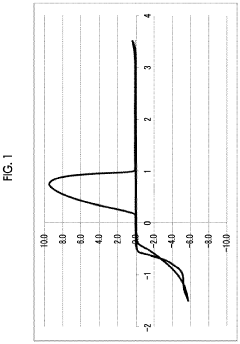
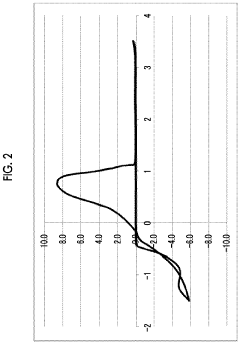
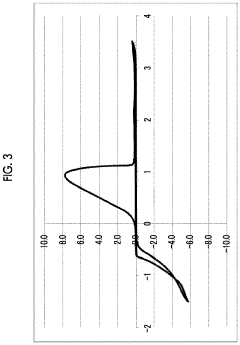
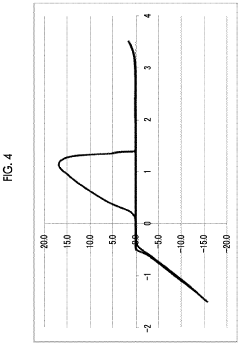
Magnesium-ion battery electrode passivation and surface film analysis
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Development Background and Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of these batteries traces back to the early 1990s, when researchers began exploring multivalent ion systems as energy storage solutions. The fundamental attraction of magnesium lies in its abundance in the Earth's crust (approximately 2.7%), making it significantly more available than lithium (0.0065%), which translates to lower raw material costs and reduced geopolitical supply risks.
The evolution of Mg-ion battery technology has been marked by several key milestones. In 2000, pioneering work by Aurbach et al. demonstrated the first rechargeable magnesium battery using organohaloaluminate electrolytes, establishing the foundational principles for this technology. Since then, research has intensified globally, with particular acceleration observed after 2010 when energy storage demands began increasing exponentially due to electric vehicle adoption and renewable energy integration.
Current technical objectives in Mg-ion battery development focus primarily on addressing the critical challenge of electrode passivation. Unlike lithium-ion systems, magnesium ions form strong chemical bonds with electrode surfaces and electrolyte components, creating resistive surface films that impede ion transport. This passivation layer significantly reduces battery performance and cycle life, representing one of the most substantial barriers to commercialization.
The analysis of these surface films has become a central research priority, with objectives including characterization of film composition, formation mechanisms, and strategies for mitigation. Researchers aim to develop electrode materials and electrolyte systems that either prevent detrimental film formation or promote the growth of ion-conductive surface layers that facilitate rather than hinder magnesium ion transport.
Industry projections suggest that solving the passivation challenge could enable Mg-ion batteries with theoretical energy densities exceeding 400 Wh/kg (compared to current Li-ion technologies at 250-300 Wh/kg). Additionally, the inherent safety advantages of magnesium systems—including non-dendritic plating and lower reactivity—position this technology as particularly valuable for large-scale applications where safety concerns are paramount.
The technological trajectory indicates that understanding and controlling the electrode-electrolyte interface phenomena will be the determining factor in whether Mg-ion batteries can transition from laboratory curiosities to commercial products. Current research is increasingly employing advanced characterization techniques such as in-situ XPS, TEM, and synchrotron-based methods to gain unprecedented insights into the surface film properties and dynamics during battery operation.
The evolution of Mg-ion battery technology has been marked by several key milestones. In 2000, pioneering work by Aurbach et al. demonstrated the first rechargeable magnesium battery using organohaloaluminate electrolytes, establishing the foundational principles for this technology. Since then, research has intensified globally, with particular acceleration observed after 2010 when energy storage demands began increasing exponentially due to electric vehicle adoption and renewable energy integration.
Current technical objectives in Mg-ion battery development focus primarily on addressing the critical challenge of electrode passivation. Unlike lithium-ion systems, magnesium ions form strong chemical bonds with electrode surfaces and electrolyte components, creating resistive surface films that impede ion transport. This passivation layer significantly reduces battery performance and cycle life, representing one of the most substantial barriers to commercialization.
The analysis of these surface films has become a central research priority, with objectives including characterization of film composition, formation mechanisms, and strategies for mitigation. Researchers aim to develop electrode materials and electrolyte systems that either prevent detrimental film formation or promote the growth of ion-conductive surface layers that facilitate rather than hinder magnesium ion transport.
