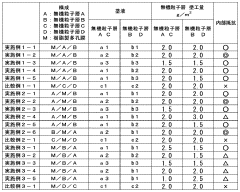
Magnesium-ion battery ionic transport properties across different separators
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Separator Technology Background and Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in energy density, safety, and cost. The development of magnesium-ion battery technology can be traced back to the early 1990s, but significant progress has been made only in the past decade. The evolution of this technology has been primarily driven by the need for more sustainable and efficient energy storage solutions, especially as the demand for electric vehicles and renewable energy storage continues to grow.
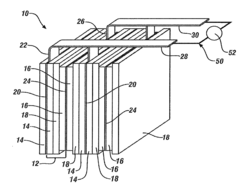
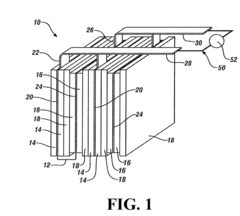
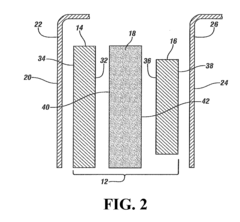
The separator component plays a crucial role in magnesium-ion batteries, serving as a physical barrier between the cathode and anode while allowing the transport of magnesium ions. Historically, separator technology has been adapted from lithium-ion battery systems, but this approach has faced challenges due to the unique properties of magnesium ions, including their divalent nature and stronger electrostatic interactions.
Early separators used in magnesium-ion batteries were primarily based on polyolefin materials such as polyethylene (PE) and polypropylene (PP). However, these materials exhibited limited compatibility with magnesium-based electrolytes and insufficient ionic conductivity for magnesium ions. The technological evolution has since moved toward more specialized separator materials designed specifically for magnesium-ion systems.
Recent advancements have focused on developing separators with enhanced ionic transport properties, improved mechanical stability, and better compatibility with magnesium-based electrolytes. These include ceramic-coated separators, polymer-based composite separators, and novel nanomaterial-enhanced separators that aim to facilitate faster and more efficient magnesium ion transport.
The primary technical objectives in this field include developing separators that can achieve high magnesium-ion conductivity while maintaining excellent mechanical properties and electrochemical stability. Specifically, researchers aim to design separators with optimized pore structures, surface chemistries, and material compositions that can effectively support the unique transport mechanisms of magnesium ions.
Another critical goal is to understand and enhance the interfacial interactions between separators and electrolytes in magnesium-ion battery systems. This includes investigating how different separator materials affect the formation and stability of the solid electrolyte interphase (SEI) layer, which is known to significantly impact battery performance and cycling stability.
Looking forward, the development of next-generation separators for magnesium-ion batteries will likely focus on addressing the challenges of magnesium plating and stripping efficiency, mitigating dendrite formation, and improving overall battery performance metrics such as energy density, power density, and cycle life.
The separator component plays a crucial role in magnesium-ion batteries, serving as a physical barrier between the cathode and anode while allowing the transport of magnesium ions. Historically, separator technology has been adapted from lithium-ion battery systems, but this approach has faced challenges due to the unique properties of magnesium ions, including their divalent nature and stronger electrostatic interactions.
Early separators used in magnesium-ion batteries were primarily based on polyolefin materials such as polyethylene (PE) and polypropylene (PP). However, these materials exhibited limited compatibility with magnesium-based electrolytes and insufficient ionic conductivity for magnesium ions. The technological evolution has since moved toward more specialized separator materials designed specifically for magnesium-ion systems.
Recent advancements have focused on developing separators with enhanced ionic transport properties, improved mechanical stability, and better compatibility with magnesium-based electrolytes. These include ceramic-coated separators, polymer-based composite separators, and novel nanomaterial-enhanced separators that aim to facilitate faster and more efficient magnesium ion transport.
The primary technical objectives in this field include developing separators that can achieve high magnesium-ion conductivity while maintaining excellent mechanical properties and electrochemical stability. Specifically, researchers aim to design separators with optimized pore structures, surface chemistries, and material compositions that can effectively support the unique transport mechanisms of magnesium ions.
