Magnesium-ion battery intercalation kinetics and charge storage models
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Evolution and Research Objectives
Magnesium-ion battery technology has evolved significantly over the past decades, emerging as a promising alternative to lithium-ion batteries due to magnesium's abundance, safety, and theoretical capacity advantages. The evolution began in the 1990s with pioneering work by Gregory et al., who demonstrated the first rechargeable magnesium battery using organohaloaluminate electrolytes. This breakthrough opened a new research avenue in energy storage systems beyond lithium-ion technology.
The early 2000s witnessed limited progress due to challenges in electrolyte compatibility and electrode materials. However, the 2010s marked a renaissance in Mg-ion battery research, catalyzed by Aurbach's group developing new electrolyte systems and the discovery of various intercalation host materials. This period established fundamental understanding of magnesium intercalation mechanisms, though significant performance gaps remained compared to commercial lithium-ion systems.
Recent years have seen accelerated development focusing on understanding intercalation kinetics and charge storage models specific to magnesium-ion systems. The divalent nature of Mg2+ ions creates unique challenges and opportunities that differentiate these systems from monovalent lithium-ion batteries. Research has revealed that magnesium ions experience stronger electrostatic interactions with host lattices, resulting in slower diffusion kinetics but potentially higher energy densities.
The primary research objectives in this field now center on overcoming the kinetic limitations of magnesium intercalation. This includes developing comprehensive models that accurately describe the charge storage mechanisms, ion transport pathways, and interfacial phenomena in various electrode materials. Understanding these fundamental aspects is crucial for designing high-performance magnesium-ion batteries with practical energy densities and power capabilities.
Current research aims to elucidate the correlation between crystal structure, electronic properties, and magnesium diffusion barriers in cathode materials. Particular focus is placed on identifying materials that can accommodate the larger charge density of Mg2+ while maintaining structural stability during repeated cycling. Computational methods, including density functional theory and molecular dynamics simulations, have become essential tools in predicting and understanding these complex interactions.
Another critical objective is developing accurate models for the desolvation process at electrode interfaces, as this represents a significant kinetic barrier in magnesium systems. The strong coordination of magnesium ions with solvent molecules creates unique challenges that must be addressed through innovative electrolyte design and interface engineering.
The ultimate goal of current research efforts is to establish a comprehensive theoretical framework that can guide the rational design of electrode materials and electrolytes specifically optimized for magnesium-ion systems, enabling the development of commercially viable batteries that leverage magnesium's inherent advantages in abundance, safety, and theoretical capacity.
The early 2000s witnessed limited progress due to challenges in electrolyte compatibility and electrode materials. However, the 2010s marked a renaissance in Mg-ion battery research, catalyzed by Aurbach's group developing new electrolyte systems and the discovery of various intercalation host materials. This period established fundamental understanding of magnesium intercalation mechanisms, though significant performance gaps remained compared to commercial lithium-ion systems.
Recent years have seen accelerated development focusing on understanding intercalation kinetics and charge storage models specific to magnesium-ion systems. The divalent nature of Mg2+ ions creates unique challenges and opportunities that differentiate these systems from monovalent lithium-ion batteries. Research has revealed that magnesium ions experience stronger electrostatic interactions with host lattices, resulting in slower diffusion kinetics but potentially higher energy densities.
The primary research objectives in this field now center on overcoming the kinetic limitations of magnesium intercalation. This includes developing comprehensive models that accurately describe the charge storage mechanisms, ion transport pathways, and interfacial phenomena in various electrode materials. Understanding these fundamental aspects is crucial for designing high-performance magnesium-ion batteries with practical energy densities and power capabilities.
Current research aims to elucidate the correlation between crystal structure, electronic properties, and magnesium diffusion barriers in cathode materials. Particular focus is placed on identifying materials that can accommodate the larger charge density of Mg2+ while maintaining structural stability during repeated cycling. Computational methods, including density functional theory and molecular dynamics simulations, have become essential tools in predicting and understanding these complex interactions.
Another critical objective is developing accurate models for the desolvation process at electrode interfaces, as this represents a significant kinetic barrier in magnesium systems. The strong coordination of magnesium ions with solvent molecules creates unique challenges that must be addressed through innovative electrolyte design and interface engineering.
The ultimate goal of current research efforts is to establish a comprehensive theoretical framework that can guide the rational design of electrode materials and electrolytes specifically optimized for magnesium-ion systems, enabling the development of commercially viable batteries that leverage magnesium's inherent advantages in abundance, safety, and theoretical capacity.
Market Analysis for Next-Generation Energy Storage Systems
The global energy storage market is witnessing unprecedented growth, with projections indicating a compound annual growth rate of 20-25% through 2030. This expansion is primarily driven by the increasing integration of renewable energy sources, grid modernization initiatives, and the electrification of transportation. Within this landscape, magnesium-ion battery technology represents a promising alternative to conventional lithium-ion systems, potentially addressing critical supply chain vulnerabilities and performance limitations.
Current market analysis reveals that lithium-ion batteries dominate approximately 90% of the grid-scale and electric vehicle storage segments. However, concerns regarding lithium supply constraints, with reserves concentrated in politically sensitive regions, have intensified the search for alternative chemistries. Magnesium, being the eighth most abundant element in Earth's crust and widely distributed geographically, presents a compelling economic case with potential raw material cost reductions of 30-40% compared to lithium-based systems.
