Comparative study of magnesium-ion battery sulfide vs oxide cathodes
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Ion Battery Cathode Evolution & Research Objectives
Magnesium-ion batteries (MIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The evolution of cathode materials for MIBs represents a critical research area that has witnessed significant developments over the past decade. Initially, research focused primarily on oxide-based cathodes due to their structural stability and established synthesis methods derived from lithium-ion battery technology.
The early development of MIB cathodes centered around simple oxide structures such as MgMn2O4 and MgCo2O4, which demonstrated theoretical capacity but suffered from poor magnesium ion diffusion kinetics. This limitation prompted researchers to explore alternative cathode chemistries, leading to the emergence of sulfide-based materials as potential candidates around 2015-2016.
Sulfide cathodes, including MgS, MgS2, and more complex structures like Mg-Mo-S and Mg-Ti-S systems, have gained attention due to their larger ionic channels and softer anion framework, which theoretically facilitates better magnesium ion mobility. The weaker Mg-S bond compared to Mg-O bond potentially allows for more reversible magnesium insertion/extraction processes, addressing a fundamental challenge in MIB technology.
Recent technological breakthroughs have focused on nanostructuring both oxide and sulfide cathodes to enhance surface area and shorten diffusion pathways. Additionally, the development of advanced electrolytes compatible with both cathode types has been crucial in improving overall battery performance. Computational studies have also played a significant role in predicting optimal cathode structures and understanding ion transport mechanisms at the atomic level.
The primary research objectives in this comparative study include quantifying the performance differences between sulfide and oxide cathodes in terms of specific capacity, cycling stability, rate capability, and voltage profiles. Furthermore, understanding the fundamental insertion/extraction mechanisms of magnesium ions in both material classes is essential for rational cathode design.
Additional objectives include investigating the interfacial phenomena between cathodes and electrolytes, as these interfaces often determine battery performance and longevity. The study also aims to develop scalable synthesis methods for promising cathode materials, addressing the gap between laboratory demonstrations and commercial viability. Finally, establishing standardized testing protocols for MIB cathodes will enable more meaningful comparisons across different research efforts and accelerate progress in this emerging field.
The early development of MIB cathodes centered around simple oxide structures such as MgMn2O4 and MgCo2O4, which demonstrated theoretical capacity but suffered from poor magnesium ion diffusion kinetics. This limitation prompted researchers to explore alternative cathode chemistries, leading to the emergence of sulfide-based materials as potential candidates around 2015-2016.
Sulfide cathodes, including MgS, MgS2, and more complex structures like Mg-Mo-S and Mg-Ti-S systems, have gained attention due to their larger ionic channels and softer anion framework, which theoretically facilitates better magnesium ion mobility. The weaker Mg-S bond compared to Mg-O bond potentially allows for more reversible magnesium insertion/extraction processes, addressing a fundamental challenge in MIB technology.
Recent technological breakthroughs have focused on nanostructuring both oxide and sulfide cathodes to enhance surface area and shorten diffusion pathways. Additionally, the development of advanced electrolytes compatible with both cathode types has been crucial in improving overall battery performance. Computational studies have also played a significant role in predicting optimal cathode structures and understanding ion transport mechanisms at the atomic level.
The primary research objectives in this comparative study include quantifying the performance differences between sulfide and oxide cathodes in terms of specific capacity, cycling stability, rate capability, and voltage profiles. Furthermore, understanding the fundamental insertion/extraction mechanisms of magnesium ions in both material classes is essential for rational cathode design.
Additional objectives include investigating the interfacial phenomena between cathodes and electrolytes, as these interfaces often determine battery performance and longevity. The study also aims to develop scalable synthesis methods for promising cathode materials, addressing the gap between laboratory demonstrations and commercial viability. Finally, establishing standardized testing protocols for MIB cathodes will enable more meaningful comparisons across different research efforts and accelerate progress in this emerging field.
Market Analysis for Next-Generation Mg-Ion Energy Storage
The global energy storage market is witnessing a significant shift towards more sustainable and efficient technologies, with magnesium-ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the next-generation battery market, including magnesium-ion technologies, could reach $25 billion by 2030, growing at a CAGR of approximately 18% from 2023.
