Magnesium-ion battery cathode structural stability analysis
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Cathode Evolution and Research Objectives
Magnesium-ion battery technology has emerged as a promising alternative to lithium-ion batteries due to the abundance of magnesium resources, enhanced safety profile, and theoretical potential for higher energy density. The evolution of cathode materials for Mg-ion batteries represents a critical research trajectory that has undergone significant transformation over the past two decades.
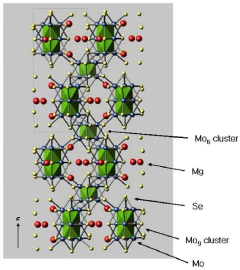
Early research in the 1990s and early 2000s focused primarily on Chevrel phases (Mo6S8), which demonstrated reversible Mg intercalation but suffered from low voltage and capacity limitations. This initial phase established fundamental understanding of Mg2+ ion mobility challenges in solid-state structures, particularly the strong electrostatic interaction between divalent Mg ions and host lattices.
The mid-2000s witnessed a shift toward oxide-based cathodes, with spinel structures such as MgMn2O4 and layered V2O5 receiving considerable attention. These materials offered higher operating voltages but encountered significant structural stability issues during cycling, leading to rapid capacity fade. The strong polarizing effect of Mg2+ ions often resulted in structural distortion and collapse during insertion/extraction processes.
Recent advancements (2015-present) have focused on developing novel cathode architectures specifically designed to accommodate the unique characteristics of Mg2+ ions. Prussian blue analogs, organic cathodes, and sulfur-based materials have demonstrated improved kinetics and structural stability. Particularly noteworthy is the development of shielding strategies to mitigate the strong coulombic interaction between Mg2+ and host frameworks.
Current research objectives center on addressing several critical challenges in cathode development. Primary among these is enhancing structural stability during repeated Mg2+ insertion/extraction cycles. This requires fundamental understanding of degradation mechanisms at the atomic level and development of novel stabilization strategies.
Another key objective involves improving Mg2+ diffusion kinetics within cathode structures. The slow diffusion of magnesium ions represents a significant barrier to practical application, necessitating innovative approaches to channel design and interface engineering.
Additionally, researchers aim to increase operating voltage while maintaining structural integrity. This objective requires careful balancing of electronic structure modification without compromising stability. Computational screening and materials genome approaches are increasingly employed to accelerate this discovery process.
The ultimate research goal remains the development of cathode materials that can deliver high energy density (>500 Wh/kg), long cycle life (>1000 cycles), and fast charging capabilities comparable to current lithium-ion technologies. Achieving these targets requires interdisciplinary approaches combining advanced characterization techniques, computational modeling, and innovative synthetic strategies focused specifically on understanding and enhancing structural stability during electrochemical cycling.
Early research in the 1990s and early 2000s focused primarily on Chevrel phases (Mo6S8), which demonstrated reversible Mg intercalation but suffered from low voltage and capacity limitations. This initial phase established fundamental understanding of Mg2+ ion mobility challenges in solid-state structures, particularly the strong electrostatic interaction between divalent Mg ions and host lattices.
The mid-2000s witnessed a shift toward oxide-based cathodes, with spinel structures such as MgMn2O4 and layered V2O5 receiving considerable attention. These materials offered higher operating voltages but encountered significant structural stability issues during cycling, leading to rapid capacity fade. The strong polarizing effect of Mg2+ ions often resulted in structural distortion and collapse during insertion/extraction processes.
Recent advancements (2015-present) have focused on developing novel cathode architectures specifically designed to accommodate the unique characteristics of Mg2+ ions. Prussian blue analogs, organic cathodes, and sulfur-based materials have demonstrated improved kinetics and structural stability. Particularly noteworthy is the development of shielding strategies to mitigate the strong coulombic interaction between Mg2+ and host frameworks.
Current research objectives center on addressing several critical challenges in cathode development. Primary among these is enhancing structural stability during repeated Mg2+ insertion/extraction cycles. This requires fundamental understanding of degradation mechanisms at the atomic level and development of novel stabilization strategies.