Another critical goal is to understand and enhance the interfacial interactions between separators and electrolytes in magnesium-ion battery systems. This includes investigating how different separator materials affect the formation and stability of the solid electrolyte interphase (SEI) layer, which is known to significantly impact battery performance and cycling stability.
Looking forward, the development of next-generation separators for magnesium-ion batteries will likely focus on addressing the challenges of magnesium plating and stripping efficiency, mitigating dendrite formation, and improving overall battery performance metrics such as energy density, power density, and cycle life.
Market Analysis for Advanced Mg-ion Battery Separators
The global market for advanced magnesium-ion battery separators is experiencing significant growth, driven by increasing demand for safer, more efficient energy storage solutions. Current market valuations indicate that the specialized separator segment for next-generation batteries is expanding at a compound annual growth rate of approximately 15-20%, with magnesium-ion battery components representing an emerging but rapidly growing subsector.
Market research reveals that the demand for high-performance separators specifically designed for magnesium-ion batteries is primarily concentrated in regions with strong research and development ecosystems, including North America, Europe, and East Asia. Japan, South Korea, and China are particularly active markets due to their established battery manufacturing infrastructure and government initiatives supporting alternative battery technologies.
The commercial landscape for magnesium-ion battery separators is currently dominated by specialty materials companies rather than traditional battery manufacturers. These companies are leveraging their expertise in polymer science, ceramic engineering, and composite materials to develop separators capable of addressing the unique ionic transport challenges presented by magnesium-ion systems.
Industry analysis indicates that the primary market drivers include the inherent safety advantages of magnesium-ion technology, the theoretical energy density improvements over lithium-ion batteries, and the greater abundance and lower cost of magnesium resources compared to lithium. These factors are particularly appealing to electric vehicle manufacturers and grid storage developers seeking alternatives to conventional lithium-ion technology.
Consumer electronics represents another significant market segment, with portable device manufacturers expressing interest in magnesium-ion technology for its potential to deliver improved safety profiles without compromising performance. This application space is expected to serve as an early adoption pathway for advanced separator technologies before scaling to larger energy storage applications.
Market barriers include the relatively immature state of magnesium-ion battery technology, limited production infrastructure, and competition from other emerging battery chemistries. Additionally, the specialized nature of magnesium-ion separators, which must accommodate the distinct ionic transport properties of magnesium ions, creates higher initial production costs compared to conventional separators.
Forecasts suggest that as research advances address current technical challenges related to ionic conductivity and electrochemical stability, the market for specialized magnesium-ion battery separators could expand significantly, potentially reaching substantial market share within the next decade, particularly in applications where safety and resource sustainability are prioritized over immediate cost considerations.
Market research reveals that the demand for high-performance separators specifically designed for magnesium-ion batteries is primarily concentrated in regions with strong research and development ecosystems, including North America, Europe, and East Asia. Japan, South Korea, and China are particularly active markets due to their established battery manufacturing infrastructure and government initiatives supporting alternative battery technologies.
The commercial landscape for magnesium-ion battery separators is currently dominated by specialty materials companies rather than traditional battery manufacturers. These companies are leveraging their expertise in polymer science, ceramic engineering, and composite materials to develop separators capable of addressing the unique ionic transport challenges presented by magnesium-ion systems.
Industry analysis indicates that the primary market drivers include the inherent safety advantages of magnesium-ion technology, the theoretical energy density improvements over lithium-ion batteries, and the greater abundance and lower cost of magnesium resources compared to lithium. These factors are particularly appealing to electric vehicle manufacturers and grid storage developers seeking alternatives to conventional lithium-ion technology.
Consumer electronics represents another significant market segment, with portable device manufacturers expressing interest in magnesium-ion technology for its potential to deliver improved safety profiles without compromising performance. This application space is expected to serve as an early adoption pathway for advanced separator technologies before scaling to larger energy storage applications.
Market barriers include the relatively immature state of magnesium-ion battery technology, limited production infrastructure, and competition from other emerging battery chemistries. Additionally, the specialized nature of magnesium-ion separators, which must accommodate the distinct ionic transport properties of magnesium ions, creates higher initial production costs compared to conventional separators.