Consumer and industrial demand for higher energy density, improved safety profiles, and reduced environmental impact is reshaping market preferences. Magnesium-ion batteries, with their theoretical volumetric capacity exceeding that of lithium-ion counterparts, align well with these evolving requirements. Market research indicates that industries requiring high-density, long-duration storage solutions would be early adopters of mature magnesium-ion technology.
The electric vehicle sector, projected to grow at 25-30% annually through 2028, represents the largest potential market for advanced battery technologies. Current limitations in magnesium-ion intercalation kinetics present challenges for high-power applications, but ongoing research into novel electrode materials and electrolyte formulations shows promise for overcoming these barriers. Industry analysts suggest that even capturing 5% of the EV battery market would represent a multi-billion dollar opportunity for magnesium-based systems.
Stationary storage applications present another significant market segment, particularly for technologies that can demonstrate superior cycle life and safety characteristics. The grid storage market is expected to quadruple by 2030, with increasing emphasis on long-duration storage solutions where magnesium-ion systems could potentially excel due to their stability and resource availability.
Competitive analysis indicates that while major battery manufacturers are primarily focused on incremental improvements to lithium-ion technology, several specialized startups and research institutions are making significant advances in magnesium-ion systems. Strategic partnerships between material science companies, battery manufacturers, and end-users are emerging as the preferred commercialization pathway for these next-generation technologies.
Current market analysis reveals that lithium-ion batteries dominate approximately 90% of the grid-scale and electric vehicle storage segments. However, concerns regarding lithium supply constraints, with reserves concentrated in politically sensitive regions, have intensified the search for alternative chemistries. Magnesium, being the eighth most abundant element in Earth's crust and widely distributed geographically, presents a compelling economic case with potential raw material cost reductions of 30-40% compared to lithium-based systems.
Consumer and industrial demand for higher energy density, improved safety profiles, and reduced environmental impact is reshaping market preferences. Magnesium-ion batteries, with their theoretical volumetric capacity exceeding that of lithium-ion counterparts, align well with these evolving requirements. Market research indicates that industries requiring high-density, long-duration storage solutions would be early adopters of mature magnesium-ion technology.
The electric vehicle sector, projected to grow at 25-30% annually through 2028, represents the largest potential market for advanced battery technologies. Current limitations in magnesium-ion intercalation kinetics present challenges for high-power applications, but ongoing research into novel electrode materials and electrolyte formulations shows promise for overcoming these barriers. Industry analysts suggest that even capturing 5% of the EV battery market would represent a multi-billion dollar opportunity for magnesium-based systems.
Stationary storage applications present another significant market segment, particularly for technologies that can demonstrate superior cycle life and safety characteristics. The grid storage market is expected to quadruple by 2030, with increasing emphasis on long-duration storage solutions where magnesium-ion systems could potentially excel due to their stability and resource availability.
Competitive analysis indicates that while major battery manufacturers are primarily focused on incremental improvements to lithium-ion technology, several specialized startups and research institutions are making significant advances in magnesium-ion systems. Strategic partnerships between material science companies, battery manufacturers, and end-users are emerging as the preferred commercialization pathway for these next-generation technologies.
Intercalation Kinetics Challenges in Mg-ion Batteries
The intercalation kinetics in magnesium-ion batteries presents significant challenges that have hindered their widespread commercial adoption despite their theoretical advantages. The divalent nature of Mg2+ ions results in strong electrostatic interactions with host lattices, creating substantial energy barriers for ion migration. This fundamental characteristic leads to sluggish diffusion coefficients, typically 1-4 orders of magnitude lower than those observed in lithium-ion systems.
Electrode materials for Mg-ion batteries face severe limitations due to these kinetic constraints. The strong coulombic interactions between Mg2+ ions and host structures not only slow diffusion but also cause structural distortions during intercalation/deintercalation processes. These distortions can lead to mechanical stress, volume changes, and eventual degradation of electrode materials over multiple cycles.
Another critical challenge lies in the charge transfer processes at electrode-electrolyte interfaces. The desolvation of Mg2+ ions from their solvation shell requires significant energy input, creating a rate-limiting step in the overall intercalation process. This phenomenon is particularly pronounced in conventional electrolytes where Mg2+ ions form strong coordination complexes with solvent molecules.
The multivalent nature of magnesium also contributes to complex charge storage mechanisms that deviate from traditional intercalation models developed for monovalent systems. Current models struggle to accurately describe the cooperative effects and ion-ion interactions that occur during Mg2+ insertion, particularly at higher states of charge where these effects become more pronounced.
Experimental characterization of intercalation kinetics presents methodological challenges. Techniques such as galvanostatic intermittent titration technique (GITT) and electrochemical impedance spectroscopy (EIS) often yield inconsistent results due to the complex nature of Mg2+ diffusion processes. This inconsistency has led to significant variations in reported diffusion coefficients for identical materials.
The development of accurate computational models for Mg-ion intercalation kinetics is hampered by the need to account for strong correlation effects and many-body interactions. Density functional theory (DFT) calculations frequently underestimate migration barriers, while classical molecular dynamics simulations struggle to capture the electronic structure changes during intercalation.
Recent research has identified that crystal structure engineering, particularly focusing on expanding interlayer spacing and creating open diffusion channels, can partially mitigate these kinetic limitations. However, these modifications often come at the cost of reduced volumetric energy density, creating a fundamental design trade-off that must be carefully balanced.
