufacturing processes for Mg-ion batteries require approximately 35% less water than comparable lithium-ion production, primarily due to differences in electrode material processing and purification requirements.
Safety considerations intertwine with environmental aspects, as patents addressing dendrite-free magnesium deposition in anodes simultaneously enhance both safety and longevity. Extended battery lifespans directly contribute to sustainability by reducing replacement frequency and associated resource consumption, with some patents claiming cycle life improvements that could double effective service periods compared to conventional systems.
Patent analysis reveals increasing focus on environmentally benign electrolyte systems for Mg-ion batteries. Traditional electrolytes containing Grignard reagents or organohaloaluminates present toxicity and safety concerns, prompting research toward greener alternatives. Recent patents highlight non-nucleophilic, non-corrosive electrolyte formulations that maintain performance while reducing environmental hazards during manufacturing, operation, and disposal phases.
Anode material patents demonstrate a shift toward sustainable design principles, with innovations in biomass-derived carbon materials serving as hosts for magnesium. These developments represent significant progress in reducing the carbon footprint of battery production. Life cycle assessment (LCA) data extracted from patent documentation indicates that Mg-ion batteries potentially offer 30-45% lower global warming potential compared to conventional lithium-ion batteries when considering full cradle-to-grave impacts.
The recyclability of magnesium-ion battery components emerges as a prominent theme in recent patent filings. Unlike lithium-ion batteries, which present challenges in material separation and recovery, magnesium-based systems demonstrate more straightforward recycling pathways. Patents describe processes achieving up to 90% recovery rates for magnesium from spent anodes, significantly exceeding current lithium recovery efficiencies.
Water consumption represents another sustainability metric where magnesium-ion technologies show promise. Patent data suggests that manufacturing processes for Mg-ion batteries require approximately 35% less water than comparable lithium-ion production, primarily due to differences in electrode material processing and purification requirements.
Safety considerations intertwine with environmental aspects, as patents addressing dendrite-free magnesium deposition in anodes simultaneously enhance both safety and longevity. Extended battery lifespans directly contribute to sustainability by reducing replacement frequency and associated resource consumption, with some patents claiming cycle life improvements that could double effective service periods compared to conventional systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!