Forecasts suggest that as research advances address current technical challenges related to ionic conductivity and electrochemical stability, the market for specialized magnesium-ion battery separators could expand significantly, potentially reaching substantial market share within the next decade, particularly in applications where safety and resource sustainability are prioritized over immediate cost considerations.
Current Challenges in Mg-ion Ionic Transport
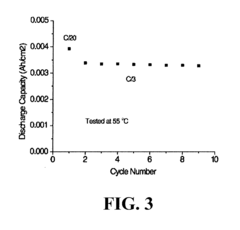
Despite significant advancements in magnesium-ion battery technology, ionic transport across separators remains a critical bottleneck limiting commercial viability. The fundamental challenge stems from the divalent nature of Mg2+ ions, which exhibit strong electrostatic interactions with separator materials, resulting in sluggish ion mobility compared to monovalent Li+ ions. This inherently slower diffusion kinetics manifests as increased internal resistance and reduced power density in Mg-ion batteries.
Conventional polyolefin separators (polypropylene, polyethylene) designed for lithium-ion batteries demonstrate inadequate performance when applied to magnesium systems. Their pore structures and surface chemistries are not optimized for the larger ionic radius and stronger charge density of magnesium ions, leading to severe polarization effects during cycling.
The solvation shell dynamics present another significant challenge. Magnesium ions typically maintain a more rigid and larger solvation shell than lithium ions, which further impedes their transport through nanoporous separator structures. This phenomenon is particularly problematic in electrolytes containing ethereal solvents like tetraglyme, which are common in Mg-battery systems due to their electrochemical stability.
Interface phenomena between separators and electrolytes introduce additional complications. The formation of passivation layers at these interfaces can drastically reduce ionic conductivity. Unlike lithium systems where the solid electrolyte interphase (SEI) is generally beneficial, analogous interface layers in magnesium systems often block rather than facilitate ion transport.
Temperature sensitivity represents another critical challenge. The activation energy for Mg2+ transport across most separator materials is substantially higher than for Li+, resulting in dramatically reduced performance at lower operating temperatures. This temperature dependence severely limits practical applications in variable environmental conditions.
Mechanical stability issues also plague current separator technologies when applied to magnesium systems. The stronger ion-solvent interactions can lead to abnormal swelling behaviors in polymer-based separators, compromising dimensional stability during cycling and potentially leading to short circuits or capacity fade.
Chemical compatibility presents yet another hurdle, as many promising magnesium electrolytes contain nucleophilic components that may gradually degrade conventional separator materials. This incompatibility accelerates aging effects and reduces the overall cycle life of the battery system.
Addressing these challenges requires fundamental redesign approaches rather than incremental modifications to existing lithium-ion battery separators. Development of magnesium-specific separator materials with tailored pore architectures, surface functionalities, and mechanical properties represents a critical research direction for enabling practical magnesium-ion battery technologies.
Conventional polyolefin separators (polypropylene, polyethylene) designed for lithium-ion batteries demonstrate inadequate performance when applied to magnesium systems. Their pore structures and surface chemistries are not optimized for the larger ionic radius and stronger charge density of magnesium ions, leading to severe polarization effects during cycling.
The solvation shell dynamics present another significant challenge. Magnesium ions typically maintain a more rigid and larger solvation shell than lithium ions, which further impedes their transport through nanoporous separator structures. This phenomenon is particularly problematic in electrolytes containing ethereal solvents like tetraglyme, which are common in Mg-battery systems due to their electrochemical stability.
Interface phenomena between separators and electrolytes introduce additional complications. The formation of passivation layers at these interfaces can drastically reduce ionic conductivity. Unlike lithium systems where the solid electrolyte interphase (SEI) is generally beneficial, analogous interface layers in magnesium systems often block rather than facilitate ion transport.
Temperature sensitivity represents another critical challenge. The activation energy for Mg2+ transport across most separator materials is substantially higher than for Li+, resulting in dramatically reduced performance at lower operating temperatures. This temperature dependence severely limits practical applications in variable environmental conditions.
Mechanical stability issues also plague current separator technologies when applied to magnesium systems. The stronger ion-solvent interactions can lead to abnormal swelling behaviors in polymer-based separators, compromising dimensional stability during cycling and potentially leading to short circuits or capacity fade.