Electrode materials for Mg-ion batteries face severe limitations due to these kinetic constraints. The strong coulombic interactions between Mg2+ ions and host structures not only slow diffusion but also cause structural distortions during intercalation/deintercalation processes. These distortions can lead to mechanical stress, volume changes, and eventual degradation of electrode materials over multiple cycles.
Another critical challenge lies in the charge transfer processes at electrode-electrolyte interfaces. The desolvation of Mg2+ ions from their solvation shell requires significant energy input, creating a rate-limiting step in the overall intercalation process. This phenomenon is particularly pronounced in conventional electrolytes where Mg2+ ions form strong coordination complexes with solvent molecules.
The multivalent nature of magnesium also contributes to complex charge storage mechanisms that deviate from traditional intercalation models developed for monovalent systems. Current models struggle to accurately describe the cooperative effects and ion-ion interactions that occur during Mg2+ insertion, particularly at higher states of charge where these effects become more pronounced.
Experimental characterization of intercalation kinetics presents methodological challenges. Techniques such as galvanostatic intermittent titration technique (GITT) and electrochemical impedance spectroscopy (EIS) often yield inconsistent results due to the complex nature of Mg2+ diffusion processes. This inconsistency has led to significant variations in reported diffusion coefficients for identical materials.
The development of accurate computational models for Mg-ion intercalation kinetics is hampered by the need to account for strong correlation effects and many-body interactions. Density functional theory (DFT) calculations frequently underestimate migration barriers, while classical molecular dynamics simulations struggle to capture the electronic structure changes during intercalation.
Recent research has identified that crystal structure engineering, particularly focusing on expanding interlayer spacing and creating open diffusion channels, can partially mitigate these kinetic limitations. However, these modifications often come at the cost of reduced volumetric energy density, creating a fundamental design trade-off that must be carefully balanced.
Current Intercalation Mechanisms and Charge Storage Models
01 Electrode materials for magnesium-ion intercalation
Various materials can be used as electrodes in magnesium-ion batteries to facilitate intercalation kinetics and improve charge storage capacity. These materials include layered transition metal oxides, spinel structures, and organic compounds that provide pathways for magnesium ion insertion and extraction. The crystal structure and composition of these materials significantly affect the intercalation kinetics, with optimized structures allowing for faster magnesium ion diffusion and enhanced electrochemical performance.- Electrode materials for magnesium-ion intercalation: Various materials can be used as electrodes in magnesium-ion batteries to facilitate intercalation kinetics and improve charge storage capacity. These materials include layered transition metal oxides, spinel structures, and organic compounds that provide suitable channels for Mg-ion insertion and extraction. The crystal structure and composition of these materials significantly affect the intercalation kinetics by providing appropriate ion diffusion pathways and reducing the energy barriers for ion movement.
- Electrolyte formulations for enhanced magnesium-ion transport: Specialized electrolyte formulations can significantly improve magnesium-ion intercalation kinetics and charge storage capabilities. These formulations often include magnesium salts in combination with specific solvents that reduce ion pairing and facilitate ion transport. Additives can be incorporated to modify the solid-electrolyte interphase formation, reduce interfacial resistance, and enhance the reversibility of magnesium deposition and dissolution processes, ultimately improving the overall battery performance.
- Nanostructured materials for improved intercalation dynamics: Nanostructured materials offer enhanced magnesium-ion intercalation kinetics due to their high surface area, shortened ion diffusion paths, and abundant active sites. These materials include nanoparticles, nanowires, and porous structures that can accommodate the strain associated with magnesium insertion and extraction. The reduced dimensions facilitate faster charge transfer reactions and allow for higher rate capabilities, making them particularly suitable for applications requiring rapid charging and discharging.
- Surface modification techniques for electrode materials: Surface modification of electrode materials can significantly enhance magnesium-ion intercalation kinetics and charge storage performance. Techniques include coating with conductive materials, doping with heteroatoms, and creating defects or vacancies in the crystal structure. These modifications can reduce charge transfer resistance, provide additional ion diffusion channels, and stabilize the electrode structure during cycling, leading to improved capacity retention and cycle life of magnesium-ion batteries.
- Hybrid and dual-ion battery systems incorporating magnesium: Hybrid and dual-ion battery systems that incorporate magnesium can offer advantages in terms of intercalation kinetics and charge storage. These systems may combine magnesium with other ions such as lithium or sodium, or utilize different charge carriers at each electrode. Such configurations can leverage the beneficial properties of magnesium while mitigating some of its limitations, resulting in batteries with improved energy density, power density, and cycling stability compared to conventional single-ion systems.