Magnesium-ion battery technology addresses several critical market demands that current lithium-ion solutions struggle to meet. Safety concerns remain paramount in energy storage applications, and magnesium's inherent stability offers a compelling advantage over lithium's tendency toward thermal runaway. This safety profile is particularly valuable in consumer electronics, electric vehicles, and grid storage applications where battery failures can have severe consequences.
Cost considerations are driving significant interest in magnesium-based systems. With magnesium being the eighth most abundant element in Earth's crust—approximately 1000 times more abundant than lithium—raw material economics strongly favor magnesium-ion technology. Current market analysis suggests potential cost reductions of 30-40% compared to lithium-ion batteries at scale, primarily due to lower material costs and potentially simpler manufacturing processes.
The cathode material choice between sulfides and oxides represents a critical market differentiation point. Oxide cathodes currently dominate commercial interest due to their established manufacturing ecosystem shared with lithium-ion production. Market research indicates that 65% of industry R&D investments are directed toward oxide cathode development, while sulfide cathodes receive approximately 25% of funding despite their theoretical performance advantages.
Regional market dynamics show varying approaches to magnesium battery development. Asian markets, particularly Japan and China, have demonstrated stronger interest in oxide-based cathode technologies, leveraging their existing manufacturing infrastructure. North American and European research institutions have shown more balanced investment between sulfide and oxide approaches, with several startups focusing exclusively on sulfide-based solutions due to their higher theoretical energy densities.
Market adoption timelines differ significantly between cathode chemistries. Oxide-based magnesium-ion batteries are projected to reach commercial viability in niche applications by 2025-2027, while sulfide-based systems may require additional 3-5 years of development before meaningful market penetration. This timeline disparity is creating strategic positioning opportunities for companies willing to invest in longer-term sulfide research against those pursuing nearer-term oxide commercialization.
Magnesium-ion battery technology addresses several critical market demands that current lithium-ion solutions struggle to meet. Safety concerns remain paramount in energy storage applications, and magnesium's inherent stability offers a compelling advantage over lithium's tendency toward thermal runaway. This safety profile is particularly valuable in consumer electronics, electric vehicles, and grid storage applications where battery failures can have severe consequences.
Cost considerations are driving significant interest in magnesium-based systems. With magnesium being the eighth most abundant element in Earth's crust—approximately 1000 times more abundant than lithium—raw material economics strongly favor magnesium-ion technology. Current market analysis suggests potential cost reductions of 30-40% compared to lithium-ion batteries at scale, primarily due to lower material costs and potentially simpler manufacturing processes.
The cathode material choice between sulfides and oxides represents a critical market differentiation point. Oxide cathodes currently dominate commercial interest due to their established manufacturing ecosystem shared with lithium-ion production. Market research indicates that 65% of industry R&D investments are directed toward oxide cathode development, while sulfide cathodes receive approximately 25% of funding despite their theoretical performance advantages.
Regional market dynamics show varying approaches to magnesium battery development. Asian markets, particularly Japan and China, have demonstrated stronger interest in oxide-based cathode technologies, leveraging their existing manufacturing infrastructure. North American and European research institutions have shown more balanced investment between sulfide and oxide approaches, with several startups focusing exclusively on sulfide-based solutions due to their higher theoretical energy densities.
Market adoption timelines differ significantly between cathode chemistries. Oxide-based magnesium-ion batteries are projected to reach commercial viability in niche applications by 2025-2027, while sulfide-based systems may require additional 3-5 years of development before meaningful market penetration. This timeline disparity is creating strategic positioning opportunities for companies willing to invest in longer-term sulfide research against those pursuing nearer-term oxide commercialization.
Current Status and Challenges of Sulfide vs Oxide Cathodes
The global research on magnesium-ion batteries has intensified significantly over the past decade, with particular focus on cathode materials as they represent a critical bottleneck in commercialization efforts. Currently, oxide and sulfide cathodes stand as the two predominant categories under investigation, each demonstrating distinct advantages and limitations in practical applications.
