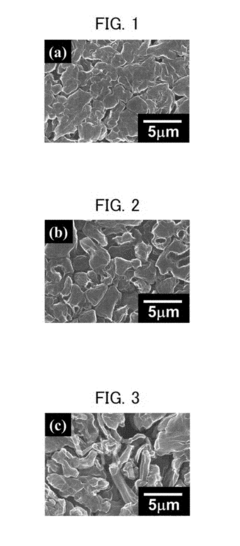
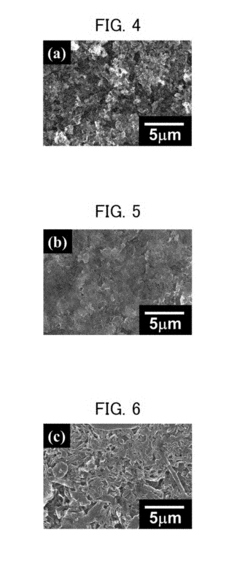
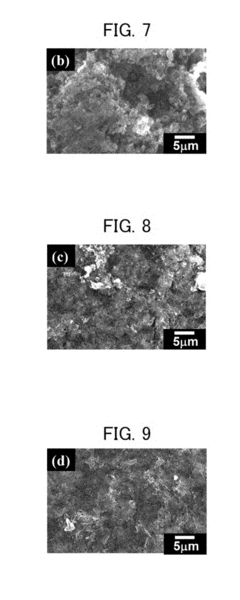
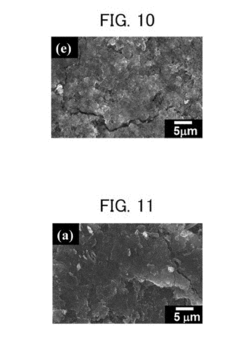
Magnesium-ion battery dendrite suppression mechanisms research
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Dendrite Formation Background & Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in energy density, safety, and cost-effectiveness. The development of these batteries traces back to the early 2000s when researchers began exploring multivalent ion systems as energy storage solutions. The evolution of this technology has been marked by significant advancements in electrode materials, electrolyte formulations, and cell design, yet dendrite formation remains a critical challenge impeding commercialization.
Dendrites are needle-like structures that form during the electrodeposition process of magnesium ions, potentially causing internal short circuits and safety hazards. Unlike lithium-ion batteries, where dendrite formation mechanisms are well-documented, the understanding of dendrite growth in magnesium systems remains limited despite their theoretical dendrite-resistant properties due to higher surface diffusion rates.
The technical trajectory shows an increasing focus on fundamental electrochemical processes at the electrode-electrolyte interface. Early research primarily concentrated on electrolyte development, while recent efforts have shifted toward understanding the nucleation and growth mechanisms of magnesium deposits. This evolution reflects the recognition that dendrite suppression requires a comprehensive approach addressing multiple aspects of the electrochemical system.
Current technical objectives in this field include elucidating the precise mechanisms of dendrite initiation and propagation in magnesium systems, developing in-situ characterization techniques to observe dendrite formation in real-time, and establishing predictive models that can guide the design of dendrite-resistant battery components. Additionally, researchers aim to identify critical parameters that influence dendrite morphology, such as current density, temperature, electrolyte composition, and surface properties of the electrode.
The ultimate goal of this research is to develop practical strategies for dendrite suppression that can be implemented in commercial magnesium-ion batteries. These strategies may include engineered electrode surfaces, advanced electrolyte formulations with optimized transport properties, or novel cell designs that mitigate dendrite growth through controlled ion flux. Success in this endeavor would remove a significant barrier to the widespread adoption of magnesium-ion technology.
Understanding and controlling dendrite formation is not merely an academic pursuit but a prerequisite for realizing the full potential of magnesium-ion batteries in applications ranging from portable electronics to grid-scale energy storage. As global demand for sustainable energy solutions continues to rise, the development of dendrite-free magnesium-ion batteries represents a critical step toward more efficient, safer, and more economical energy storage systems.
Dendrites are needle-like structures that form during the electrodeposition process of magnesium ions, potentially causing internal short circuits and safety hazards. Unlike lithium-ion batteries, where dendrite formation mechanisms are well-documented, the understanding of dendrite growth in magnesium systems remains limited despite their theoretical dendrite-resistant properties due to higher surface diffusion rates.