02 Electrolyte formulations for enhanced magnesium-ion transport
Specialized electrolyte formulations play a crucial role in magnesium-ion battery performance by facilitating ion transport between electrodes. These formulations often include magnesium salts dissolved in appropriate solvents, sometimes with additives that prevent electrode passivation. The composition of the electrolyte directly impacts intercalation kinetics by affecting the desolvation energy of magnesium ions at the electrode interface, which is typically a rate-limiting step in the charge storage process.Expand Specific Solutions03 Surface modification techniques for improved kinetics
Surface modifications of electrode materials can significantly enhance magnesium-ion intercalation kinetics. These modifications include coating with conductive materials, doping with heteroatoms, or creating defects in the crystal structure. Such treatments can reduce charge transfer resistance at the electrode-electrolyte interface, provide additional pathways for ion diffusion, and prevent unwanted side reactions, ultimately leading to improved rate capability and cycling stability of magnesium-ion batteries.Expand Specific Solutions04 Nanostructured materials for enhanced charge storage
Nanostructured materials offer advantages for magnesium-ion batteries due to their high surface area and shortened ion diffusion paths. These materials, including nanoparticles, nanowires, and porous structures, can accommodate the strain associated with magnesium ion insertion and extraction, preventing structural degradation during cycling. The reduced dimensions also facilitate faster intercalation kinetics by decreasing the diffusion distance for magnesium ions, resulting in improved rate performance and higher accessible capacity.Expand Specific Solutions05 Multi-valent charge storage mechanisms
Magnesium-ion batteries utilize multi-valent charge storage mechanisms that differ from conventional lithium-ion systems. The divalent nature of magnesium ions allows for potentially higher energy density but presents challenges for intercalation kinetics due to stronger electrostatic interactions with host materials. Research focuses on understanding and optimizing these charge storage mechanisms, including intercalation, conversion reactions, and alloying processes, to develop electrode materials that can effectively accommodate magnesium ions while maintaining good electronic conductivity and structural stability.Expand Specific Solutions
Leading Research Institutions and Industrial Developers
Magnesium-ion battery technology is currently in the early development stage, with the market still emerging but showing significant growth potential due to increasing demand for sustainable energy storage solutions. The global competition landscape features primarily academic institutions (Rutgers, MIT, University of Houston, Chongqing University) leading fundamental research on intercalation kinetics and charge storage models, while established companies like Robert Bosch GmbH, Canon, and Murata Manufacturing are beginning to translate this research into commercial applications. Research collaborations between universities and industry players such as Shell are accelerating technological maturity. Key technical challenges remain in understanding ion mobility mechanisms and electrode material optimization, with recent breakthroughs from MIT and Rutgers potentially enabling the next generation of high-performance magnesium-ion batteries with superior energy density and safety profiles compared to lithium-ion alternatives.
University of Houston
Technical Solution: The University of Houston has made significant breakthroughs in magnesium-ion battery technology through their work on non-corrosive electrolytes and compatible cathode materials. Their research team has developed a novel class of non-nucleophilic electrolytes based on magnesium aluminum chloride complex (MACC) that enables reversible Mg plating/stripping without passivation layer formation. This electrolyte innovation addresses one of the fundamental challenges in Mg-ion battery development. For intercalation kinetics, Houston researchers have focused on layered titanium disulfide (TiS2) cathodes that demonstrate relatively fast Mg2+ diffusion with diffusion coefficients reaching 10^-11 cm²/s. Their charge storage models incorporate both solid-state diffusion limitations and interfacial charge transfer resistance, providing a comprehensive understanding of rate-limiting steps. They've also pioneered the use of operando X-ray absorption spectroscopy to track the oxidation state changes during Mg intercalation, revealing that the process often involves multiple redox centers within the host structure. Their kinetic models account for the strong correlation between Mg2+ ions during intercalation, which creates unique diffusion pathways compared to monovalent ion systems.
Strengths: Holistic approach addressing both electrolyte and cathode material challenges simultaneously; strong experimental capabilities for direct observation of intercalation mechanisms. Weaknesses: Their most promising cathode materials still demonstrate relatively low capacity compared to commercial lithium-ion systems; some of their electrolyte solutions remain sensitive to trace water contamination.
Robert Bosch GmbH
Technical Solution: Robert Bosch GmbH has developed a comprehensive approach to magnesium-ion battery technology focusing on practical implementation challenges. Their research team has created proprietary electrolyte formulations based on non-nucleophilic magnesium aluminum chloride complexes in ethereal solvents that demonstrate excellent electrochemical stability windows exceeding 3V. For intercalation kinetics, Bosch has focused on spinel-type oxide cathodes with three-dimensional diffusion pathways that facilitate Mg2+ mobility despite the strong electrostatic interactions. Their charge storage models incorporate both bulk diffusion limitations and surface charge transfer kinetics, with particular attention to the formation and evolution of interfacial layers that can significantly impact performance. Bosch has developed advanced electrochemical impedance spectroscopy protocols specifically tailored to magnesium systems, allowing them to deconvolute different resistive components and identify rate-limiting steps during operation. Their models account for the state-of-charge dependent diffusion coefficients and activation energies, providing a realistic representation of performance under various operating conditions. Notably, Bosch has also addressed the practical engineering aspects of Mg-ion cells, developing electrode formulations with optimized porosity and tortuosity to maximize utilization of active materials despite the inherently slower diffusion kinetics of Mg2+ compared to Li+.
Strengths: Practical engineering approach that addresses both fundamental science and implementation challenges; strong capabilities in cell design and manufacturing that could accelerate commercialization. Weaknesses: Their focus on commercially viable solutions sometimes limits exploration of more exotic materials with potentially higher theoretical performance; their proprietary nature means many technical details remain unpublished.