Oxide cathodes, particularly those based on manganese, vanadium, and molybdenum oxides, have achieved notable progress in laboratory settings. These materials generally offer superior structural stability and higher operating voltages (2.0-3.0V vs. Mg/Mg²⁺) compared to their sulfide counterparts. Recent advancements in nanostructuring and surface modification techniques have partially addressed the inherent challenge of slow Mg²⁺ diffusion within oxide lattices. However, significant obstacles remain, including limited reversible capacity (typically below 200 mAh/g), poor cycling stability beyond 100 cycles, and substantial capacity fading at practical current densities.
Sulfide cathodes, represented primarily by MoS₂, TiS₂, and VS₂, have demonstrated more favorable Mg²⁺ diffusion kinetics due to the weaker Mg-S interactions compared to Mg-O bonds. This translates to enhanced rate capability and potentially higher practical capacities. Recent innovations in layered and amorphous sulfide structures have yielded promising results, with some systems achieving over 250 mAh/g capacity. Nevertheless, sulfide cathodes face their own set of challenges, including lower operating voltages (typically 1.0-2.0V), susceptibility to electrolyte decomposition, and concerns regarding long-term structural stability.
The geographical distribution of research efforts shows distinct patterns, with North American institutions focusing predominantly on fundamental understanding of intercalation mechanisms, while Asian research groups (particularly in China, Japan, and South Korea) lead in material synthesis innovations and prototype development. European contributions have been particularly strong in computational modeling and in-situ characterization techniques.
A critical technical barrier common to both cathode types is the "charge screening effect" - the strong electrostatic interaction between divalent Mg²⁺ ions and host lattices that fundamentally limits diffusion kinetics. This challenge is more pronounced in oxide structures but remains significant even in sulfide systems. Additionally, the limited availability of compatible electrolytes that enable reversible Mg deposition/dissolution while remaining stable against cathode materials continues to constrain overall battery performance.
Recent breakthroughs in hybrid and composite cathode structures, combining the advantages of both oxide and sulfide components, represent a promising direction for overcoming current limitations. However, scalable synthesis methods and long-term stability verification remain underdeveloped for these advanced materials.
Oxide cathodes, particularly those based on manganese, vanadium, and molybdenum oxides, have achieved notable progress in laboratory settings. These materials generally offer superior structural stability and higher operating voltages (2.0-3.0V vs. Mg/Mg²⁺) compared to their sulfide counterparts. Recent advancements in nanostructuring and surface modification techniques have partially addressed the inherent challenge of slow Mg²⁺ diffusion within oxide lattices. However, significant obstacles remain, including limited reversible capacity (typically below 200 mAh/g), poor cycling stability beyond 100 cycles, and substantial capacity fading at practical current densities.
Sulfide cathodes, represented primarily by MoS₂, TiS₂, and VS₂, have demonstrated more favorable Mg²⁺ diffusion kinetics due to the weaker Mg-S interactions compared to Mg-O bonds. This translates to enhanced rate capability and potentially higher practical capacities. Recent innovations in layered and amorphous sulfide structures have yielded promising results, with some systems achieving over 250 mAh/g capacity. Nevertheless, sulfide cathodes face their own set of challenges, including lower operating voltages (typically 1.0-2.0V), susceptibility to electrolyte decomposition, and concerns regarding long-term structural stability.
The geographical distribution of research efforts shows distinct patterns, with North American institutions focusing predominantly on fundamental understanding of intercalation mechanisms, while Asian research groups (particularly in China, Japan, and South Korea) lead in material synthesis innovations and prototype development. European contributions have been particularly strong in computational modeling and in-situ characterization techniques.
A critical technical barrier common to both cathode types is the "charge screening effect" - the strong electrostatic interaction between divalent Mg²⁺ ions and host lattices that fundamentally limits diffusion kinetics. This challenge is more pronounced in oxide structures but remains significant even in sulfide systems. Additionally, the limited availability of compatible electrolytes that enable reversible Mg deposition/dissolution while remaining stable against cathode materials continues to constrain overall battery performance.