The technical trajectory shows an increasing focus on fundamental electrochemical processes at the electrode-electrolyte interface. Early research primarily concentrated on electrolyte development, while recent efforts have shifted toward understanding the nucleation and growth mechanisms of magnesium deposits. This evolution reflects the recognition that dendrite suppression requires a comprehensive approach addressing multiple aspects of the electrochemical system.
Current technical objectives in this field include elucidating the precise mechanisms of dendrite initiation and propagation in magnesium systems, developing in-situ characterization techniques to observe dendrite formation in real-time, and establishing predictive models that can guide the design of dendrite-resistant battery components. Additionally, researchers aim to identify critical parameters that influence dendrite morphology, such as current density, temperature, electrolyte composition, and surface properties of the electrode.
The ultimate goal of this research is to develop practical strategies for dendrite suppression that can be implemented in commercial magnesium-ion batteries. These strategies may include engineered electrode surfaces, advanced electrolyte formulations with optimized transport properties, or novel cell designs that mitigate dendrite growth through controlled ion flux. Success in this endeavor would remove a significant barrier to the widespread adoption of magnesium-ion technology.
Understanding and controlling dendrite formation is not merely an academic pursuit but a prerequisite for realizing the full potential of magnesium-ion batteries in applications ranging from portable electronics to grid-scale energy storage. As global demand for sustainable energy solutions continues to rise, the development of dendrite-free magnesium-ion batteries represents a critical step toward more efficient, safer, and more economical energy storage systems.
Market Analysis for Dendrite-Free Mg-ion Batteries
The global market for magnesium-ion batteries is experiencing significant growth potential, primarily driven by the increasing demand for safer and more efficient energy storage solutions. Current lithium-ion battery technologies face limitations in terms of safety, cost, and resource availability, creating a substantial market opportunity for alternative battery chemistries like magnesium-ion batteries, particularly those with dendrite suppression capabilities.
Market research indicates that the energy storage sector is projected to grow at a compound annual growth rate (CAGR) of approximately 20% through 2030, with grid storage and electric vehicles representing the largest application segments. Within this broader market, dendrite-free magnesium-ion batteries could capture a significant share due to their inherent safety advantages and potential for higher energy density compared to conventional lithium-ion batteries.
The automotive industry represents a particularly promising market for dendrite-free magnesium-ion batteries. As electric vehicle adoption accelerates globally, manufacturers are actively seeking battery technologies that offer improved safety profiles and reduced fire risks. Magnesium-ion batteries with effective dendrite suppression mechanisms address these concerns directly, positioning them as valuable alternatives in this high-growth segment.
Consumer electronics constitutes another substantial market opportunity, where safety concerns regarding current lithium-ion batteries have led to numerous product recalls and significant financial losses for manufacturers. The potential for dendrite-free magnesium-ion batteries to eliminate these safety risks creates strong market pull in this sector.
Geographically, Asia-Pacific currently dominates the advanced battery market landscape, with China, Japan, and South Korea leading in both production capacity and research initiatives. However, significant research and development activities in North America and Europe indicate growing interest in next-generation battery technologies, including magnesium-ion systems.
Market barriers include the current cost premium associated with new battery technologies and the established manufacturing infrastructure for lithium-ion batteries. However, increasing concerns about lithium supply chain vulnerabilities and price volatility are driving interest in magnesium-based alternatives, as magnesium is more abundant and geographically distributed than lithium.
Industry analysts predict that as dendrite suppression technologies mature and manufacturing scales up, magnesium-ion batteries could achieve cost parity with lithium-ion batteries within the next decade, potentially capturing up to 15% of the energy storage market by 2035. This represents a substantial commercial opportunity for companies that can successfully develop and commercialize dendrite-free magnesium-ion battery technologies.
Market research indicates that the energy storage sector is projected to grow at a compound annual growth rate (CAGR) of approximately 20% through 2030, with grid storage and electric vehicles representing the largest application segments. Within this broader market, dendrite-free magnesium-ion batteries could capture a significant share due to their inherent safety advantages and potential for higher energy density compared to conventional lithium-ion batteries.
The automotive industry represents a particularly promising market for dendrite-free magnesium-ion batteries. As electric vehicle adoption accelerates globally, manufacturers are actively seeking battery technologies that offer improved safety profiles and reduced fire risks. Magnesium-ion batteries with effective dendrite suppression mechanisms address these concerns directly, positioning them as valuable alternatives in this high-growth segment.