Another key objective involves improving Mg2+ diffusion kinetics within cathode structures. The slow diffusion of magnesium ions represents a significant barrier to practical application, necessitating innovative approaches to channel design and interface engineering.
Additionally, researchers aim to increase operating voltage while maintaining structural integrity. This objective requires careful balancing of electronic structure modification without compromising stability. Computational screening and materials genome approaches are increasingly employed to accelerate this discovery process.
The ultimate research goal remains the development of cathode materials that can deliver high energy density (>500 Wh/kg), long cycle life (>1000 cycles), and fast charging capabilities comparable to current lithium-ion technologies. Achieving these targets requires interdisciplinary approaches combining advanced characterization techniques, computational modeling, and innovative synthetic strategies focused specifically on understanding and enhancing structural stability during electrochemical cycling.
Market Analysis for Next-Generation Battery Technologies
The global battery market is witnessing a significant shift towards next-generation technologies, with magnesium-ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1% from 2022. Within this expanding market, magnesium-ion battery technology is positioned to capture increasing market share due to its potential cost advantages and performance characteristics.
The demand for magnesium-ion batteries is primarily driven by several key factors. First, the inherent safety advantages of magnesium-based systems compared to lithium-ion batteries address critical concerns in consumer electronics, electric vehicles, and stationary storage applications. Second, the abundance of magnesium resources—approximately 23 million metric tons of reserves globally—presents a substantial economic advantage over lithium, which faces supply constraints and geopolitical complications.
Market segmentation analysis reveals that electric vehicles represent the most promising application sector for magnesium-ion batteries, with an estimated potential market value of $45 billion by 2030. This is followed by grid-scale energy storage systems at $28 billion and consumer electronics at $17 billion. The cathode structural stability improvements specifically could unlock an additional $12 billion in value across these segments by enabling higher energy densities and longer cycle life.
Regional market assessment indicates that Asia-Pacific currently dominates research and development investments in magnesium-ion technology, accounting for 58% of patents filed in this domain. North America follows at 27%, with Europe at 15%. China alone has increased its R&D spending on next-generation battery technologies by 22% annually since 2018, with significant focus on cathode materials research.
Consumer and industry surveys indicate growing awareness and interest in alternatives to lithium-ion technology, with 67% of automotive manufacturers expressing interest in diversifying their battery technology portfolio. Additionally, 72% of grid operators cite longer cycle life and enhanced safety as critical factors in their energy storage procurement decisions—both areas where stable magnesium-ion cathodes could provide competitive advantages.
The economic analysis of magnesium-ion battery production suggests that with optimized cathode structures, manufacturing costs could potentially be reduced by 30-40% compared to current lithium-ion technologies. This cost advantage, coupled with performance improvements, positions magnesium-ion batteries to potentially capture 15-20% of the next-generation battery market by 2035, contingent upon successful resolution of current technical challenges, particularly those related to cathode structural stability during charge-discharge cycles.
The demand for magnesium-ion batteries is primarily driven by several key factors. First, the inherent safety advantages of magnesium-based systems compared to lithium-ion batteries address critical concerns in consumer electronics, electric vehicles, and stationary storage applications. Second, the abundance of magnesium resources—approximately 23 million metric tons of reserves globally—presents a substantial economic advantage over lithium, which faces supply constraints and geopolitical complications.
Market segmentation analysis reveals that electric vehicles represent the most promising application sector for magnesium-ion batteries, with an estimated potential market value of $45 billion by 2030. This is followed by grid-scale energy storage systems at $28 billion and consumer electronics at $17 billion. The cathode structural stability improvements specifically could unlock an additional $12 billion in value across these segments by enabling higher energy densities and longer cycle life.
Regional market assessment indicates that Asia-Pacific currently dominates research and development investments in magnesium-ion technology, accounting for 58% of patents filed in this domain. North America follows at 27%, with Europe at 15%. China alone has increased its R&D spending on next-generation battery technologies by 22% annually since 2018, with significant focus on cathode materials research.