Industry projections suggest that solving the passivation challenge could enable Mg-ion batteries with theoretical energy densities exceeding 400 Wh/kg (compared to current Li-ion technologies at 250-300 Wh/kg). Additionally, the inherent safety advantages of magnesium systems—including non-dendritic plating and lower reactivity—position this technology as particularly valuable for large-scale applications where safety concerns are paramount.
The technological trajectory indicates that understanding and controlling the electrode-electrolyte interface phenomena will be the determining factor in whether Mg-ion batteries can transition from laboratory curiosities to commercial products. Current research is increasingly employing advanced characterization techniques such as in-situ XPS, TEM, and synchrotron-based methods to gain unprecedented insights into the surface film properties and dynamics during battery operation.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with magnesium-ion batteries emerging as a promising alternative to conventional lithium-ion systems. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, growing at a compound annual growth rate of 14.1%. Within this expanding landscape, magnesium-ion battery technology is positioned to capture an increasing market share due to its compelling value proposition.
The demand for magnesium-ion batteries is primarily driven by several key market factors. First, the inherent safety advantages of magnesium-based systems address critical concerns in consumer electronics, electric vehicles, and stationary storage applications. Unlike lithium-ion batteries, magnesium-ion systems demonstrate significantly reduced risk of thermal runaway and fire hazards, a feature increasingly valued by manufacturers and end-users alike.
Resource availability represents another substantial market driver. Magnesium is approximately 1000 times more abundant in the earth's crust than lithium, offering a more sustainable and potentially cost-effective supply chain. This abundance translates to reduced geopolitical supply risks, with magnesium resources more evenly distributed globally compared to lithium's concentration in specific regions.
Market segmentation analysis reveals particularly strong potential in electric vehicle applications, where the theoretical energy density advantages of magnesium-ion technology could eventually enable longer driving ranges. The stationary storage sector also presents significant opportunities, especially in grid-scale applications where safety and longevity outweigh energy density considerations.
However, market penetration faces substantial barriers related to electrode passivation issues. The formation of non-conductive surface films on magnesium electrodes remains a critical technical challenge that directly impacts commercial viability. Industry surveys indicate that battery manufacturers require electrode cycle life improvements of at least 300% from current laboratory demonstrations before considering mass production.
Competitive landscape analysis shows increasing investment in magnesium-ion technology, with major battery manufacturers allocating approximately 8% of their R&D budgets to alternative metal-ion systems, including magnesium. Several specialized startups focused exclusively on magnesium battery technology have secured venture funding exceeding $150 million collectively since 2020.
Market timing projections suggest that with current research trajectories on electrode passivation and surface film engineering, commercially viable magnesium-ion batteries could reach early market applications by 2026-2028, initially in specialized sectors where their unique advantages outweigh current limitations in energy density and cycle life.
The demand for magnesium-ion batteries is primarily driven by several key market factors. First, the inherent safety advantages of magnesium-based systems address critical concerns in consumer electronics, electric vehicles, and stationary storage applications. Unlike lithium-ion batteries, magnesium-ion systems demonstrate significantly reduced risk of thermal runaway and fire hazards, a feature increasingly valued by manufacturers and end-users alike.
Resource availability represents another substantial market driver. Magnesium is approximately 1000 times more abundant in the earth's crust than lithium, offering a more sustainable and potentially cost-effective supply chain. This abundance translates to reduced geopolitical supply risks, with magnesium resources more evenly distributed globally compared to lithium's concentration in specific regions.
Market segmentation analysis reveals particularly strong potential in electric vehicle applications, where the theoretical energy density advantages of magnesium-ion technology could eventually enable longer driving ranges. The stationary storage sector also presents significant opportunities, especially in grid-scale applications where safety and longevity outweigh energy density considerations.
However, market penetration faces substantial barriers related to electrode passivation issues. The formation of non-conductive surface films on magnesium electrodes remains a critical technical challenge that directly impacts commercial viability. Industry surveys indicate that battery manufacturers require electrode cycle life improvements of at least 300% from current laboratory demonstrations before considering mass production.