Consumer electronics constitutes another substantial market opportunity, where safety concerns regarding current lithium-ion batteries have led to numerous product recalls and significant financial losses for manufacturers. The potential for dendrite-free magnesium-ion batteries to eliminate these safety risks creates strong market pull in this sector.
Geographically, Asia-Pacific currently dominates the advanced battery market landscape, with China, Japan, and South Korea leading in both production capacity and research initiatives. However, significant research and development activities in North America and Europe indicate growing interest in next-generation battery technologies, including magnesium-ion systems.
Market barriers include the current cost premium associated with new battery technologies and the established manufacturing infrastructure for lithium-ion batteries. However, increasing concerns about lithium supply chain vulnerabilities and price volatility are driving interest in magnesium-based alternatives, as magnesium is more abundant and geographically distributed than lithium.
Industry analysts predict that as dendrite suppression technologies mature and manufacturing scales up, magnesium-ion batteries could achieve cost parity with lithium-ion batteries within the next decade, potentially capturing up to 15% of the energy storage market by 2035. This represents a substantial commercial opportunity for companies that can successfully develop and commercialize dendrite-free magnesium-ion battery technologies.
Current Challenges in Mg Dendrite Suppression Technology
Despite significant advancements in magnesium-ion battery technology, dendrite formation remains a critical challenge impeding commercial viability. Unlike lithium-ion batteries, magnesium dendrite formation follows different mechanisms due to the divalent nature of Mg2+ ions and their distinct electrochemical properties. Current research indicates that dendrite growth in Mg batteries occurs primarily through non-uniform deposition at high current densities, creating surface irregularities that evolve into dendrites.
The electrolyte composition presents a major challenge, as most conventional electrolytes form passivation layers on magnesium anodes, leading to localized deposition and subsequent dendrite growth. Chloride-containing electrolytes, while offering improved deposition kinetics, often promote dendrite formation due to their aggressive nature toward electrode surfaces. Additionally, the high reduction potential of magnesium electrolytes limits the selection of suitable solvents and salts that can simultaneously enable efficient Mg2+ transport and suppress dendrite formation.
Interface stability between the magnesium anode and electrolyte represents another significant hurdle. Unlike lithium batteries where the solid electrolyte interphase (SEI) typically protects against dendrite growth, magnesium interfaces often develop non-uniform, resistive layers that concentrate current at defect sites, accelerating dendrite nucleation. The chemistry of these interfacial layers remains poorly understood, complicating efforts to engineer stable interfaces.
Current density distribution across the electrode surface significantly influences dendrite formation. Geometric irregularities, impurities, and grain boundaries on magnesium anodes create preferential nucleation sites for dendrites. Controlling surface morphology at the nanoscale has proven challenging, particularly under the practical current densities required for commercial applications.
Temperature management presents additional complications, as magnesium deposition kinetics are highly temperature-dependent. At lower temperatures, increased electrolyte viscosity and reduced ion mobility create conditions favorable for dendrite growth. Conversely, elevated temperatures can accelerate side reactions that compromise interface stability.
The lack of standardized testing protocols for evaluating dendrite formation in magnesium systems hampers progress in this field. Different research groups employ varying methodologies, making direct comparisons between suppression strategies difficult. Advanced in-situ characterization techniques capable of monitoring dendrite nucleation and growth in real-time remain limited, constraining mechanistic understanding.
Computational modeling of magnesium dendrite formation lags behind experimental work, with most models failing to capture the complex interplay between electrolyte chemistry, interface phenomena, and electrodeposition kinetics. This gap between theoretical understanding and experimental observation slows the development of effective suppression strategies.
The electrolyte composition presents a major challenge, as most conventional electrolytes form passivation layers on magnesium anodes, leading to localized deposition and subsequent dendrite growth. Chloride-containing electrolytes, while offering improved deposition kinetics, often promote dendrite formation due to their aggressive nature toward electrode surfaces. Additionally, the high reduction potential of magnesium electrolytes limits the selection of suitable solvents and salts that can simultaneously enable efficient Mg2+ transport and suppress dendrite formation.