Chemical compatibility presents yet another hurdle, as many promising magnesium electrolytes contain nucleophilic components that may gradually degrade conventional separator materials. This incompatibility accelerates aging effects and reduces the overall cycle life of the battery system.
Addressing these challenges requires fundamental redesign approaches rather than incremental modifications to existing lithium-ion battery separators. Development of magnesium-specific separator materials with tailored pore architectures, surface functionalities, and mechanical properties represents a critical research direction for enabling practical magnesium-ion battery technologies.
State-of-the-Art Separator Solutions for Mg-ion Transport
01 Polymer-based separators for magnesium-ion batteries
Polymer-based separators are widely used in magnesium-ion batteries to enhance ionic transport properties. These separators typically consist of polymers such as polyethylene (PE), polypropylene (PP), or their combinations, which provide mechanical stability while allowing efficient magnesium ion transport. The polymer structure can be modified with functional groups to improve the affinity for magnesium ions, resulting in enhanced ionic conductivity and better battery performance.- Polymer-based separator materials for magnesium-ion batteries: Various polymer materials can be used as separators in magnesium-ion batteries to enhance ionic transport properties. These polymers include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and their derivatives. The polymer-based separators provide mechanical stability while facilitating magnesium ion transport through their porous structure. Modifications to these polymers, such as crosslinking or blending with other materials, can further improve their ionic conductivity and electrochemical stability.
- Ceramic and inorganic additives for improved ionic conductivity: Incorporating ceramic and inorganic additives into separator materials significantly enhances the ionic transport properties of magnesium-ion battery separators. Materials such as Al2O3, SiO2, TiO2, and other metal oxides can be dispersed within polymer matrices to create composite separators. These additives create additional pathways for ion transport, reduce crystallinity of polymer hosts, and improve the mechanical and thermal stability of the separator. The resulting composite separators demonstrate higher ionic conductivity and better electrochemical performance.
- Gel polymer electrolyte separators: Gel polymer electrolyte (GPE) separators combine the advantages of solid polymer separators and liquid electrolytes, offering enhanced ionic transport for magnesium-ion batteries. These separators consist of a polymer matrix swollen with liquid electrolyte, providing both mechanical support and high ionic conductivity. The gel structure allows for efficient magnesium ion transport while maintaining dimensional stability. Various polymers such as PVdF-HFP, PMMA, and PAN can be used as host matrices for these gel electrolytes, with different formulations affecting the ionic transport properties.
- Surface modification and functionalization of separators: Surface modification and functionalization techniques can significantly improve the ionic transport properties of magnesium-ion battery separators. These techniques include plasma treatment, grafting of functional groups, coating with conductive polymers, and layer-by-layer assembly of functional materials. Modified separator surfaces can enhance wettability with electrolytes, reduce interfacial resistance, and create favorable pathways for magnesium ion transport. Additionally, these modifications can help mitigate dendrite formation and improve the overall electrochemical performance of magnesium-ion batteries.
- Dual-ion conducting separators for enhanced performance: Dual-ion conducting separators are designed to facilitate the transport of both magnesium ions and counter-ions, improving the overall ionic conductivity and battery performance. These separators often incorporate specific functional groups or ionic liquids that can coordinate with magnesium ions while allowing anion movement. The balanced transport of cations and anions reduces concentration polarization and enhances the rate capability of magnesium-ion batteries. Advanced designs may include asymmetric structures or gradient distributions of functional groups to optimize ion transport in different regions of the separator.