Competitive landscape analysis shows increasing investment in magnesium-ion technology, with major battery manufacturers allocating approximately 8% of their R&D budgets to alternative metal-ion systems, including magnesium. Several specialized startups focused exclusively on magnesium battery technology have secured venture funding exceeding $150 million collectively since 2020.
Market timing projections suggest that with current research trajectories on electrode passivation and surface film engineering, commercially viable magnesium-ion batteries could reach early market applications by 2026-2028, initially in specialized sectors where their unique advantages outweigh current limitations in energy density and cycle life.
Current Challenges in Mg-ion Battery Electrode Passivation
Despite significant advancements in magnesium-ion battery technology, electrode passivation remains one of the most critical challenges hindering commercial viability. The formation of surface films on magnesium electrodes fundamentally differs from lithium systems, presenting unique obstacles. Unlike the beneficial SEI (Solid Electrolyte Interphase) layer in lithium-ion batteries that allows Li+ transport while preventing further electrolyte decomposition, surface films on magnesium electrodes are typically non-conductive to Mg2+ ions, effectively blocking electrochemical reactions.
The primary challenge stems from the divalent nature of magnesium ions, which create stronger electrostatic interactions with the host lattice and surrounding chemical species. This results in sluggish diffusion kinetics and high desolvation energy barriers at electrode interfaces. Conventional electrolytes based on Grignard reagents or magnesium organohaloaluminates form passivation layers that are impermeable to Mg2+ ions, causing rapid capacity fading and poor cycling performance.
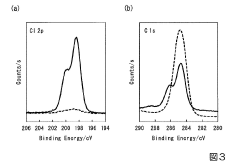
Surface film characterization presents another significant hurdle. The complex and often unstable nature of these films makes them difficult to analyze using standard techniques. X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) analyses reveal that these films typically consist of MgO, MgCO3, and various organic decomposition products, but their exact formation mechanisms remain poorly understood.
Electrolyte decomposition at operating potentials contributes substantially to passivation issues. Most electrolytes demonstrate narrow electrochemical stability windows, leading to continuous decomposition during cycling. This ongoing decomposition progressively thickens the passivation layer, increasing internal resistance and degrading battery performance over time.
The compatibility between cathode materials and electrolytes presents another critical challenge. High-voltage cathode materials often catalyze electrolyte decomposition, forming resistive surface films that impede Mg2+ insertion/extraction. This cathode-side passivation is particularly problematic as it directly impacts energy density and rate capability.
Environmental factors such as moisture and oxygen contamination significantly exacerbate passivation issues. Even trace amounts of water can trigger side reactions leading to Mg(OH)2 formation, which is highly resistive to Mg2+ transport. This extreme sensitivity necessitates stringent manufacturing conditions, adding complexity and cost to production processes.
Current research approaches focus on developing novel electrolyte formulations with additives that form Mg2+-conductive interfaces, but progress remains limited by insufficient fundamental understanding of interfacial chemistry in magnesium systems. The lack of in-situ and operando characterization techniques specifically optimized for magnesium electrochemistry further complicates efforts to develop effective passivation mitigation strategies.
The primary challenge stems from the divalent nature of magnesium ions, which create stronger electrostatic interactions with the host lattice and surrounding chemical species. This results in sluggish diffusion kinetics and high desolvation energy barriers at electrode interfaces. Conventional electrolytes based on Grignard reagents or magnesium organohaloaluminates form passivation layers that are impermeable to Mg2+ ions, causing rapid capacity fading and poor cycling performance.
Surface film characterization presents another significant hurdle. The complex and often unstable nature of these films makes them difficult to analyze using standard techniques. X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) analyses reveal that these films typically consist of MgO, MgCO3, and various organic decomposition products, but their exact formation mechanisms remain poorly understood.
Electrolyte decomposition at operating potentials contributes substantially to passivation issues. Most electrolytes demonstrate narrow electrochemical stability windows, leading to continuous decomposition during cycling. This ongoing decomposition progressively thickens the passivation layer, increasing internal resistance and degrading battery performance over time.
The compatibility between cathode materials and electrolytes presents another critical challenge. High-voltage cathode materials often catalyze electrolyte decomposition, forming resistive surface films that impede Mg2+ insertion/extraction. This cathode-side passivation is particularly problematic as it directly impacts energy density and rate capability.