Interface stability between the magnesium anode and electrolyte represents another significant hurdle. Unlike lithium batteries where the solid electrolyte interphase (SEI) typically protects against dendrite growth, magnesium interfaces often develop non-uniform, resistive layers that concentrate current at defect sites, accelerating dendrite nucleation. The chemistry of these interfacial layers remains poorly understood, complicating efforts to engineer stable interfaces.
Current density distribution across the electrode surface significantly influences dendrite formation. Geometric irregularities, impurities, and grain boundaries on magnesium anodes create preferential nucleation sites for dendrites. Controlling surface morphology at the nanoscale has proven challenging, particularly under the practical current densities required for commercial applications.
Temperature management presents additional complications, as magnesium deposition kinetics are highly temperature-dependent. At lower temperatures, increased electrolyte viscosity and reduced ion mobility create conditions favorable for dendrite growth. Conversely, elevated temperatures can accelerate side reactions that compromise interface stability.
The lack of standardized testing protocols for evaluating dendrite formation in magnesium systems hampers progress in this field. Different research groups employ varying methodologies, making direct comparisons between suppression strategies difficult. Advanced in-situ characterization techniques capable of monitoring dendrite nucleation and growth in real-time remain limited, constraining mechanistic understanding.
Computational modeling of magnesium dendrite formation lags behind experimental work, with most models failing to capture the complex interplay between electrolyte chemistry, interface phenomena, and electrodeposition kinetics. This gap between theoretical understanding and experimental observation slows the development of effective suppression strategies.
State-of-the-Art Dendrite Suppression Strategies
01 Electrolyte additives for dendrite suppression
Various additives can be incorporated into the electrolyte of magnesium-ion batteries to suppress dendrite formation. These additives can modify the solid electrolyte interphase (SEI) layer, improve ion transport, and promote uniform magnesium deposition. Common additives include fluorinated compounds, ionic liquids, and certain salts that can effectively inhibit dendrite growth during charging and discharging cycles, thereby enhancing battery safety and longevity.- Electrolyte additives for dendrite suppression: Various electrolyte additives can be incorporated into magnesium-ion batteries to suppress dendrite formation during charging and discharging cycles. These additives modify the solid electrolyte interphase (SEI) layer formation, promote uniform magnesium deposition, and prevent dendritic growth. Common additives include fluorinated compounds, ionic liquids, and certain salts that can stabilize the electrode-electrolyte interface and enhance cycling performance.
- Structured electrode designs: Specialized electrode architectures can effectively mitigate dendrite formation in magnesium-ion batteries. These designs include three-dimensional structures, porous frameworks, and patterned surfaces that distribute current density more evenly across the electrode surface. By controlling the nucleation and growth of magnesium deposits, these structured electrodes promote uniform metal deposition and reduce the likelihood of dendrite formation, thereby enhancing battery safety and cycle life.
- Protective coatings and artificial SEI layers: Applying protective coatings or artificial solid electrolyte interphase (SEI) layers on magnesium metal anodes can effectively suppress dendrite growth. These coatings serve as physical barriers that guide uniform magnesium deposition and prevent localized high current densities. Materials used include polymers, ceramics, and composite layers that are magnesium-ion conductive but can mechanically inhibit dendrite penetration, thereby improving battery safety and longevity.
- Advanced electrolyte formulations: Novel electrolyte formulations play a crucial role in suppressing dendrite formation in magnesium-ion batteries. These include non-nucleophilic electrolytes, dual-salt systems, and high-concentration electrolytes that modify the solvation structure of magnesium ions. By altering the ion transport mechanisms and deposition kinetics, these electrolytes promote more uniform magnesium plating and stripping, reducing the tendency for dendrite growth and improving cycling stability.
- Interface engineering approaches: Interface engineering between the electrode and electrolyte is a critical strategy for dendrite suppression in magnesium-ion batteries. This involves modifying the electrode surface chemistry, introducing functional interlayers, or incorporating gradient structures that control the ion flux and deposition behavior. These approaches help to achieve homogeneous current distribution, regulate the nucleation process, and prevent the formation of high-energy sites where dendrites typically initiate.