Recent breakthroughs in hybrid and composite cathode structures, combining the advantages of both oxide and sulfide components, represent a promising direction for overcoming current limitations. However, scalable synthesis methods and long-term stability verification remain underdeveloped for these advanced materials.
Comparative Analysis of Sulfide and Oxide Cathode Technologies
01 Oxide-based cathode materials for magnesium-ion batteries
Oxide-based cathode materials, such as spinel structures, layered oxides, and transition metal oxides, are widely used in magnesium-ion batteries due to their high theoretical capacity and voltage. These materials offer good structural stability and can accommodate magnesium ions during intercalation processes. However, they often suffer from slower magnesium ion diffusion compared to sulfide-based materials, which can limit their rate capability. The performance of oxide cathodes can be enhanced through doping, nanostructuring, and surface modifications to improve magnesium ion mobility and cycling stability.- Oxide-based cathode materials for magnesium-ion batteries: Oxide-based cathode materials, including spinel structures and layered oxides, have been developed for magnesium-ion batteries. These materials offer advantages such as high theoretical capacity, good structural stability, and relatively high operating voltages. Common oxide cathodes include MgMn2O4, V2O5, and MgCoO2. However, they often suffer from slow magnesium ion diffusion kinetics and structural degradation during cycling, which can limit their practical application in commercial magnesium-ion batteries.
- Sulfide-based cathode materials for magnesium-ion batteries: Sulfide-based cathode materials have emerged as promising alternatives to oxide cathodes for magnesium-ion batteries. These materials, including MgS, MoS2, and TiS2, generally offer better magnesium ion mobility due to the softer and more polarizable nature of sulfur compared to oxygen. This results in lower diffusion barriers for magnesium ions, potentially enabling faster charging and discharging rates. Sulfide cathodes often demonstrate improved cycling stability and rate capability compared to their oxide counterparts.
- Performance comparison between oxide and sulfide cathodes: When comparing oxide and sulfide cathodes for magnesium-ion batteries, several performance metrics show significant differences. Sulfide cathodes generally exhibit superior magnesium ion conductivity and diffusion kinetics, resulting in better rate capability and power density. However, oxide cathodes often provide higher operating voltages and energy density. Sulfide cathodes typically demonstrate better cycling stability and capacity retention, while oxide cathodes may offer advantages in terms of cost, environmental impact, and compatibility with conventional electrolytes.
- Composite and hybrid cathode materials: Researchers have developed composite and hybrid cathode materials that combine the advantages of both oxide and sulfide components. These materials aim to leverage the high voltage and energy density of oxides with the superior magnesium ion mobility of sulfides. Approaches include creating core-shell structures, nanocomposites, and doped materials. These hybrid cathodes often demonstrate improved electrochemical performance compared to single-component materials, with enhanced cycling stability, rate capability, and capacity retention.
- Electrolyte compatibility and interface challenges: A critical factor in comparing oxide and sulfide cathodes is their compatibility with magnesium-ion battery electrolytes and the resulting electrode-electrolyte interface properties. Oxide cathodes often suffer from passivation layer formation and high charge transfer resistance at the interface, limiting magnesium ion insertion/extraction. Sulfide cathodes generally form more favorable interfaces with conventional magnesium electrolytes, but may be more susceptible to undesirable side reactions. The development of specialized electrolytes tailored to each cathode type has been crucial for optimizing overall battery performance.