Environmental factors such as moisture and oxygen contamination significantly exacerbate passivation issues. Even trace amounts of water can trigger side reactions leading to Mg(OH)2 formation, which is highly resistive to Mg2+ transport. This extreme sensitivity necessitates stringent manufacturing conditions, adding complexity and cost to production processes.
Current research approaches focus on developing novel electrolyte formulations with additives that form Mg2+-conductive interfaces, but progress remains limited by insufficient fundamental understanding of interfacial chemistry in magnesium systems. The lack of in-situ and operando characterization techniques specifically optimized for magnesium electrochemistry further complicates efforts to develop effective passivation mitigation strategies.
Current Analytical Methods for Surface Film Characterization
01 Surface film formation and passivation mechanisms in Mg-ion batteries
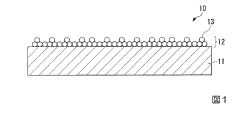
Surface films formed on magnesium-ion battery electrodes play a crucial role in battery performance. These films can either passivate the electrode surface, hindering ion transport, or form beneficial solid electrolyte interphases (SEI) that protect the electrode while allowing Mg-ion diffusion. The composition and properties of these films depend on electrolyte components, electrode materials, and operating conditions. Understanding these mechanisms is essential for developing high-performance magnesium-ion batteries with improved cycling stability.- Surface film formation and passivation mechanisms in Mg-ion batteries: Surface films formed on magnesium-ion battery electrodes play a crucial role in battery performance. These films can either passivate the electrode surface, hindering ion transport, or form beneficial solid electrolyte interphases (SEI) that protect the electrode while allowing Mg-ion diffusion. Understanding the formation mechanisms and composition of these films is essential for developing high-performance magnesium-ion batteries with improved cycling stability and efficiency.
- Electrode materials with enhanced surface stability for Mg-ion batteries: Specific electrode materials can be engineered to minimize detrimental surface film formation in magnesium-ion batteries. These materials include modified cathode structures, nanostructured electrodes, and composite materials that resist passivation while maintaining high electrochemical activity. By selecting appropriate electrode materials and optimizing their surface properties, the formation of blocking passivation layers can be reduced, leading to improved magnesium-ion transport and battery performance.
- Electrolyte formulations to control surface film properties: The composition of electrolytes significantly influences the nature of surface films formed on magnesium-ion battery electrodes. Specialized electrolyte formulations containing specific salts, solvents, and additives can promote the formation of favorable surface films that allow efficient magnesium-ion transport while protecting the electrode from degradation. These electrolytes can help prevent the formation of blocking passivation layers and enhance the overall electrochemical performance of magnesium-ion batteries.
- Surface modification techniques to mitigate passivation: Various surface modification approaches can be employed to mitigate passivation issues in magnesium-ion battery electrodes. These techniques include coating electrodes with protective layers, surface functionalization, and introduction of dopants that enhance magnesium-ion diffusion through surface films. By modifying the electrode surface, the formation of blocking passivation layers can be controlled, leading to improved cycling performance and rate capability in magnesium-ion batteries.
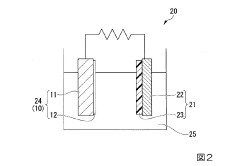
- Characterization and analysis of surface films in Mg-ion batteries: Advanced analytical techniques are essential for understanding the composition, structure, and properties of surface films formed on magnesium-ion battery electrodes. These techniques include spectroscopic methods, microscopy, and electrochemical analysis that provide insights into film formation mechanisms and their impact on battery performance. By characterizing surface films, researchers can develop strategies to control their properties and optimize the electrochemical performance of magnesium-ion batteries.