02 Solid-state electrolytes for dendrite prevention
Solid-state electrolytes offer a physical barrier to dendrite growth in magnesium-ion batteries. These materials, including ceramic electrolytes, polymer electrolytes, and composite systems, provide mechanical resistance against dendrite penetration while maintaining sufficient magnesium ion conductivity. The rigid structure of solid electrolytes helps to ensure uniform ion distribution and deposition, significantly reducing the risk of dendrite formation compared to liquid electrolyte systems.Expand Specific Solutions03 Electrode surface modification techniques
Modifying the surface of magnesium battery electrodes can effectively suppress dendrite formation. Techniques include coating electrodes with protective layers, introducing nanostructured materials, or applying surface treatments that alter the electrode's physical and chemical properties. These modifications can control the nucleation and growth of magnesium deposits, promote uniform deposition, and create a more stable interface between the electrode and electrolyte, thereby inhibiting dendrite formation.Expand Specific Solutions04 Advanced electrode architectures
Innovative electrode designs and architectures can significantly reduce dendrite formation in magnesium-ion batteries. Three-dimensional electrode structures, porous frameworks, and gradient designs help distribute current density more evenly across the electrode surface. These advanced architectures provide more nucleation sites for magnesium deposition and facilitate uniform ion flux, which prevents localized high current densities that typically lead to dendrite growth.Expand Specific Solutions05 Current density control and charging protocols
Implementing specialized charging protocols and controlling current density are effective strategies for suppressing dendrite growth in magnesium-ion batteries. Pulse charging, current density limitations, and temperature-controlled charging can promote more uniform magnesium deposition. These approaches manage the kinetics of ion transport and deposition, preventing the rapid, uneven growth that leads to dendrite formation, thereby extending battery life and improving safety performance.Expand Specific Solutions
Leading Research Groups and Industry Players
Magnesium-ion battery dendrite suppression research is currently in an early development stage, with growing interest due to magnesium's theoretical advantages over lithium-ion technology. The market remains relatively small but is expanding as companies seek safer, higher-energy-density alternatives. Technical maturity varies significantly across key players, with automotive manufacturers like Nissan, Renault, and Hyundai investing substantially in this field. Research institutions including Beijing Institute of Technology and Northwestern Polytechnical University are making significant contributions to fundamental understanding. Battery manufacturers such as CATL, LG Chem, and Panasonic are developing practical applications, while specialized companies like 3DOM Alliance and Iontra focus on innovative dendrite suppression technologies, indicating a diverse competitive landscape with both established corporations and emerging specialists.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology (CATL) has developed innovative approaches to magnesium-ion battery dendrite suppression through their dual-salt electrolyte system. Their technology utilizes a combination of magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) and magnesium chloride (MgCl2) in ether-based solvents to form a stable and uniform solid electrolyte interphase (SEI) layer. This SEI layer effectively prevents direct contact between magnesium metal and the electrolyte, significantly reducing dendrite formation. CATL's research has demonstrated that their electrolyte formulation enables homogeneous magnesium deposition with a Coulombic efficiency exceeding 99% over 500 cycles. Additionally, they've incorporated nanoscale ceramic particles into their electrolyte to create artificial SEI layers that further inhibit dendrite growth by modifying the surface energy and ion flux distribution at the electrode-electrolyte interface.
Strengths: Superior cycling stability with high Coulombic efficiency; scalable manufacturing process compatible with existing production lines; effective dendrite suppression without sacrificing energy density. Weaknesses: Higher cost compared to conventional lithium-ion batteries; limited temperature operating range; potential challenges with electrolyte stability in long-term applications.
UT-Battelle LLC
Technical Solution: UT-Battelle, operating Oak Ridge National Laboratory (ORNL), has conducted extensive research on magnesium-ion battery dendrite suppression through their integrated computational and experimental approach. Their work focuses on understanding the fundamental mechanisms of dendrite formation at atomic and mesoscopic scales using advanced characterization techniques and multi-scale modeling. UT-Battelle has developed specialized in situ transmission electron microscopy methods to directly observe magnesium deposition processes in real-time, providing unprecedented insights into dendrite nucleation and growth. Their research has identified critical parameters controlling dendrite formation, including electrolyte composition, current density distribution, and surface defect concentration. Based on these insights, they've engineered novel electrolyte formulations incorporating specific additives that modify the solvation structure of magnesium ions and promote more uniform deposition. UT-Battelle has also pioneered the development of artificial solid-electrolyte interphases using atomic layer deposition techniques to create ultrathin, conformal protective layers on magnesium anodes that effectively suppress dendrite formation while maintaining high ionic conductivity.