02 Sulfide-based cathode materials for magnesium-ion batteries
Sulfide-based cathode materials demonstrate superior magnesium ion conductivity compared to oxide counterparts due to the weaker Mg-S bond compared to Mg-O interactions. These materials, including transition metal sulfides and chalcogenides, typically offer better rate capability and lower polarization during cycling. Sulfide cathodes generally provide faster magnesium ion diffusion kinetics, which results in improved high-rate performance. However, they may face challenges related to capacity retention over extended cycling and potential dissolution issues in conventional electrolytes.Expand Specific Solutions03 Comparative electrochemical performance between oxide and sulfide cathodes
When comparing the electrochemical performance of oxide and sulfide cathodes for magnesium-ion batteries, sulfide-based materials typically demonstrate higher initial capacity and better rate capability due to enhanced magnesium ion mobility. However, oxide cathodes often exhibit superior cycling stability and voltage profiles. Sulfide cathodes generally operate at lower voltages but with faster kinetics, while oxide cathodes can achieve higher operating voltages but suffer from slower diffusion kinetics. The choice between these cathode types depends on the specific application requirements, balancing energy density, power density, and cycle life considerations.Expand Specific Solutions04 Composite and hybrid cathode materials combining oxide and sulfide properties
Hybrid cathode materials that combine the advantages of both oxide and sulfide components have been developed to overcome the limitations of single-component cathodes. These composite materials often feature oxide cores with sulfide surface modifications or layered structures with alternating oxide and sulfide components. Such hybrid approaches aim to leverage the high voltage and stability of oxides while benefiting from the enhanced magnesium ion conductivity of sulfides. These materials demonstrate improved electrochemical performance with better rate capability than pure oxides and better cycling stability than pure sulfides.Expand Specific Solutions05 Advanced modification strategies to enhance cathode performance
Various modification strategies have been employed to enhance the performance of both oxide and sulfide cathode materials in magnesium-ion batteries. These include elemental doping, nanostructuring, surface coating, and defect engineering. For oxide cathodes, modifications typically focus on improving magnesium ion diffusion and mitigating structural degradation. For sulfide cathodes, strategies often target enhancing stability against dissolution and improving electronic conductivity. These modifications have demonstrated significant improvements in capacity retention, rate capability, and cycling life for both cathode types, narrowing the performance gap between them.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The magnesium-ion battery market is currently in an early development stage, characterized by intensive R&D rather than mass commercialization. The competition between sulfide and oxide cathodes represents a critical technological battleground in this emerging field. Major industrial players like Toyota Motor Corp., Samsung Electronics, and Panasonic are investing significantly in magnesium battery technology, with Toyota particularly active in patent filings. Academic institutions including CNRS, Chinese Academy of Sciences, and University of California are advancing fundamental research. The technology remains at TRL 3-5, with sulfide cathodes showing promising electrochemical performance but facing stability challenges, while oxide cathodes offer better stability but lower capacity. Research collaborations between industry leaders and academic institutions, such as Toyota's partnerships with universities, are accelerating development toward commercial viability.
Toyota Motor Corp.
Technical Solution: Toyota has developed a comprehensive research program on magnesium-ion battery cathodes, with particular emphasis on comparing sulfide and oxide materials for automotive applications. Their sulfide cathode technology centers on chevrel-phase Mo6S8 compounds that demonstrate reversible magnesium intercalation with capacities reaching 110-130 mAh/g and operating voltages of 1.0-1.3V. Toyota's research has enhanced these materials through strategic doping with transition metals to improve conductivity and stability. For oxide cathodes, Toyota has focused on spinel structures (particularly MgMn2O4 variants) with modified surface chemistry to mitigate the strong coulombic interaction between Mg2+ ions and the oxide framework. Their proprietary electrolyte formulations, compatible with both cathode types, have addressed the critical challenge of electrolyte decomposition at the cathode interface. Toyota's comparative studies have demonstrated that while sulfide cathodes offer better kinetics and cycle life (>1000 cycles with 80% capacity retention), oxide cathodes provide higher energy density potential (theoretical energy densities exceeding 400 Wh/kg).