02 Electrode materials with enhanced surface stability for Mg-ion batteries
Specific electrode materials can be designed or modified to minimize detrimental surface film formation in magnesium-ion batteries. These materials include specially engineered cathodes and anodes with surface coatings or dopants that resist passivation while maintaining electrochemical activity. Some approaches involve nanostructured materials that provide favorable surface chemistry and improved magnesium-ion diffusion kinetics, reducing the formation of insulating surface layers and enhancing overall battery performance.Expand Specific Solutions03 Electrolyte formulations to control surface film properties
Advanced electrolyte formulations can significantly influence the nature of surface films formed on magnesium-ion battery electrodes. By incorporating specific additives, salts, or solvents, the electrolyte can promote the formation of ion-conductive surface layers while preventing the growth of passivating films. These formulations often include components that decompose to form beneficial interface layers, chelating agents that prevent unwanted side reactions, or compounds that scavenge impurities that would otherwise contribute to electrode passivation.Expand Specific Solutions04 Interface engineering strategies to mitigate passivation
Interface engineering approaches focus on modifying the electrode-electrolyte interface to control surface film formation in magnesium-ion batteries. These strategies include artificial SEI layers applied before battery assembly, surface functionalization of electrode materials, and the use of interlayers that mediate ion transport while blocking unwanted reactions. Such engineering techniques can significantly reduce passivation effects, improve coulombic efficiency, and extend battery cycle life by maintaining efficient magnesium-ion transport across the electrode surface.Expand Specific Solutions05 Characterization and analysis of surface films in Mg-ion systems
Advanced analytical techniques are essential for understanding the composition, structure, and formation mechanisms of surface films in magnesium-ion batteries. These methods include spectroscopic techniques, microscopy, electrochemical impedance spectroscopy, and in-situ/operando measurements that reveal the dynamic nature of surface film evolution during battery operation. Such characterization provides critical insights into passivation processes, helping researchers develop effective strategies to control surface film properties and optimize battery performance.Expand Specific Solutions
Leading Research Groups and Industrial Players
The magnesium-ion battery electrode passivation and surface film analysis market is in an early growth phase, characterized by intensive R&D rather than commercial maturity. With the global push for alternative battery technologies beyond lithium-ion, this sector shows promising growth potential estimated at $300-500 million annually. Technologically, the field remains developing with key players demonstrating varying levels of advancement. Leading companies like LG Energy Solution, Samsung SDI, and KIST are making significant strides in electrode material development and surface film characterization. Research institutions including Yamaguchi University and SINANO are contributing fundamental knowledge, while specialized materials companies such as Capchem Technology and FUJIFILM Wako Pure Chemical are developing electrolyte formulations to address passivation challenges. The competitive landscape reflects a mix of established battery manufacturers and specialized research entities working to overcome the technical barriers to commercialization.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced surface film analysis techniques for magnesium-ion battery electrodes using a combination of X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS). Their approach focuses on understanding the chemical composition and structural evolution of the electrode-electrolyte interphase during cycling. The company has pioneered the use of in-situ and operando characterization methods to monitor passivation layer formation in real-time, allowing for deeper insights into degradation mechanisms. Their research has identified that fluoride-rich surface films can effectively suppress magnesium dendrite formation while maintaining reasonable ionic conductivity, leading to enhanced cycling stability and safety in their prototype Mg-ion batteries.
Strengths: Superior analytical capabilities for real-time surface film characterization; proprietary electrolyte formulations that promote beneficial passivation layer formation. Weaknesses: The passivation layers still exhibit higher resistance compared to lithium-ion systems, limiting power performance; technology remains at pre-commercial research stage.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed a novel approach to magnesium-ion battery electrode passivation through their "controlled interface engineering" technology. Their research focuses on creating artificial protective layers on electrode surfaces prior to cell assembly, rather than relying solely on in-situ formed passivation films. Using atomic layer deposition (ALD) techniques, Samsung applies ultra-thin (2-5 nm) coatings of aluminum oxide and titanium dioxide on cathode materials to prevent direct contact with corrosive electrolytes while still allowing Mg-ion transport. This pre-passivation strategy is complemented by electrolyte additives that further modify the electrode-electrolyte interface during cycling. Samsung's surface analysis protocol combines electrochemical impedance spectroscopy with advanced microscopy techniques to characterize film morphology, composition, and ion transport properties throughout battery life.
Strengths: Precise control over protective film thickness and composition; improved cycle life through engineered interfaces; reduced initial capacity loss compared to naturally formed films. Weaknesses: Added manufacturing complexity and cost from ALD coating processes; challenges in maintaining coating integrity during long-term cycling and volume changes.
Key Patents and Literature on Passivation Layer Mitigation
Negative electrode material for magnesium secondary battery and magnesium secondary battery
PatentInactiveJP2020135981A
Innovation
- A coating layer containing carbonaceous material particles with halogen and magnesium is applied to the magnesium material surface, preventing passive film formation and enabling efficient electrodepositing with low overvoltage.