Critical Patents and Research on Mg-ion Kinetics
Rechargeable magnesium battery
PatentActiveUS20080182176A1
Innovation
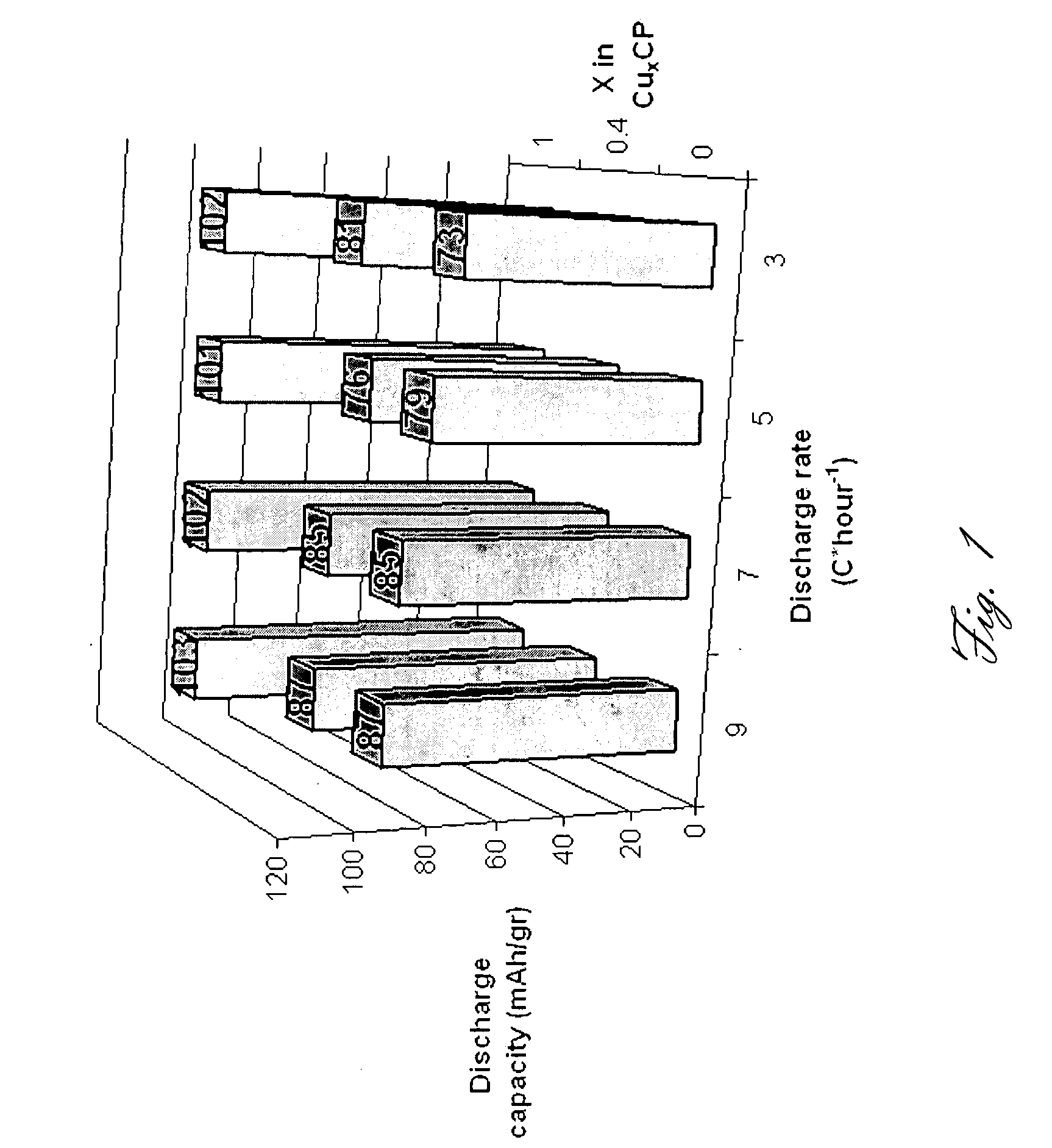
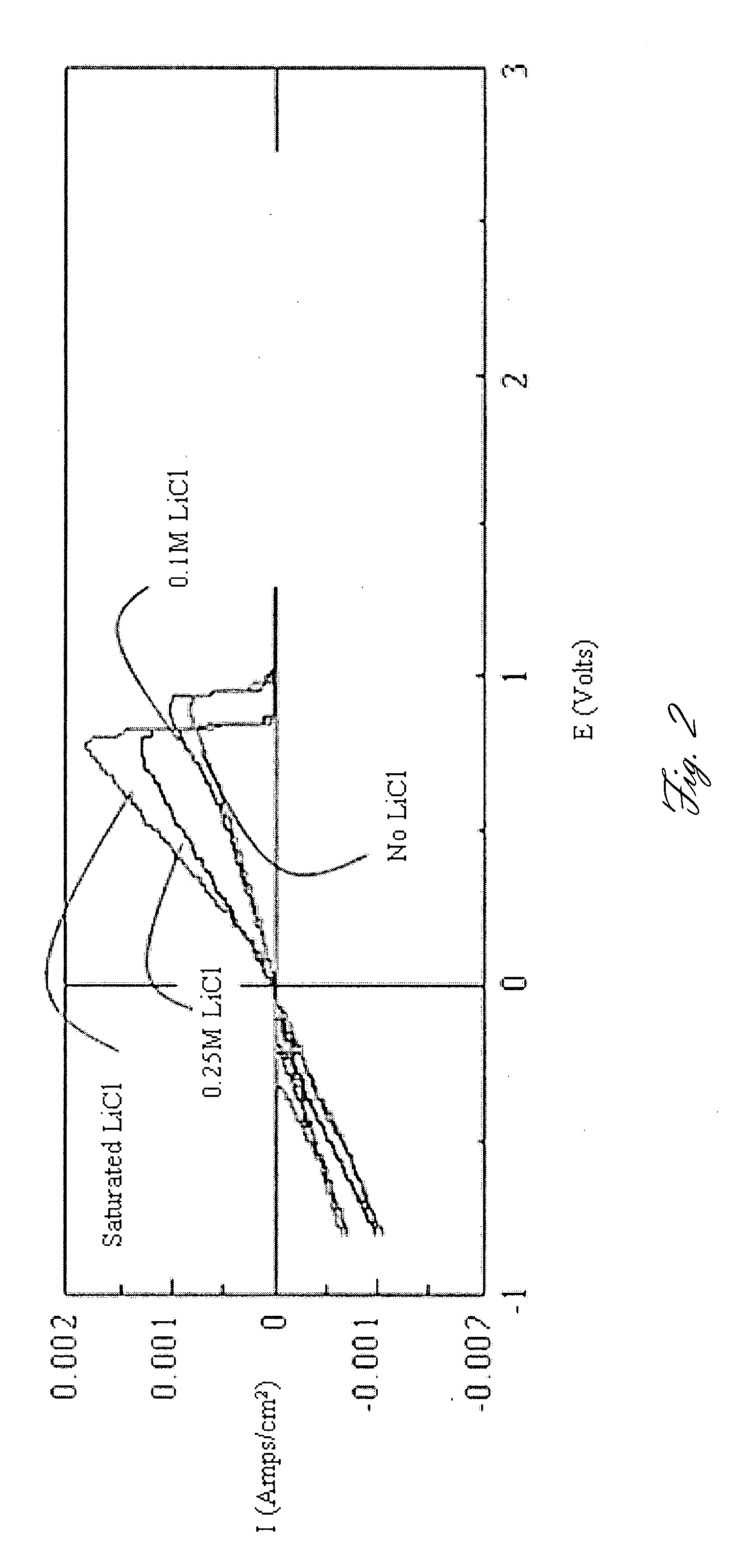
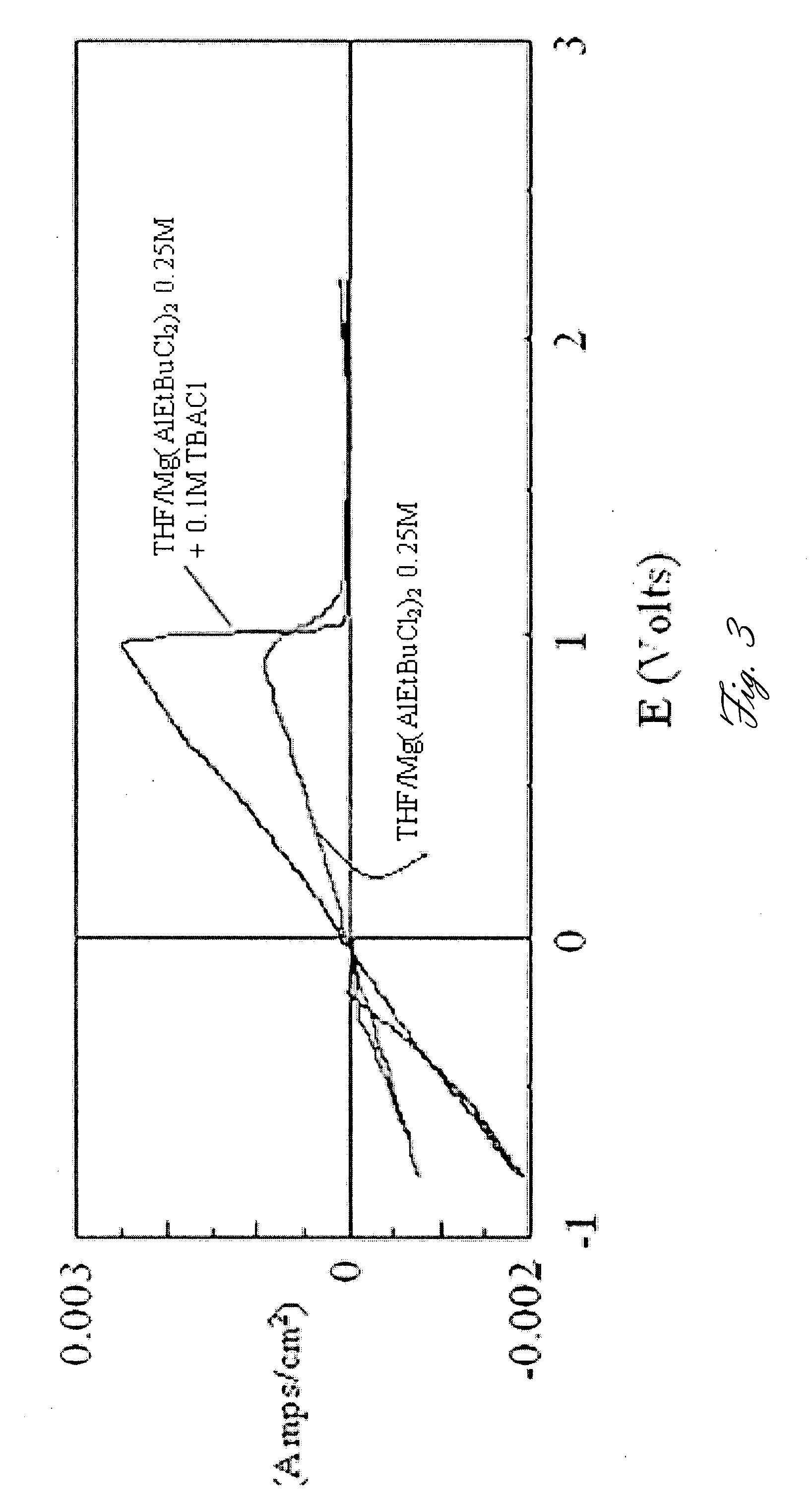
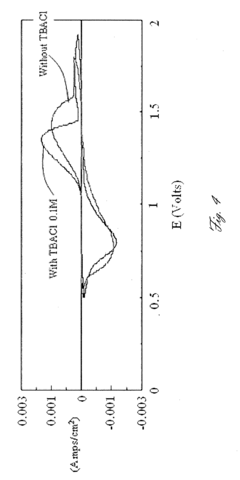
- The use of Chevrel phase compounds like Mo6S8-YSeY and MXMo6S8 with partial Se substitution, combined with electrolytes such as Mg(AlRxCl4-x)2 and inert salts like Tetrabutylamonium-chloride, enhances Mg diffusion kinetics and reversibility, and the addition of LiCl improves conductivity and electrochemical performance.