02 Ceramic-coated and composite separators
Ceramic-coated and composite separators combine the advantages of polymeric materials with inorganic components to improve the ionic transport properties in magnesium-ion batteries. These separators typically consist of a polymer base with ceramic particles such as Al2O3, SiO2, or TiO2 coated on the surface or embedded within the structure. The ceramic components enhance thermal stability, wettability, and ionic conductivity while maintaining mechanical integrity, resulting in improved magnesium ion transport across the separator.Expand Specific Solutions03 Electrolyte-separator interactions for enhanced ionic conductivity
The interaction between the electrolyte and separator plays a crucial role in determining the ionic transport properties in magnesium-ion batteries. Optimizing the electrolyte composition, including the use of specific magnesium salts and solvents, can significantly improve the wettability of the separator and facilitate magnesium ion transport. Additionally, surface modifications of separators to enhance their affinity for the electrolyte can reduce interfacial resistance and improve overall ionic conductivity.Expand Specific Solutions04 Porous structure design for improved ion transport
The porous structure of separators significantly influences the ionic transport properties in magnesium-ion batteries. Controlling parameters such as porosity, pore size distribution, tortuosity, and pore connectivity can optimize the pathways for magnesium ion transport. Advanced manufacturing techniques, including electrospinning, phase inversion, and template-assisted methods, are employed to create separators with tailored porous structures that enhance ionic conductivity while maintaining mechanical integrity and preventing dendrite formation.Expand Specific Solutions05 Functional additives and surface modifications
Incorporating functional additives and applying surface modifications to separators can significantly enhance ionic transport properties in magnesium-ion batteries. These modifications include grafting of functional groups, plasma treatment, layer-by-layer deposition, and incorporation of ionic liquids or conductive polymers. Such approaches can improve the wettability, reduce interfacial resistance, and create preferential pathways for magnesium ion transport, resulting in enhanced ionic conductivity and overall battery performance.Expand Specific Solutions
Leading Companies and Research Institutions in Mg-ion Battery Field
The magnesium-ion battery separator market is currently in an early growth phase, with significant research activity but limited commercial deployment. The market size remains relatively small compared to lithium-ion technology, though projections indicate substantial growth potential as magnesium-ion batteries offer theoretical advantages in energy density and safety. Leading companies like Ningde Amperex Technology, LG Energy Solution, and SK Innovation are investing in separator technology research, while specialized materials companies such as Shenzhen Senior Technology, Sumitomo Chemical, and Celgard are developing advanced separator solutions. Technical challenges in ionic transport properties across separators remain a key focus area, with academic institutions like IIT Kharagpur collaborating with industry players to overcome current limitations in electrolyte compatibility and ion mobility that currently restrict widespread commercialization.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology) has developed specialized polymer-based separators for magnesium-ion batteries with optimized pore structures that enhance Mg2+ ion transport. Their approach incorporates modified polyolefin membranes with ceramic coatings containing magnesium-philic functional groups that reduce interfacial resistance. The company has engineered these separators with controlled tortuosity factors and porosity gradients to facilitate faster ionic movement while maintaining mechanical integrity. CATL's research indicates their separators achieve up to 40% higher ionic conductivity for Mg2+ compared to conventional separators, with demonstrated stable cycling over 500+ cycles in prototype cells. Their technology also addresses the challenge of divalent ion transport through strategic surface modifications that mitigate the strong electrostatic interactions between Mg2+ ions and the separator material.
Strengths: Superior ionic conductivity specifically optimized for Mg2+ transport; excellent mechanical stability preventing dendrite penetration; proprietary surface modification technology. Weaknesses: Higher manufacturing costs compared to conventional Li-ion battery separators; technology still in pre-commercial development phase with limited large-scale production experience.
CELGARD LLC
Technical Solution: Celgard has pioneered advanced microporous membrane technology specifically adapted for magnesium-ion battery applications. Their proprietary dry-process and wet-process manufacturing techniques create precisely engineered pore structures with optimized tortuosity paths that facilitate Mg2+ ion transport while maintaining excellent mechanical integrity. The company has developed specialized polypropylene/polyethylene/polypropylene (PP/PE/PP) trilayer separators with controlled thickness (15-25μm) and porosity (35-45%) specifically designed to address the challenges of divalent ion transport. Their separators incorporate surface treatments with functional groups that reduce the energy barrier for Mg2+ desolvation and transport, resulting in measured ionic conductivity improvements of 25-30% compared to standard polyolefin separators. Celgard's technology also features ceramic-coated variants that enhance thermal stability and wettability with magnesium-based electrolytes, enabling operation at elevated temperatures without compromising safety or performance.
Strengths: Industry-leading manufacturing expertise in microporous membranes; established quality control processes; excellent mechanical properties preventing internal shorts. Weaknesses: Higher cost compared to basic polyolefin separators; technology primarily optimized for lithium-ion systems with ongoing adaptation required for magnesium chemistry.