Sulfur-based electrolyte solution for magnesium cell
PatentInactiveUS20220069354A1
Innovation
- A sulfonic acid-based magnesium salt combined with a Lewis acid in a solvent forms an electrolyte solution that enhances oxidative stability to 3 V or more, facilitating stable and repeated magnesium dissolution-precipitation in magnesium batteries.
Environmental Impact and Sustainability Assessment
The development of magnesium-ion batteries represents a significant advancement in energy storage technology, offering potential advantages over lithium-ion systems. However, comprehensive environmental impact and sustainability assessment is essential to determine their true ecological footprint and long-term viability.
Magnesium-ion battery electrode passivation and surface film formation processes present distinct environmental considerations compared to conventional lithium-ion technologies. The extraction of magnesium is generally less environmentally damaging than lithium mining, with magnesium being the eighth most abundant element in Earth's crust (2.1% by mass). This abundance translates to reduced land disruption, water usage, and habitat destruction associated with resource extraction.
The electrode passivation processes in magnesium-ion batteries typically involve less toxic materials compared to those used in lithium-ion batteries. Many conventional lithium battery electrolytes contain fluorinated compounds that pose significant environmental hazards when improperly disposed of. In contrast, magnesium battery systems often utilize chloride-based or non-fluorinated electrolytes, reducing potential environmental contamination risks during manufacturing and end-of-life disposal.
Life cycle assessment (LCA) studies indicate that magnesium-ion batteries may have a 15-30% lower carbon footprint during production compared to equivalent lithium-ion systems, primarily due to differences in electrode material processing requirements. However, the surface film formation on magnesium electrodes often requires specialized electrolyte formulations that may introduce other environmental considerations, particularly regarding solvent toxicity and biodegradability.
Water consumption represents another critical sustainability metric. Preliminary analyses suggest magnesium battery production could reduce water usage by approximately 25% compared to lithium technologies, though this advantage varies significantly depending on specific electrode passivation techniques employed and electrolyte compositions.
End-of-life management presents both challenges and opportunities. The surface films formed on magnesium electrodes typically contain fewer heavy metals and toxic components than those in lithium systems, potentially simplifying recycling processes. Current recycling efficiency estimates for magnesium battery components range from 60-75%, compared to 50-60% for lithium-ion technologies, though these figures continue to evolve as recycling technologies advance.
Regulatory frameworks worldwide are beginning to acknowledge these differences, with the European Battery Directive and similar legislation in Asia and North America increasingly differentiating between battery chemistries based on comprehensive environmental impact assessments. This regulatory evolution will likely accelerate as magnesium-ion technologies mature and their environmental profiles become better understood through expanded commercial deployment and long-term performance evaluation.
Magnesium-ion battery electrode passivation and surface film formation processes present distinct environmental considerations compared to conventional lithium-ion technologies. The extraction of magnesium is generally less environmentally damaging than lithium mining, with magnesium being the eighth most abundant element in Earth's crust (2.1% by mass). This abundance translates to reduced land disruption, water usage, and habitat destruction associated with resource extraction.
The electrode passivation processes in magnesium-ion batteries typically involve less toxic materials compared to those used in lithium-ion batteries. Many conventional lithium battery electrolytes contain fluorinated compounds that pose significant environmental hazards when improperly disposed of. In contrast, magnesium battery systems often utilize chloride-based or non-fluorinated electrolytes, reducing potential environmental contamination risks during manufacturing and end-of-life disposal.
Life cycle assessment (LCA) studies indicate that magnesium-ion batteries may have a 15-30% lower carbon footprint during production compared to equivalent lithium-ion systems, primarily due to differences in electrode material processing requirements. However, the surface film formation on magnesium electrodes often requires specialized electrolyte formulations that may introduce other environmental considerations, particularly regarding solvent toxicity and biodegradability.
Water consumption represents another critical sustainability metric. Preliminary analyses suggest magnesium battery production could reduce water usage by approximately 25% compared to lithium technologies, though this advantage varies significantly depending on specific electrode passivation techniques employed and electrolyte compositions.
End-of-life management presents both challenges and opportunities. The surface films formed on magnesium electrodes typically contain fewer heavy metals and toxic components than those in lithium systems, potentially simplifying recycling processes. Current recycling efficiency estimates for magnesium battery components range from 60-75%, compared to 50-60% for lithium-ion technologies, though these figures continue to evolve as recycling technologies advance.