Consumer and industry surveys indicate growing awareness and interest in alternatives to lithium-ion technology, with 67% of automotive manufacturers expressing interest in diversifying their battery technology portfolio. Additionally, 72% of grid operators cite longer cycle life and enhanced safety as critical factors in their energy storage procurement decisions—both areas where stable magnesium-ion cathodes could provide competitive advantages.
The economic analysis of magnesium-ion battery production suggests that with optimized cathode structures, manufacturing costs could potentially be reduced by 30-40% compared to current lithium-ion technologies. This cost advantage, coupled with performance improvements, positions magnesium-ion batteries to potentially capture 15-20% of the next-generation battery market by 2035, contingent upon successful resolution of current technical challenges, particularly those related to cathode structural stability during charge-discharge cycles.
Current Challenges in Mg-ion Cathode Structural Stability
Despite significant advancements in magnesium-ion battery research, cathode materials continue to present formidable challenges for commercialization. The primary obstacle lies in the strong electrostatic interaction between Mg2+ ions and host lattices, which significantly impedes ion diffusion and insertion/extraction processes. This fundamental issue manifests as structural instability during cycling, severely limiting practical applications of Mg-ion batteries.
Chevrel phases (Mo6S8) and spinel structures, while demonstrating reasonable Mg2+ mobility, suffer from gradual structural degradation during extended cycling. X-ray diffraction analyses reveal that repeated Mg2+ insertion/extraction causes irreversible phase transformations and amorphization in many promising cathode materials, particularly in layered structures like V2O5 and MoS2.
The multivalent nature of magnesium ions creates another significant challenge. The divalent Mg2+ induces stronger coulombic interactions with the host framework compared to monovalent Li+ ions, resulting in substantial structural distortions during intercalation. These distortions frequently lead to irreversible structural changes, capacity fading, and eventual cathode failure. High-resolution transmission electron microscopy studies have documented lattice expansion/contraction exceeding 10% in several oxide-based cathodes, far beyond the structural tolerance limits.
Water co-intercalation presents another critical challenge for cathode stability. Many Mg-ion battery systems utilize water-containing electrolytes to enhance Mg2+ mobility. However, this approach introduces water molecules into cathode structures, causing hydration/dehydration cycles that accelerate structural degradation. Recent research using in-situ environmental TEM has demonstrated that even trace amounts of water can trigger dissolution-precipitation mechanisms that permanently alter cathode morphology and composition.
Transition metal dissolution represents a particularly insidious failure mechanism. During cycling, cathode transition metals (particularly Mn, V, and Co) can dissolve into the electrolyte, creating vacancies in the crystal structure that collapse over time. This phenomenon has been extensively documented in spinel structures and contributes significantly to capacity fade. Spectroscopic analyses indicate that dissolved species can migrate to the anode surface, further compromising electrochemical performance.
Interface stability between cathodes and electrolytes remains poorly understood. The high reduction potential of Mg2+ often leads to electrolyte decomposition at the cathode surface, forming resistive interphases that hinder ion transport. Unlike the beneficial SEI formation in lithium-ion systems, these interface layers in Mg-ion batteries typically increase impedance and accelerate capacity fade. Advanced surface characterization techniques have revealed complex degradation products containing magnesium fluorides, carbonates, and organic species that progressively compromise cathode structural integrity.
Chevrel phases (Mo6S8) and spinel structures, while demonstrating reasonable Mg2+ mobility, suffer from gradual structural degradation during extended cycling. X-ray diffraction analyses reveal that repeated Mg2+ insertion/extraction causes irreversible phase transformations and amorphization in many promising cathode materials, particularly in layered structures like V2O5 and MoS2.
The multivalent nature of magnesium ions creates another significant challenge. The divalent Mg2+ induces stronger coulombic interactions with the host framework compared to monovalent Li+ ions, resulting in substantial structural distortions during intercalation. These distortions frequently lead to irreversible structural changes, capacity fading, and eventual cathode failure. High-resolution transmission electron microscopy studies have documented lattice expansion/contraction exceeding 10% in several oxide-based cathodes, far beyond the structural tolerance limits.