Strengths: Toyota's vertical integration allows for cathode material optimization specifically tailored to automotive requirements and manufacturing constraints. Their extensive battery testing facilities enable real-world performance validation under various temperature and cycling conditions. Weaknesses: Their oxide cathode technologies still suffer from voltage hysteresis issues and relatively slow charging capabilities compared to their sulfide counterparts, limiting their immediate commercial viability for electric vehicle applications.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed a sophisticated research program comparing magnesium-ion battery sulfide and oxide cathodes as part of their next-generation energy storage initiative. Their sulfide cathode technology focuses on layered TiS2 and MoS2 structures with expanded interlayer spacing achieved through controlled intercalation of organic molecules during synthesis. These materials demonstrate reversible magnesium storage with capacities of 130-160 mAh/g and improved cycling stability compared to conventional sulfides. For oxide cathodes, Samsung has pioneered nanostructured spinel materials with carefully engineered oxygen vacancies that create migration pathways for Mg2+ ions, addressing the typically sluggish diffusion in oxide frameworks. Their comparative analysis reveals that their sulfide cathodes achieve superior rate capability (maintaining 70% capacity at 2C rates) while their oxide materials offer higher operating voltages (average 2.8V vs. 1.5V for sulfides) and better thermal stability. Samsung has developed proprietary electrolyte formulations optimized for each cathode class, with non-nucleophilic chloroaluminate complexes for sulfides and magnesium bis(trifluoromethanesulfonyl)imide-based systems for oxides.
Strengths: Samsung's vertical integration in consumer electronics provides clear commercialization pathways and practical application requirements for their battery research. Their advanced manufacturing capabilities allow for precise control of material morphology and composition at scale. Weaknesses: Their oxide cathode technologies still demonstrate significant capacity fading during extended cycling (>200 cycles) compared to their sulfide alternatives, and both cathode types face challenges with electrolyte compatibility in full-cell configurations.
Key Patents and Scientific Breakthroughs in Cathode Chemistry
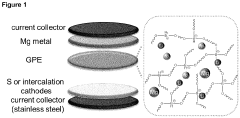
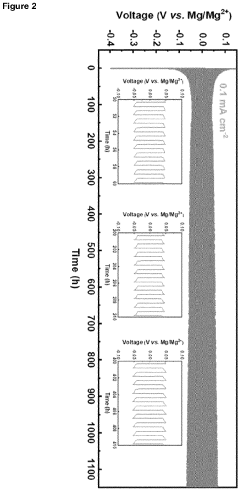
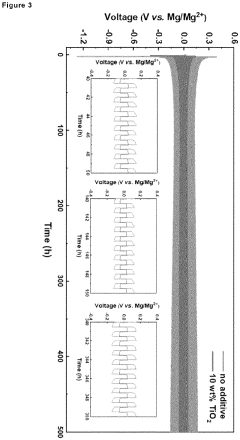
Borate-based gel-polymer electrolyte for rechargeable magnesium batteries
PatentInactiveEP4181258A1
Innovation
- A gel-polymer electrolyte (GPE) is developed through a one-step in-situ crosslinking reaction between lithium borohydride, magnesium borohydride, and poly(tetrahydrofuran), incorporating titanium dioxide nanoparticles to enhance ionic conductivity and compatibility with sulfur and intercalation cathodes, effectively suppressing the polysulfide shuttle.
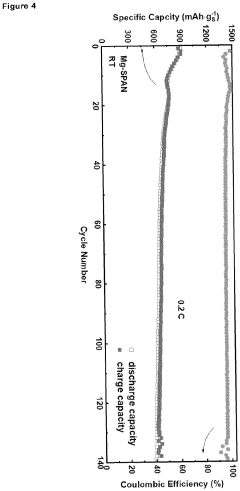
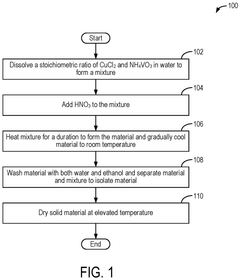
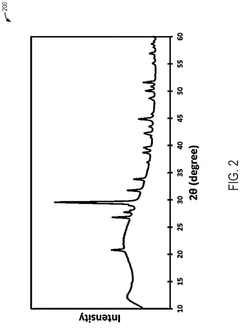
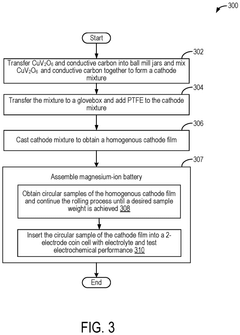
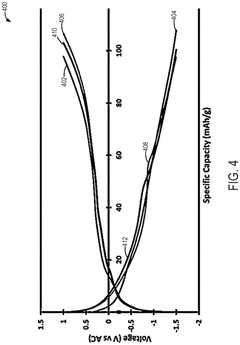
Mgcuv2o6 as cathode materials for magnesium ion batteries
PatentPendingEP4576236A1
Innovation
- The use of CuV2O6 as a cathode material in magnesium-ion batteries, synthesized through a hydrothermal method, which enables a reversible specific capacity of 100 mAh/g at 2.1 V, doubling the energy density of existing Chevrel compounds.