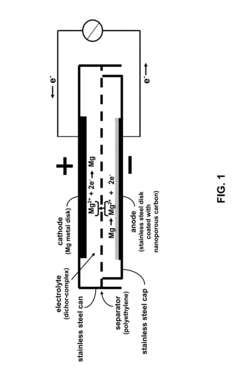
Rechargeable magnesium ion battery with nanoporous-carbon electrode for reversible magnesium ion intercalation
PatentInactiveUS20170294654A1
Innovation
- A rechargeable magnesium battery design featuring a nanoporous carbon electrode with tailored interplanar spacings for reversible magnesium ion intercalation, using a magnesium organohaloaluminate electrolyte to facilitate ion movement and enable high-capacity, high-voltage cathode operation.
Materials Science Innovations for Mg-ion Electrodes
Recent advancements in materials science have opened new frontiers for magnesium-ion battery electrode development. The fundamental challenge in Mg-ion technology lies in the strong electrostatic interaction between Mg2+ ions and host materials, which significantly impedes intercalation kinetics. Traditional cathode materials designed for lithium-ion systems often perform poorly with magnesium due to these sluggish kinetics and structural limitations.
Innovative electrode materials with optimized crystal structures represent a critical breakthrough area. Layered materials with expanded interlayer spacing have demonstrated enhanced Mg2+ diffusion capabilities. Specifically, vanadium-based compounds such as V2O5 and MgxV2O5 have shown promising reversible Mg2+ insertion properties when their structures are properly engineered. The incorporation of water molecules or other spacing agents between layers has proven effective in reducing diffusion barriers.
Nanostructuring approaches have emerged as another vital innovation pathway. Reducing particle dimensions to nanoscale significantly shortens diffusion paths for Mg2+ ions, thereby improving rate capability. Mesoporous architectures, nanotubes, and two-dimensional nanosheets have all demonstrated enhanced electrochemical performance compared to their bulk counterparts. These nanostructured materials provide additional benefits including better accommodation of structural changes during cycling and improved electrolyte accessibility.
Composite electrode formulations represent another promising direction. Combining active materials with conductive additives like graphene, carbon nanotubes, or MXenes has shown remarkable improvements in electronic conductivity and structural stability. These composites often exhibit synergistic effects where the carbon component not only enhances conductivity but also buffers volume changes and sometimes participates in charge storage mechanisms.
Surface modification strategies have proven particularly valuable for Mg-ion electrodes. Atomic layer deposition of ultrathin coatings, surface functionalization with specific chemical groups, and interface engineering techniques have all demonstrated effectiveness in mitigating interfacial resistance. These approaches help prevent unwanted side reactions while facilitating Mg2+ transport across the critical electrode-electrolyte interface.
Computational materials design has accelerated the discovery of novel Mg-ion electrode materials. High-throughput screening methods combined with density functional theory calculations have identified promising candidates with favorable Mg2+ diffusion channels and reasonable voltage profiles. Machine learning approaches are increasingly being employed to predict structure-property relationships and identify materials with optimal combinations of capacity, rate capability, and cycling stability.
Innovative electrode materials with optimized crystal structures represent a critical breakthrough area. Layered materials with expanded interlayer spacing have demonstrated enhanced Mg2+ diffusion capabilities. Specifically, vanadium-based compounds such as V2O5 and MgxV2O5 have shown promising reversible Mg2+ insertion properties when their structures are properly engineered. The incorporation of water molecules or other spacing agents between layers has proven effective in reducing diffusion barriers.
Nanostructuring approaches have emerged as another vital innovation pathway. Reducing particle dimensions to nanoscale significantly shortens diffusion paths for Mg2+ ions, thereby improving rate capability. Mesoporous architectures, nanotubes, and two-dimensional nanosheets have all demonstrated enhanced electrochemical performance compared to their bulk counterparts. These nanostructured materials provide additional benefits including better accommodation of structural changes during cycling and improved electrolyte accessibility.