Water co-intercalation presents another critical challenge for cathode stability. Many Mg-ion battery systems utilize water-containing electrolytes to enhance Mg2+ mobility. However, this approach introduces water molecules into cathode structures, causing hydration/dehydration cycles that accelerate structural degradation. Recent research using in-situ environmental TEM has demonstrated that even trace amounts of water can trigger dissolution-precipitation mechanisms that permanently alter cathode morphology and composition.
Transition metal dissolution represents a particularly insidious failure mechanism. During cycling, cathode transition metals (particularly Mn, V, and Co) can dissolve into the electrolyte, creating vacancies in the crystal structure that collapse over time. This phenomenon has been extensively documented in spinel structures and contributes significantly to capacity fade. Spectroscopic analyses indicate that dissolved species can migrate to the anode surface, further compromising electrochemical performance.
Interface stability between cathodes and electrolytes remains poorly understood. The high reduction potential of Mg2+ often leads to electrolyte decomposition at the cathode surface, forming resistive interphases that hinder ion transport. Unlike the beneficial SEI formation in lithium-ion systems, these interface layers in Mg-ion batteries typically increase impedance and accelerate capacity fade. Advanced surface characterization techniques have revealed complex degradation products containing magnesium fluorides, carbonates, and organic species that progressively compromise cathode structural integrity.
Contemporary Approaches to Cathode Structural Enhancement
01 Layered oxide cathode materials for structural stability
Layered oxide materials, particularly those with transition metal elements, provide excellent structural stability for magnesium-ion battery cathodes. These materials maintain their crystal structure during repeated magnesium ion insertion and extraction, preventing collapse during cycling. The layered structure offers channels for efficient magnesium ion diffusion, while specific elemental compositions can enhance the binding energy between the cathode material and magnesium ions, further improving structural integrity during operation.- Layered oxide cathode materials for structural stability: Layered oxide materials, particularly those with transition metal elements, provide excellent structural stability for magnesium-ion battery cathodes. These materials maintain their crystal structure during repeated magnesium ion insertion and extraction, preventing collapse even at high cycling rates. The layered structure offers channels for efficient magnesium ion diffusion while maintaining structural integrity, resulting in improved cycling performance and battery longevity.
- Spinel structure cathode materials for enhanced stability: Spinel-type cathode materials demonstrate superior structural stability in magnesium-ion batteries due to their three-dimensional framework. These materials, often based on manganese oxides, provide robust channels for magnesium ion migration while resisting structural deformation during charge-discharge cycles. The cubic spinel structure maintains stability even with the larger size of magnesium ions compared to lithium ions, offering excellent capacity retention and extended cycle life.
- Doping strategies to enhance cathode structural stability: Introducing dopant elements into magnesium-ion battery cathode materials significantly improves their structural stability. Strategic doping with elements such as aluminum, zinc, or other transition metals strengthens the crystal lattice, preventing structural collapse during magnesium insertion/extraction. These dopants can occupy specific lattice sites, reducing volume changes during cycling and mitigating phase transitions that would otherwise lead to capacity fading and structural degradation.
- Carbon-based composite cathodes for structural reinforcement: Carbon-based composite cathodes incorporate carbon materials such as graphene, carbon nanotubes, or amorphous carbon to enhance the structural stability of magnesium-ion battery cathodes. These carbon components create a conductive network that buffers volume changes during cycling while improving electron transport. The carbon matrix prevents agglomeration of active materials and maintains structural integrity even during deep discharge, resulting in improved cycling stability and rate capability.
- Surface coating and modification techniques: Surface coating and modification techniques significantly enhance the structural stability of magnesium-ion battery cathodes. Applying protective layers of metal oxides, fluorides, or phosphates on cathode particles creates a barrier against electrolyte attack while stabilizing the surface structure. These coatings prevent unwanted side reactions at the cathode-electrolyte interface, reduce dissolution of active materials, and maintain structural integrity during cycling, leading to improved capacity retention and extended battery life.