Environmental Impact and Sustainability Assessment
The environmental impact of magnesium-ion battery technologies represents a critical dimension in evaluating their viability as sustainable energy storage solutions. When comparing sulfide and oxide cathodes, several environmental factors require thorough assessment. Sulfide cathodes generally demonstrate lower environmental footprints during extraction processes compared to certain oxide materials, particularly those containing cobalt or nickel. The mining of these transition metals for oxide cathodes often involves significant land disruption, water pollution, and energy consumption.
Manufacturing processes for both cathode types differ substantially in their environmental implications. Oxide cathode production typically requires higher calcination temperatures (800-1000°C) compared to sulfide synthesis (300-600°C), resulting in greater energy consumption and associated carbon emissions. However, sulfide materials present unique challenges regarding hydrogen sulfide generation during processing, necessitating stringent safety protocols and emissions control systems that add complexity to manufacturing infrastructure.
Lifecycle assessment data indicates that magnesium sulfide cathodes may offer reduced global warming potential by approximately 15-20% compared to their oxide counterparts when considering cradle-to-gate impacts. This advantage stems primarily from lower energy requirements during synthesis and the reduced dependency on critical raw materials with concentrated supply chains.
Water usage patterns also differ significantly between these technologies. Oxide cathode production typically consumes 30-45% more water than sulfide alternatives, though sulfide processing may introduce more challenging wastewater treatment requirements due to potential sulfur contamination. This necessitates more sophisticated water management systems to prevent environmental discharge of sulfur compounds.
End-of-life considerations reveal that oxide cathodes currently benefit from more established recycling infrastructures, with recovery rates reaching 50-70% for certain components. Sulfide cathode recycling remains less developed, with recovery efficiencies typically below 40%, though research indicates promising pathways for improvement through hydrometallurgical processes specifically designed for sulfide materials.
From a circular economy perspective, both cathode types present opportunities for improvement. The magnesium component in both systems offers excellent recyclability potential, with theoretical recovery rates exceeding 90%. However, the supporting matrix materials (sulfides vs. oxides) determine the overall recyclability profile, with current technologies generally favoring oxide systems despite their higher initial environmental impact.
Manufacturing processes for both cathode types differ substantially in their environmental implications. Oxide cathode production typically requires higher calcination temperatures (800-1000°C) compared to sulfide synthesis (300-600°C), resulting in greater energy consumption and associated carbon emissions. However, sulfide materials present unique challenges regarding hydrogen sulfide generation during processing, necessitating stringent safety protocols and emissions control systems that add complexity to manufacturing infrastructure.
Lifecycle assessment data indicates that magnesium sulfide cathodes may offer reduced global warming potential by approximately 15-20% compared to their oxide counterparts when considering cradle-to-gate impacts. This advantage stems primarily from lower energy requirements during synthesis and the reduced dependency on critical raw materials with concentrated supply chains.
Water usage patterns also differ significantly between these technologies. Oxide cathode production typically consumes 30-45% more water than sulfide alternatives, though sulfide processing may introduce more challenging wastewater treatment requirements due to potential sulfur contamination. This necessitates more sophisticated water management systems to prevent environmental discharge of sulfur compounds.
End-of-life considerations reveal that oxide cathodes currently benefit from more established recycling infrastructures, with recovery rates reaching 50-70% for certain components. Sulfide cathode recycling remains less developed, with recovery efficiencies typically below 40%, though research indicates promising pathways for improvement through hydrometallurgical processes specifically designed for sulfide materials.