Composite electrode formulations represent another promising direction. Combining active materials with conductive additives like graphene, carbon nanotubes, or MXenes has shown remarkable improvements in electronic conductivity and structural stability. These composites often exhibit synergistic effects where the carbon component not only enhances conductivity but also buffers volume changes and sometimes participates in charge storage mechanisms.
Surface modification strategies have proven particularly valuable for Mg-ion electrodes. Atomic layer deposition of ultrathin coatings, surface functionalization with specific chemical groups, and interface engineering techniques have all demonstrated effectiveness in mitigating interfacial resistance. These approaches help prevent unwanted side reactions while facilitating Mg2+ transport across the critical electrode-electrolyte interface.
Computational materials design has accelerated the discovery of novel Mg-ion electrode materials. High-throughput screening methods combined with density functional theory calculations have identified promising candidates with favorable Mg2+ diffusion channels and reasonable voltage profiles. Machine learning approaches are increasingly being employed to predict structure-property relationships and identify materials with optimal combinations of capacity, rate capability, and cycling stability.
Sustainability and Resource Considerations for Mg-based Technologies
The sustainability profile of magnesium-ion battery technologies presents significant advantages over conventional lithium-ion systems. Magnesium is the eighth most abundant element in Earth's crust, with reserves approximately 3000 times greater than lithium, ensuring long-term resource availability. This abundance translates to lower extraction costs and reduced geopolitical supply risks, as magnesium resources are more evenly distributed globally compared to lithium's concentration in regions like South America's "Lithium Triangle."
From an environmental perspective, magnesium extraction typically generates a smaller ecological footprint than lithium mining operations. Traditional lithium extraction requires extensive water usage—approximately 500,000 gallons per ton of lithium—in often water-scarce regions, while magnesium can be sourced from seawater through less resource-intensive processes. The carbon footprint associated with magnesium production has been estimated at 10-15% lower than equivalent lithium processing when considering full lifecycle assessments.
Recycling infrastructure represents another critical sustainability dimension. Current research indicates that magnesium-based battery components may offer superior recyclability compared to lithium counterparts. Laboratory studies demonstrate recovery rates exceeding 90% for magnesium from spent battery materials using hydrometallurgical processes, compared to typical 50-70% recovery rates for lithium using similar methods. However, commercial-scale recycling systems for magnesium batteries remain underdeveloped, requiring significant investment to realize this theoretical advantage.
Safety considerations further enhance the sustainability profile of magnesium-ion technologies. Unlike lithium-ion batteries, magnesium systems demonstrate substantially reduced fire and explosion risks due to magnesium's lower reactivity with atmospheric components. This inherent safety reduces the need for complex thermal management systems, potentially decreasing overall material requirements and simplifying end-of-life handling.
Economic sustainability analysis reveals that while current magnesium battery production costs exceed lithium counterparts by approximately 30-40%, long-term projections suggest this gap will narrow as manufacturing scales. The primary cost drivers include electrolyte complexity and cathode material synthesis, both areas where recent research has identified promising cost-reduction pathways through alternative material formulations and simplified production techniques.
Future sustainability improvements for magnesium-ion technologies will likely focus on developing aqueous electrolyte systems to replace current organic solvents, reducing toxicity and environmental impact. Additionally, research into biomass-derived carbon materials as potential electrode components offers pathways to further reduce the technology's environmental footprint while potentially enhancing performance characteristics.
From an environmental perspective, magnesium extraction typically generates a smaller ecological footprint than lithium mining operations. Traditional lithium extraction requires extensive water usage—approximately 500,000 gallons per ton of lithium—in often water-scarce regions, while magnesium can be sourced from seawater through less resource-intensive processes. The carbon footprint associated with magnesium production has been estimated at 10-15% lower than equivalent lithium processing when considering full lifecycle assessments.
Recycling infrastructure represents another critical sustainability dimension. Current research indicates that magnesium-based battery components may offer superior recyclability compared to lithium counterparts. Laboratory studies demonstrate recovery rates exceeding 90% for magnesium from spent battery materials using hydrometallurgical processes, compared to typical 50-70% recovery rates for lithium using similar methods. However, commercial-scale recycling systems for magnesium batteries remain underdeveloped, requiring significant investment to realize this theoretical advantage.
Safety considerations further enhance the sustainability profile of magnesium-ion technologies. Unlike lithium-ion batteries, magnesium systems demonstrate substantially reduced fire and explosion risks due to magnesium's lower reactivity with atmospheric components. This inherent safety reduces the need for complex thermal management systems, potentially decreasing overall material requirements and simplifying end-of-life handling.
Economic sustainability analysis reveals that while current magnesium battery production costs exceed lithium counterparts by approximately 30-40%, long-term projections suggest this gap will narrow as manufacturing scales. The primary cost drivers include electrolyte complexity and cathode material synthesis, both areas where recent research has identified promising cost-reduction pathways through alternative material formulations and simplified production techniques.
Future sustainability improvements for magnesium-ion technologies will likely focus on developing aqueous electrolyte systems to replace current organic solvents, reducing toxicity and environmental impact. Additionally, research into biomass-derived carbon materials as potential electrode components offers pathways to further reduce the technology's environmental footprint while potentially enhancing performance characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!