02 Spinel structure cathode materials for enhanced stability
Spinel-type structures provide three-dimensional ion diffusion pathways that maintain structural integrity during magnesium ion insertion and extraction. These materials typically contain transition metals in specific crystallographic arrangements that resist structural deformation during cycling. The robust framework of spinel structures accommodates the strong electrostatic interactions between magnesium ions and the host lattice, preventing cathode degradation and maintaining performance over extended cycling periods.Expand Specific Solutions03 Surface coating and modification techniques
Surface coating and modification techniques significantly enhance the structural stability of magnesium-ion battery cathodes. Applying protective layers of metal oxides, fluorides, or carbon-based materials prevents direct contact between the cathode material and electrolyte, reducing unwanted side reactions. These coatings also help maintain structural integrity by minimizing volume changes during cycling and preventing dissolution of active materials into the electrolyte, thereby extending battery life and improving cycling stability.Expand Specific Solutions04 Doping strategies for improved cathode stability
Incorporating dopant elements into the crystal structure of cathode materials enhances structural stability in magnesium-ion batteries. Strategic doping with elements such as aluminum, titanium, or zirconium strengthens the host lattice, preventing structural collapse during magnesium insertion and extraction. These dopants can occupy specific crystallographic sites, stabilizing the framework, reducing lattice strain, and minimizing phase transitions during cycling, which collectively improve the long-term structural integrity of the cathode materials.Expand Specific Solutions05 Nanostructured cathode materials for structural resilience
Nanostructured cathode materials offer enhanced structural stability for magnesium-ion batteries through reduced diffusion distances and improved strain accommodation. Nanoscale architectures such as nanoparticles, nanowires, and nanosheets provide shorter ion transport paths and larger surface areas for magnesium ion interaction. These structures can better accommodate volume changes during cycling and provide more stable interfaces with the electrolyte, resulting in improved structural integrity and longer cycle life compared to bulk materials.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The magnesium-ion battery cathode structural stability market is currently in an early growth phase, characterized by intensive R&D activities rather than widespread commercialization. The global market size remains relatively small but is projected to expand significantly as magnesium-ion technology offers a promising alternative to lithium-ion batteries due to magnesium's abundance and theoretical energy density advantages. Technical maturity remains low, with key players focusing on overcoming cathode stability challenges. Leading organizations include established automotive manufacturers (Toyota, GM), chemical companies (LG Chem, DuPont, Solvay), specialized battery developers (Nano One Materials, Jiangsu Zenergy), and research institutions (Chinese Academy of Sciences, Naval Research Laboratory). Academic-industrial partnerships are accelerating development, with universities like Chongqing University and Texas A&M collaborating with industry to address fundamental structural stability issues.
Toyota Motor Corp.
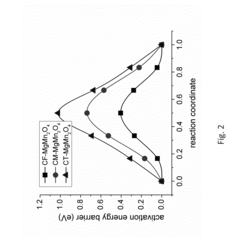
Technical Solution: Toyota Motor Corporation has developed a systematic approach to magnesium-ion battery cathode stability analysis as part of their broader alternative energy storage research program. Their proprietary "Multi-Scale Structural Evolution Analysis" methodology combines computational modeling with advanced characterization techniques to predict and validate structural changes during magnesium intercalation/deintercalation[2]. Toyota's research has focused particularly on vanadium-based cathode materials, including V2O5 and MgxV2O5 systems, where they've achieved significant improvements in structural stability through precise control of oxygen vacancy concentrations and crystal orientation[4]. Their materials engineering team has pioneered the development of composite cathode structures featuring mechanically reinforcing secondary phases that maintain structural integrity during the significant volume changes associated with Mg2+ cycling. Toyota has also developed proprietary in-situ characterization cells that enable simultaneous electrochemical testing and structural analysis using synchrotron X-ray diffraction, providing unprecedented insights into degradation mechanisms during actual battery operation[7].