Strengths: World-class characterization capabilities and computational resources; holistic understanding of dendrite formation mechanisms across multiple length scales; strong collaboration network with industry partners. Weaknesses: Focus primarily on fundamental research rather than commercial applications; technologies may require significant further development for practical implementation; potential challenges with scaling specialized characterization techniques to production environments.
Critical Patents and Research on Mg Dendrite Control
Method for producing all-solid-state battery
PatentPendingUS20250174738A1
Innovation
- A method for producing an all-solid state battery that includes a power generating element with a positive electrode active material layer, a negative electrode current collector with lithium metal deposition, a solid electrolyte layer, and a negative electrode intermediate layer containing materials capable of occluding and releasing lithium ions or alloying with lithium. The battery undergoes a two-step charging process with specific current density conditions to enhance lithium-ion conductivity and suppress dendrite growth.
Separator for metal secondary batteries
PatentInactiveUS20170279101A1
Innovation
- A porous separator with a polymer electrolyte layer formed on a polyimide film is used to homogenize the electric field at the negative electrode, preventing dendrite growth by employing a 3-dimensional ordered macroporous structure and a polymer electrolyte layer.
Safety and Performance Implications of Dendrite Formation
Dendrite formation in magnesium-ion batteries presents significant safety and performance challenges that must be addressed for widespread commercial adoption. The growth of dendritic structures during charging cycles creates potential internal short circuits, which can lead to catastrophic thermal runaway events. Unlike lithium-ion batteries, magnesium dendrites typically form more slowly but still pose serious safety concerns, particularly in high-energy density applications where thermal management is already challenging.
Performance degradation resulting from dendrite formation manifests in several ways. Capacity fade accelerates as dendrites consume active magnesium, reducing the available material for electrochemical reactions. Coulombic efficiency decreases progressively as more energy is diverted to dendrite formation rather than useful charge storage. Additionally, impedance rise occurs as dendrites create irregular interfaces that impede ion transport, leading to power capability reduction over time.
Cycle life limitations represent another critical implication of dendrite growth. Research indicates that magnesium batteries experiencing dendrite formation may lose 20-30% of their initial capacity within 100-200 cycles, significantly underperforming their theoretical potential. This premature aging effect directly impacts the economic viability of magnesium-ion technology for applications requiring long service life.
The mechanical stress induced by dendrite growth further compounds these issues. As dendrites penetrate the separator material, they create microscopic deformations that compromise structural integrity. This mechanical degradation can accelerate even when the battery is not in active use, presenting challenges for long-term storage stability.
From an industrial perspective, dendrite formation significantly impacts manufacturing processes and quality control requirements. Production facilities must implement sophisticated monitoring systems to detect early signs of dendrite nucleation, adding complexity and cost to manufacturing operations. The unpredictability of dendrite growth patterns also complicates warranty and reliability predictions for commercial products.
Recent research has quantified the economic impact of dendrite-related failures, estimating that addressing dendrite formation could reduce lifetime battery costs by 15-25% through improved longevity and reduced safety engineering requirements. This economic incentive has accelerated research into novel electrolyte formulations and interface engineering approaches specifically targeting dendrite suppression mechanisms in magnesium-ion systems.
Performance degradation resulting from dendrite formation manifests in several ways. Capacity fade accelerates as dendrites consume active magnesium, reducing the available material for electrochemical reactions. Coulombic efficiency decreases progressively as more energy is diverted to dendrite formation rather than useful charge storage. Additionally, impedance rise occurs as dendrites create irregular interfaces that impede ion transport, leading to power capability reduction over time.
Cycle life limitations represent another critical implication of dendrite growth. Research indicates that magnesium batteries experiencing dendrite formation may lose 20-30% of their initial capacity within 100-200 cycles, significantly underperforming their theoretical potential. This premature aging effect directly impacts the economic viability of magnesium-ion technology for applications requiring long service life.
The mechanical stress induced by dendrite growth further compounds these issues. As dendrites penetrate the separator material, they create microscopic deformations that compromise structural integrity. This mechanical degradation can accelerate even when the battery is not in active use, presenting challenges for long-term storage stability.
From an industrial perspective, dendrite formation significantly impacts manufacturing processes and quality control requirements. Production facilities must implement sophisticated monitoring systems to detect early signs of dendrite nucleation, adding complexity and cost to manufacturing operations. The unpredictability of dendrite growth patterns also complicates warranty and reliability predictions for commercial products.