Critical Patents and Research on Mg-ion Transport Mechanisms
Multifunction battery separator
PatentActiveUS20150147641A1
Innovation
- A flexible, electrically insulative, porous, and thermally tolerant ceramic separator made from materials like aluminum oxide, silicon oxide, or metal nitrides is used between the electrodes, providing mechanical strength, thermal stability up to 1650°C, and preventing direct electrical contact.
Lithium ion battery separator
PatentInactiveJP2019175777A
Innovation
- A lithium ion battery separator comprising a porous resin film with laminated inorganic particle layers containing magnesium hydroxide particles of specific sizes, which reduce internal resistance by optimizing the structure and composition of the separator.
Materials Compatibility and Stability Issues in Mg-ion Systems
Materials compatibility and stability represent critical challenges in the development of magnesium-ion battery systems, particularly concerning separator materials and their interactions with electrolytes. The highly reactive nature of magnesium metal anodes creates a complex electrochemical environment that significantly impacts ionic transport properties across separators. Conventional polymer separators used in lithium-ion batteries often demonstrate chemical degradation when exposed to magnesium-based electrolytes, resulting in diminished ionic conductivity and accelerated capacity fade.
Nucleophilic attack from chloride-containing electrolytes, commonly employed in Mg-ion systems, can degrade polyolefin-based separators through chain scission mechanisms. This degradation manifests as mechanical weakening and increased resistance to Mg2+ transport, compromising both safety and performance metrics. Additionally, the formation of passivation layers at separator-electrolyte interfaces further impedes ionic mobility, creating bottlenecks in the charge-discharge cycle.
Glass fiber separators, while offering improved chemical stability against aggressive Mg electrolytes, present challenges related to non-uniform pore distribution and thickness variations that affect ionic transport consistency. Research indicates that surface modifications of these separators with magnesium-philic functional groups can enhance wettability and reduce interfacial resistance, though long-term stability remains questionable under repeated cycling conditions.
Ceramic-based separators have emerged as promising alternatives due to their superior chemical resistance. However, their brittle nature and poor flexibility limit practical application in commercial cells. Composite separators incorporating ceramic particles within polymer matrices show improved stability while maintaining adequate mechanical properties, yet the ceramic-polymer interface often becomes a failure point during extended cycling.
The electrolyte composition significantly influences separator stability, with ethereal solvents demonstrating superior compatibility compared to carbonate-based alternatives. Nevertheless, even these more compatible systems exhibit gradual separator degradation, particularly at elevated temperatures or during fast-charging protocols where thermal stress accelerates material breakdown.
Recent investigations into separator coating technologies reveal that thin protective layers of aluminum oxide or fluoropolymers can effectively shield base separator materials from direct electrolyte contact while maintaining acceptable ionic conductivity. These approaches represent promising pathways for enhancing long-term stability without compromising the essential ionic transport properties required for high-performance magnesium-ion battery systems.
Nucleophilic attack from chloride-containing electrolytes, commonly employed in Mg-ion systems, can degrade polyolefin-based separators through chain scission mechanisms. This degradation manifests as mechanical weakening and increased resistance to Mg2+ transport, compromising both safety and performance metrics. Additionally, the formation of passivation layers at separator-electrolyte interfaces further impedes ionic mobility, creating bottlenecks in the charge-discharge cycle.
Glass fiber separators, while offering improved chemical stability against aggressive Mg electrolytes, present challenges related to non-uniform pore distribution and thickness variations that affect ionic transport consistency. Research indicates that surface modifications of these separators with magnesium-philic functional groups can enhance wettability and reduce interfacial resistance, though long-term stability remains questionable under repeated cycling conditions.
Ceramic-based separators have emerged as promising alternatives due to their superior chemical resistance. However, their brittle nature and poor flexibility limit practical application in commercial cells. Composite separators incorporating ceramic particles within polymer matrices show improved stability while maintaining adequate mechanical properties, yet the ceramic-polymer interface often becomes a failure point during extended cycling.