Regulatory frameworks worldwide are beginning to acknowledge these differences, with the European Battery Directive and similar legislation in Asia and North America increasingly differentiating between battery chemistries based on comprehensive environmental impact assessments. This regulatory evolution will likely accelerate as magnesium-ion technologies mature and their environmental profiles become better understood through expanded commercial deployment and long-term performance evaluation.
Safety Standards and Performance Benchmarking
The development of safety standards for magnesium-ion batteries represents a critical aspect of their commercialization pathway. Currently, these standards remain in nascent stages compared to the well-established frameworks for lithium-ion technologies. Organizations including the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) are beginning to evaluate how existing battery safety protocols might be adapted for magnesium-ion systems, with particular attention to their unique electrode passivation characteristics.
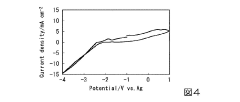
Performance benchmarking methodologies for magnesium-ion batteries must account for the distinctive surface film formation processes that differ significantly from lithium-ion counterparts. Standard testing protocols are being developed to evaluate capacity retention, rate capability, and cycle life with specific consideration for how electrode passivation affects these metrics. The correlation between surface film properties and safety performance represents a key focus area, as preliminary research indicates that magnesium-ion systems may offer inherent safety advantages due to lower reactivity of magnesium metal compared to lithium.
Thermal runaway thresholds for magnesium-ion cells appear higher in initial testing, though comprehensive data remains limited. Accelerating rate calorimetry (ARC) and differential scanning calorimetry (DSC) tests suggest that the nature of the surface films formed on magnesium electrodes contributes to improved thermal stability profiles compared to conventional lithium-ion systems. However, standardized abuse testing protocols specifically designed for magnesium-ion technology are still under development.
Industry consortia including the Battery Safety Council and academic-industrial partnerships are working to establish performance benchmarks that accurately reflect the operational characteristics of magnesium-ion systems. These efforts include developing normalized metrics for comparing energy density, power capability, and cycle life across different electrode formulations and surface film compositions. The establishment of these benchmarks is complicated by the diversity of approaches to managing electrode passivation.
Regulatory bodies in major markets including the European Union, United States, and China are monitoring developments in magnesium-ion technology with the intention of incorporating specific provisions into existing battery safety frameworks. The UN Transportation Testing requirements (UN 38.3) will require adaptation to address the unique characteristics of magnesium-ion systems, particularly regarding the stability of surface films under mechanical stress and thermal cycling conditions that may be encountered during transport.
Performance benchmarking methodologies for magnesium-ion batteries must account for the distinctive surface film formation processes that differ significantly from lithium-ion counterparts. Standard testing protocols are being developed to evaluate capacity retention, rate capability, and cycle life with specific consideration for how electrode passivation affects these metrics. The correlation between surface film properties and safety performance represents a key focus area, as preliminary research indicates that magnesium-ion systems may offer inherent safety advantages due to lower reactivity of magnesium metal compared to lithium.
Thermal runaway thresholds for magnesium-ion cells appear higher in initial testing, though comprehensive data remains limited. Accelerating rate calorimetry (ARC) and differential scanning calorimetry (DSC) tests suggest that the nature of the surface films formed on magnesium electrodes contributes to improved thermal stability profiles compared to conventional lithium-ion systems. However, standardized abuse testing protocols specifically designed for magnesium-ion technology are still under development.
Industry consortia including the Battery Safety Council and academic-industrial partnerships are working to establish performance benchmarks that accurately reflect the operational characteristics of magnesium-ion systems. These efforts include developing normalized metrics for comparing energy density, power capability, and cycle life across different electrode formulations and surface film compositions. The establishment of these benchmarks is complicated by the diversity of approaches to managing electrode passivation.
Regulatory bodies in major markets including the European Union, United States, and China are monitoring developments in magnesium-ion technology with the intention of incorporating specific provisions into existing battery safety frameworks. The UN Transportation Testing requirements (UN 38.3) will require adaptation to address the unique characteristics of magnesium-ion systems, particularly regarding the stability of surface films under mechanical stress and thermal cycling conditions that may be encountered during transport.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!