Recent research has quantified the economic impact of dendrite-related failures, estimating that addressing dendrite formation could reduce lifetime battery costs by 15-25% through improved longevity and reduced safety engineering requirements. This economic incentive has accelerated research into novel electrolyte formulations and interface engineering approaches specifically targeting dendrite suppression mechanisms in magnesium-ion systems.
Materials Science Advancements for Mg Electrodes
Recent advancements in materials science have significantly contributed to addressing the dendrite formation challenges in magnesium-ion batteries. The development of novel electrode materials with optimized microstructures has emerged as a critical factor in suppressing dendrite growth. Researchers have focused on creating nanostructured magnesium electrodes with controlled porosity and grain boundaries, which effectively distribute current density and minimize localized ion deposition that typically leads to dendrite initiation.
Surface modification techniques have proven particularly effective in enhancing the electrochemical performance of magnesium electrodes. Atomic layer deposition (ALD) and molecular layer deposition (MLD) methods enable the creation of ultrathin protective layers that maintain ion conductivity while preventing side reactions with electrolytes. These protective coatings, often composed of metal oxides or fluorides, create artificial solid electrolyte interphases (SEI) that guide uniform magnesium deposition.
Composite electrode designs incorporating carbon-based materials have shown promising results in dendrite suppression. The integration of graphene, carbon nanotubes, or mesoporous carbon with magnesium creates hierarchical structures that facilitate homogeneous ion flux and mechanical stability. These composites effectively reduce the local current density hotspots that typically initiate dendrite formation while maintaining high specific capacity and rate capability.
Alloying strategies represent another significant advancement, where magnesium is combined with elements such as bismuth, tin, or antimony to form intermetallic compounds. These alloys demonstrate modified surface energetics and deposition kinetics that inherently resist dendritic growth. The controlled phase transformation during cycling provides additional mechanisms to maintain structural integrity and prevent morphological instabilities.
3D electrode architectures have emerged as a revolutionary approach to dendrite suppression. By designing electrodes with precise three-dimensional structures, researchers can control ion transport pathways and create physical barriers to dendrite propagation. Techniques such as 3D printing, template-assisted synthesis, and directed assembly enable the fabrication of complex electrode geometries with optimized surface area and mechanical properties.
Self-healing materials represent the cutting edge of electrode design, incorporating dynamic components that can respond to early-stage dendrite formation. These materials contain functional groups or nanoparticles that migrate to potential dendrite nucleation sites, effectively smoothing the electrode surface during operation and preventing the growth of protrusions that would otherwise develop into dendrites.
Surface modification techniques have proven particularly effective in enhancing the electrochemical performance of magnesium electrodes. Atomic layer deposition (ALD) and molecular layer deposition (MLD) methods enable the creation of ultrathin protective layers that maintain ion conductivity while preventing side reactions with electrolytes. These protective coatings, often composed of metal oxides or fluorides, create artificial solid electrolyte interphases (SEI) that guide uniform magnesium deposition.
Composite electrode designs incorporating carbon-based materials have shown promising results in dendrite suppression. The integration of graphene, carbon nanotubes, or mesoporous carbon with magnesium creates hierarchical structures that facilitate homogeneous ion flux and mechanical stability. These composites effectively reduce the local current density hotspots that typically initiate dendrite formation while maintaining high specific capacity and rate capability.
Alloying strategies represent another significant advancement, where magnesium is combined with elements such as bismuth, tin, or antimony to form intermetallic compounds. These alloys demonstrate modified surface energetics and deposition kinetics that inherently resist dendritic growth. The controlled phase transformation during cycling provides additional mechanisms to maintain structural integrity and prevent morphological instabilities.
3D electrode architectures have emerged as a revolutionary approach to dendrite suppression. By designing electrodes with precise three-dimensional structures, researchers can control ion transport pathways and create physical barriers to dendrite propagation. Techniques such as 3D printing, template-assisted synthesis, and directed assembly enable the fabrication of complex electrode geometries with optimized surface area and mechanical properties.
Self-healing materials represent the cutting edge of electrode design, incorporating dynamic components that can respond to early-stage dendrite formation. These materials contain functional groups or nanoparticles that migrate to potential dendrite nucleation sites, effectively smoothing the electrode surface during operation and preventing the growth of protrusions that would otherwise develop into dendrites.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!