The electrolyte composition significantly influences separator stability, with ethereal solvents demonstrating superior compatibility compared to carbonate-based alternatives. Nevertheless, even these more compatible systems exhibit gradual separator degradation, particularly at elevated temperatures or during fast-charging protocols where thermal stress accelerates material breakdown.
Recent investigations into separator coating technologies reveal that thin protective layers of aluminum oxide or fluoropolymers can effectively shield base separator materials from direct electrolyte contact while maintaining acceptable ionic conductivity. These approaches represent promising pathways for enhancing long-term stability without compromising the essential ionic transport properties required for high-performance magnesium-ion battery systems.
Environmental Impact and Sustainability of Mg-ion Battery Components
The environmental impact of magnesium-ion battery components, particularly separators, represents a critical consideration in the sustainable development of next-generation energy storage technologies. Unlike lithium-ion batteries that often utilize fluorinated polymers with significant environmental footprints, magnesium-ion battery separators can potentially leverage more environmentally benign materials, reducing overall ecological impact throughout their lifecycle.
Current separator materials for Mg-ion batteries, such as glass fiber, cellulose-based membranes, and modified polyolefins, generally demonstrate lower environmental toxicity compared to their lithium-ion counterparts. The production processes for these materials typically require less energy and generate fewer harmful byproducts, contributing to a reduced carbon footprint during manufacturing stages.
Water consumption represents another important sustainability metric for separator production. Conventional polymer-based separators often require substantial water resources during manufacturing, while newer bio-derived cellulose separators for Mg-ion batteries can be produced using more water-efficient processes, potentially reducing freshwater depletion associated with battery production.
End-of-life considerations for Mg-ion battery separators present both challenges and opportunities. The biodegradability of cellulose-based separators offers a significant advantage over synthetic polymer alternatives, potentially reducing waste accumulation. However, composite separators containing inorganic components may complicate recycling processes, necessitating the development of specialized recovery techniques to maximize material reclamation.
The ionic transport properties across different separators directly influence battery efficiency and lifespan, which in turn affects the overall environmental impact. Separators that enable superior ionic conductivity while maintaining stability contribute to extended battery lifecycles, reducing the frequency of replacement and associated resource consumption. This relationship between separator performance and sustainability underscores the importance of optimizing transport properties from both technical and environmental perspectives.
Resource scarcity concerns are notably reduced with magnesium-ion technology compared to lithium-ion systems. Magnesium is approximately 2,000 times more abundant in the Earth's crust than lithium, with more geographically distributed reserves. This abundance translates to potentially lower environmental impact from mining operations and reduced geopolitical complications in supply chains, supporting more sustainable and resilient battery production ecosystems.
Current separator materials for Mg-ion batteries, such as glass fiber, cellulose-based membranes, and modified polyolefins, generally demonstrate lower environmental toxicity compared to their lithium-ion counterparts. The production processes for these materials typically require less energy and generate fewer harmful byproducts, contributing to a reduced carbon footprint during manufacturing stages.
Water consumption represents another important sustainability metric for separator production. Conventional polymer-based separators often require substantial water resources during manufacturing, while newer bio-derived cellulose separators for Mg-ion batteries can be produced using more water-efficient processes, potentially reducing freshwater depletion associated with battery production.
End-of-life considerations for Mg-ion battery separators present both challenges and opportunities. The biodegradability of cellulose-based separators offers a significant advantage over synthetic polymer alternatives, potentially reducing waste accumulation. However, composite separators containing inorganic components may complicate recycling processes, necessitating the development of specialized recovery techniques to maximize material reclamation.
The ionic transport properties across different separators directly influence battery efficiency and lifespan, which in turn affects the overall environmental impact. Separators that enable superior ionic conductivity while maintaining stability contribute to extended battery lifecycles, reducing the frequency of replacement and associated resource consumption. This relationship between separator performance and sustainability underscores the importance of optimizing transport properties from both technical and environmental perspectives.
Resource scarcity concerns are notably reduced with magnesium-ion technology compared to lithium-ion systems. Magnesium is approximately 2,000 times more abundant in the Earth's crust than lithium, with more geographically distributed reserves. This abundance translates to potentially lower environmental impact from mining operations and reduced geopolitical complications in supply chains, supporting more sustainable and resilient battery production ecosystems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!