Strengths: Toyota's integrated approach combining fundamental materials science with practical automotive battery requirements results in cathode materials optimized for real-world applications. Their extensive resources allow for comprehensive testing across multiple scales from laboratory to prototype cells. Weaknesses: Their focus on automotive applications sometimes results in cathode designs that prioritize safety and longevity over maximum energy density, potentially limiting applications in other sectors requiring higher energy storage capabilities.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has developed a comprehensive approach to magnesium-ion battery cathode stability analysis utilizing advanced computational modeling coupled with experimental validation. Their research focuses on chevrel phase Mo6S8 and spinel-type oxide structures that demonstrate promising stability during Mg2+ intercalation[2]. Battelle employs density functional theory (DFT) calculations to predict structural changes during cycling and identify potential degradation mechanisms at the atomic level. Their proprietary "Structure-Property Relationship Framework" enables rapid screening of candidate cathode materials by correlating electronic structure with magnesium diffusion kinetics and structural stability parameters[4]. The institute has pioneered the use of operando X-ray absorption spectroscopy and neutron diffraction techniques to monitor real-time structural evolution during charge-discharge cycles, providing crucial insights into degradation mechanisms. Battelle's recent breakthrough involves a novel surface modification strategy using atomic layer deposition to create protective coatings that significantly enhance structural integrity while maintaining efficient ion transport pathways[5].
Strengths: Battelle's integrated computational-experimental approach provides comprehensive understanding of degradation mechanisms, allowing for targeted material design. Their access to national laboratory facilities enables cutting-edge characterization techniques unavailable to many competitors. Weaknesses: Their focus on fundamental research sometimes results in materials that excel in stability but underperform in practical energy density metrics required for commercial applications.
Critical Patents and Research on Mg-ion Intercalation Mechanisms
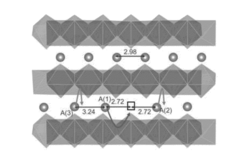
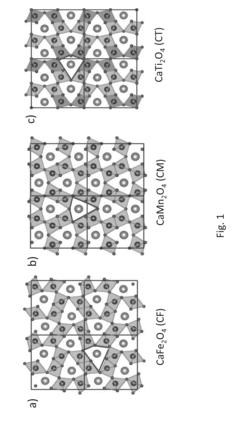
MgMn2O4 WITH A CRYSTAL STRUCTURE ANALOGUE TO CaFe2O4, CaMn2O4, OR CaTi2O4 AS RECHARGEABLE MAGNESIUM BATTERY CATHODE
PatentInactiveUS20140295280A1
Innovation
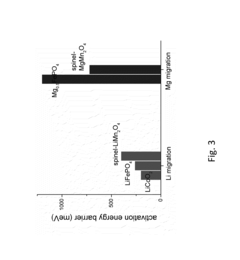
- A cathode active material with the formula MgxMn2O4, featuring a crystal structure analogous to CaFe2O4, CaMn2O4, or CaTi2O4, which creates an open channel for single-dimensional ionic diffusion, facilitating lower activation energy barriers and cooperative ion hopping, thereby enhancing magnesium ion mobility.
Novel cathode active material and magnesium secondary battery comprising the same
PatentInactiveKR1020120073757A
Innovation
- A novel magnesium transition metal chalcogenide compound with a specific crystal structure (MgxMo15-yMySe19-zAz) is developed, allowing for stable Mg ion desorption and insertion without structural change, using a tunnel structure for Mg ions and incorporating suitable substitutions to enhance bonding and stability.
Environmental Impact and Sustainability Assessment
The environmental impact of magnesium-ion battery cathode materials represents a critical consideration in the broader context of sustainable energy storage solutions. Unlike lithium-ion batteries, magnesium-ion systems potentially offer reduced environmental footprints due to the greater natural abundance of magnesium resources. Magnesium is the eighth most abundant element in Earth's crust, with reserves approximately 3000 times greater than lithium, significantly reducing extraction-related environmental concerns and resource depletion risks.