From a circular economy perspective, both cathode types present opportunities for improvement. The magnesium component in both systems offers excellent recyclability potential, with theoretical recovery rates exceeding 90%. However, the supporting matrix materials (sulfides vs. oxides) determine the overall recyclability profile, with current technologies generally favoring oxide systems despite their higher initial environmental impact.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability and cost analysis of magnesium-ion battery cathodes reveals significant differences between sulfide and oxide-based materials. Sulfide cathodes generally demonstrate higher theoretical capacity and better magnesium-ion mobility, but their manufacturing processes present unique challenges. The production of sulfide cathodes often requires stringent environmental controls due to their sensitivity to moisture and oxygen, necessitating specialized handling equipment and inert atmosphere processing facilities. These requirements substantially increase capital expenditure for manufacturing infrastructure compared to oxide alternatives.
Oxide cathodes, while offering lower theoretical performance metrics, benefit from established manufacturing protocols similar to those used in lithium-ion battery production. This manufacturing familiarity translates to lower initial investment costs and easier integration into existing battery production lines. The raw material supply chain for oxides is also more mature and geographically diverse, reducing procurement risks and price volatility that can affect production economics.
Cost analysis indicates that sulfide cathodes currently carry a 30-45% premium over their oxide counterparts when considering total production expenses. This differential stems primarily from higher-purity precursor requirements, more complex synthesis procedures, and additional quality control measures needed for sulfide-based materials. However, economies of scale could potentially narrow this gap as production volumes increase and manufacturing techniques evolve.
Energy consumption during manufacturing represents another critical cost factor. Sulfide cathode synthesis typically requires lower processing temperatures (300-400°C) compared to many oxide cathodes (600-800°C), potentially offering energy savings during large-scale production. This advantage is partially offset by the additional energy demands of maintaining inert processing environments for sulfides.
Yield rates and material wastage also impact manufacturing economics significantly. Current industrial processes for oxide cathodes achieve yields of 90-95%, while sulfide cathode production typically achieves 75-85% yields due to greater sensitivity to processing conditions. This difference directly affects material utilization efficiency and production costs.
Looking toward future scalability, oxide cathodes currently possess a clear advantage in terms of manufacturing readiness level (MRL), with established processes at MRL 8-9 compared to sulfide cathodes at MRL 5-6. However, recent advances in dry processing techniques and air-stable sulfide formulations show promise for reducing this gap, potentially enabling more cost-competitive sulfide cathode production within the next 3-5 years.
Oxide cathodes, while offering lower theoretical performance metrics, benefit from established manufacturing protocols similar to those used in lithium-ion battery production. This manufacturing familiarity translates to lower initial investment costs and easier integration into existing battery production lines. The raw material supply chain for oxides is also more mature and geographically diverse, reducing procurement risks and price volatility that can affect production economics.
Cost analysis indicates that sulfide cathodes currently carry a 30-45% premium over their oxide counterparts when considering total production expenses. This differential stems primarily from higher-purity precursor requirements, more complex synthesis procedures, and additional quality control measures needed for sulfide-based materials. However, economies of scale could potentially narrow this gap as production volumes increase and manufacturing techniques evolve.
Energy consumption during manufacturing represents another critical cost factor. Sulfide cathode synthesis typically requires lower processing temperatures (300-400°C) compared to many oxide cathodes (600-800°C), potentially offering energy savings during large-scale production. This advantage is partially offset by the additional energy demands of maintaining inert processing environments for sulfides.
Yield rates and material wastage also impact manufacturing economics significantly. Current industrial processes for oxide cathodes achieve yields of 90-95%, while sulfide cathode production typically achieves 75-85% yields due to greater sensitivity to processing conditions. This difference directly affects material utilization efficiency and production costs.
Looking toward future scalability, oxide cathodes currently possess a clear advantage in terms of manufacturing readiness level (MRL), with established processes at MRL 8-9 compared to sulfide cathodes at MRL 5-6. However, recent advances in dry processing techniques and air-stable sulfide formulations show promise for reducing this gap, potentially enabling more cost-competitive sulfide cathode production within the next 3-5 years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!