The cathode materials used in magnesium-ion batteries typically contain fewer toxic heavy metals compared to conventional lithium-ion counterparts. Many promising cathode candidates utilize manganese, vanadium, or molybdenum-based compounds, which generally present lower environmental toxicity profiles than cobalt or nickel-rich materials commonly found in lithium-ion batteries. This reduced toxicity translates to decreased environmental contamination risks during manufacturing, usage, and end-of-life disposal.
Life cycle assessment (LCA) studies indicate that the structural stability of magnesium-ion cathodes directly influences their environmental impact. Cathodes with superior structural integrity during repeated magnesium insertion/extraction cycles demonstrate extended operational lifespans, reducing replacement frequency and associated manufacturing emissions. However, current challenges in cathode structural stability often necessitate additional stabilizing compounds or complex synthesis methods, potentially offsetting some environmental benefits through increased processing requirements.
Water consumption represents another important environmental consideration in cathode production. Many magnesium cathode synthesis routes employ aqueous processes that, while generally less toxic than organic solvent-based methods, may require significant water inputs. Improving structural stability through hydrothermal or solvothermal processes must balance performance gains against water resource impacts, particularly in water-stressed regions.
From a circular economy perspective, the recyclability of magnesium-ion cathode materials remains underdeveloped compared to established lithium-ion recycling pathways. The structural transformations that occur during cycling can complicate material recovery, though the inherently lower economic value of constituent elements has limited recycling infrastructure development. Designing cathode structures with both operational stability and end-of-life recoverability represents an emerging research direction with significant sustainability implications.
Carbon footprint analyses suggest that manufacturing energy requirements for structurally stable magnesium cathodes currently exceed those of commercial lithium-ion cathodes, primarily due to less optimized production processes. However, the potential for longer cycle life could reduce lifetime emissions through decreased replacement frequency, highlighting the importance of balancing immediate manufacturing impacts against long-term usage benefits.
The cathode materials used in magnesium-ion batteries typically contain fewer toxic heavy metals compared to conventional lithium-ion counterparts. Many promising cathode candidates utilize manganese, vanadium, or molybdenum-based compounds, which generally present lower environmental toxicity profiles than cobalt or nickel-rich materials commonly found in lithium-ion batteries. This reduced toxicity translates to decreased environmental contamination risks during manufacturing, usage, and end-of-life disposal.
Life cycle assessment (LCA) studies indicate that the structural stability of magnesium-ion cathodes directly influences their environmental impact. Cathodes with superior structural integrity during repeated magnesium insertion/extraction cycles demonstrate extended operational lifespans, reducing replacement frequency and associated manufacturing emissions. However, current challenges in cathode structural stability often necessitate additional stabilizing compounds or complex synthesis methods, potentially offsetting some environmental benefits through increased processing requirements.
Water consumption represents another important environmental consideration in cathode production. Many magnesium cathode synthesis routes employ aqueous processes that, while generally less toxic than organic solvent-based methods, may require significant water inputs. Improving structural stability through hydrothermal or solvothermal processes must balance performance gains against water resource impacts, particularly in water-stressed regions.
From a circular economy perspective, the recyclability of magnesium-ion cathode materials remains underdeveloped compared to established lithium-ion recycling pathways. The structural transformations that occur during cycling can complicate material recovery, though the inherently lower economic value of constituent elements has limited recycling infrastructure development. Designing cathode structures with both operational stability and end-of-life recoverability represents an emerging research direction with significant sustainability implications.
Carbon footprint analyses suggest that manufacturing energy requirements for structurally stable magnesium cathodes currently exceed those of commercial lithium-ion cathodes, primarily due to less optimized production processes. However, the potential for longer cycle life could reduce lifetime emissions through decreased replacement frequency, highlighting the importance of balancing immediate manufacturing impacts against long-term usage benefits.
Safety Standards and Performance Benchmarking
The development of safety standards for magnesium-ion battery cathodes represents a critical aspect of their commercial viability. Currently, the safety protocols established for lithium-ion batteries serve as reference points, though magnesium-specific standards are still evolving. Organizations such as the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) are working to adapt existing frameworks to accommodate the unique characteristics of magnesium-ion systems, particularly focusing on thermal stability parameters and structural integrity under stress conditions.
Performance benchmarking for magnesium-ion battery cathodes primarily evaluates structural stability through accelerated aging tests, thermal cycling, and mechanical stress simulations. These assessments typically measure capacity retention after 1000+ cycles, structural integrity following thermal exposure between -20°C and 60°C, and resistance to mechanical deformation under various pressure conditions. The industry standard increasingly requires cathode materials to maintain at least 80% capacity retention after 1000 cycles and structural coherence after exposure to temperatures up to 150°C.
Recent comparative analyses between conventional lithium-ion cathodes and emerging magnesium-ion alternatives reveal significant differences in safety profiles. Magnesium-ion systems generally demonstrate superior thermal stability, with decomposition temperatures typically 50-100°C higher than their lithium counterparts. This enhanced thermal resilience translates to reduced fire and explosion risks, a critical advantage for large-scale energy storage applications.
Standardized testing protocols for cathode structural stability now incorporate advanced analytical techniques including in-situ X-ray diffraction during cycling, transmission electron microscopy for microstructural evolution monitoring, and differential scanning calorimetry for thermal behavior characterization. These methods provide quantitative metrics for comparing different cathode formulations and establishing minimum performance thresholds.
The benchmarking landscape is further complicated by the diversity of magnesium-ion cathode chemistries under development. Chevrel phases, spinel structures, and layered oxides each present distinct stability profiles and failure modes, necessitating tailored assessment methodologies. Industry consensus is gradually forming around a multi-parameter evaluation framework that considers not only cycle life and thermal stability but also rate capability, voltage stability, and structural resilience against magnesium plating and stripping processes.
Regulatory bodies in major markets including the European Union, United States, and China are currently developing certification requirements specifically for magnesium-based energy storage systems, with particular emphasis on structural stability under abuse conditions. These emerging standards will likely establish minimum performance criteria for commercial deployment, potentially accelerating the transition from research prototypes to market-ready products.
Performance benchmarking for magnesium-ion battery cathodes primarily evaluates structural stability through accelerated aging tests, thermal cycling, and mechanical stress simulations. These assessments typically measure capacity retention after 1000+ cycles, structural integrity following thermal exposure between -20°C and 60°C, and resistance to mechanical deformation under various pressure conditions. The industry standard increasingly requires cathode materials to maintain at least 80% capacity retention after 1000 cycles and structural coherence after exposure to temperatures up to 150°C.
Recent comparative analyses between conventional lithium-ion cathodes and emerging magnesium-ion alternatives reveal significant differences in safety profiles. Magnesium-ion systems generally demonstrate superior thermal stability, with decomposition temperatures typically 50-100°C higher than their lithium counterparts. This enhanced thermal resilience translates to reduced fire and explosion risks, a critical advantage for large-scale energy storage applications.
Standardized testing protocols for cathode structural stability now incorporate advanced analytical techniques including in-situ X-ray diffraction during cycling, transmission electron microscopy for microstructural evolution monitoring, and differential scanning calorimetry for thermal behavior characterization. These methods provide quantitative metrics for comparing different cathode formulations and establishing minimum performance thresholds.
The benchmarking landscape is further complicated by the diversity of magnesium-ion cathode chemistries under development. Chevrel phases, spinel structures, and layered oxides each present distinct stability profiles and failure modes, necessitating tailored assessment methodologies. Industry consensus is gradually forming around a multi-parameter evaluation framework that considers not only cycle life and thermal stability but also rate capability, voltage stability, and structural resilience against magnesium plating and stripping processes.
Regulatory bodies in major markets including the European Union, United States, and China are currently developing certification requirements specifically for magnesium-based energy storage systems, with particular emphasis on structural stability under abuse conditions. These emerging standards will likely establish minimum performance criteria for commercial deployment, potentially accelerating the transition from research prototypes to market-ready products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!