Magnesium-ion battery solid electrolyte development and performance analysis
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-ion Battery Solid Electrolyte Background and Objectives
Magnesium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of magnesium-ion battery technology can be traced back to the early 1990s, but significant progress has been made only in the past decade. The evolution of this technology has been primarily driven by the need for safer, more sustainable, and higher energy density energy storage solutions to meet the growing demands of electric vehicles, renewable energy storage, and portable electronics.
The fundamental challenge in magnesium-ion battery development has been the sluggish kinetics of magnesium ions in conventional electrolytes and electrode materials. Unlike lithium ions, magnesium ions carry a divalent charge, resulting in stronger electrostatic interactions with host lattices and slower diffusion rates. This has led researchers to focus on developing novel solid electrolytes that can facilitate efficient magnesium-ion transport.
Solid electrolytes for magnesium-ion batteries have gained attention due to their potential to overcome the limitations of liquid electrolytes, including safety concerns, narrow electrochemical stability windows, and compatibility issues with magnesium metal anodes. The technological trajectory has shifted from simple magnesium salts dissolved in organic solvents to more sophisticated solid-state systems, including polymer electrolytes, ceramic electrolytes, and hybrid systems.
Recent technological trends indicate a growing interest in inorganic solid electrolytes, particularly those based on magnesium-conducting ceramics such as magnesium borohydrides, magnesium phosphates, and magnesium-doped fast-ion conductors. These materials offer promising ionic conductivities at room temperature and enhanced electrochemical stability compared to their liquid counterparts.
The primary technical objectives for magnesium-ion solid electrolyte development include achieving room temperature ionic conductivity exceeding 10^-4 S/cm, maintaining electrochemical stability within a wide voltage window (0-4V vs. Mg/Mg^2+), ensuring compatibility with magnesium metal anodes, and demonstrating long-term cycling stability. Additionally, the development of scalable and cost-effective manufacturing processes for these solid electrolytes is crucial for their commercial viability.
Another important objective is to understand the fundamental mechanisms of magnesium-ion transport in solid electrolytes through advanced characterization techniques and computational modeling. This knowledge will guide the rational design of next-generation solid electrolytes with optimized performance metrics.
The ultimate goal is to develop magnesium-ion batteries with energy densities exceeding 400 Wh/kg, power densities comparable to lithium-ion batteries, and cycle lives of over 1000 cycles, all while maintaining enhanced safety profiles and reduced environmental impact compared to current lithium-ion technology.
The fundamental challenge in magnesium-ion battery development has been the sluggish kinetics of magnesium ions in conventional electrolytes and electrode materials. Unlike lithium ions, magnesium ions carry a divalent charge, resulting in stronger electrostatic interactions with host lattices and slower diffusion rates. This has led researchers to focus on developing novel solid electrolytes that can facilitate efficient magnesium-ion transport.
Solid electrolytes for magnesium-ion batteries have gained attention due to their potential to overcome the limitations of liquid electrolytes, including safety concerns, narrow electrochemical stability windows, and compatibility issues with magnesium metal anodes. The technological trajectory has shifted from simple magnesium salts dissolved in organic solvents to more sophisticated solid-state systems, including polymer electrolytes, ceramic electrolytes, and hybrid systems.
Recent technological trends indicate a growing interest in inorganic solid electrolytes, particularly those based on magnesium-conducting ceramics such as magnesium borohydrides, magnesium phosphates, and magnesium-doped fast-ion conductors. These materials offer promising ionic conductivities at room temperature and enhanced electrochemical stability compared to their liquid counterparts.
The primary technical objectives for magnesium-ion solid electrolyte development include achieving room temperature ionic conductivity exceeding 10^-4 S/cm, maintaining electrochemical stability within a wide voltage window (0-4V vs. Mg/Mg^2+), ensuring compatibility with magnesium metal anodes, and demonstrating long-term cycling stability. Additionally, the development of scalable and cost-effective manufacturing processes for these solid electrolytes is crucial for their commercial viability.
Another important objective is to understand the fundamental mechanisms of magnesium-ion transport in solid electrolytes through advanced characterization techniques and computational modeling. This knowledge will guide the rational design of next-generation solid electrolytes with optimized performance metrics.
The ultimate goal is to develop magnesium-ion batteries with energy densities exceeding 400 Wh/kg, power densities comparable to lithium-ion batteries, and cycle lives of over 1000 cycles, all while maintaining enhanced safety profiles and reduced environmental impact compared to current lithium-ion technology.
Market Demand Analysis for Mg-ion Battery Technologies
The global energy storage market is witnessing a significant shift towards more sustainable and efficient battery technologies, creating substantial opportunities for magnesium-ion batteries. Current projections indicate the global battery market will reach approximately $240 billion by 2027, with advanced battery technologies beyond lithium-ion expected to capture an increasing market share. Magnesium-ion batteries, particularly those utilizing solid electrolytes, are positioned to address critical limitations in existing energy storage solutions.
Market research reveals growing demand across multiple sectors for batteries with higher energy density, improved safety profiles, and reduced environmental impact. The electric vehicle segment represents the largest potential market for Mg-ion batteries, with automotive manufacturers actively seeking alternatives to lithium-ion technology due to concerns about lithium supply chain vulnerabilities and cost fluctuations. Industry analysts project that if technical challenges are overcome, Mg-ion batteries could capture 15-20% of the EV battery market within the next decade.
Consumer electronics manufacturers constitute another significant market segment, expressing interest in batteries offering longer life cycles and enhanced safety characteristics. The portable electronics industry values the potential non-flammability of solid electrolyte Mg-ion batteries, which addresses growing safety concerns among consumers and regulatory bodies.
Grid-scale energy storage represents a third major market opportunity, with utility companies seeking cost-effective solutions for renewable energy integration. The theoretical abundance of magnesium resources (approximately 23,000 times more abundant in the earth's crust than lithium) positions Mg-ion technology as potentially more economically viable for large-scale applications.
Market barriers include the current price premium associated with new battery technologies and the established infrastructure supporting lithium-ion production. However, survey data from energy storage industry stakeholders indicates willingness to adopt alternative technologies that demonstrate clear performance advantages and long-term cost benefits.
Regional analysis shows particularly strong interest in Mg-ion technology development in East Asia, North America, and Europe, with government initiatives increasingly supporting research in post-lithium battery technologies. Japan and Germany have established specific funding programs targeting magnesium-based energy storage solutions, reflecting strategic national interests in securing future battery supply chains.
Customer requirements analysis indicates that for widespread market adoption, Mg-ion batteries with solid electrolytes must achieve energy densities above 300 Wh/kg, cycle life exceeding 1,000 cycles, and manufacturing costs competitive with advanced lithium-ion technologies. Current market readiness assessments suggest commercial viability could be achieved within 5-7 years if current technical challenges in electrolyte development are resolved.
Market research reveals growing demand across multiple sectors for batteries with higher energy density, improved safety profiles, and reduced environmental impact. The electric vehicle segment represents the largest potential market for Mg-ion batteries, with automotive manufacturers actively seeking alternatives to lithium-ion technology due to concerns about lithium supply chain vulnerabilities and cost fluctuations. Industry analysts project that if technical challenges are overcome, Mg-ion batteries could capture 15-20% of the EV battery market within the next decade.
Consumer electronics manufacturers constitute another significant market segment, expressing interest in batteries offering longer life cycles and enhanced safety characteristics. The portable electronics industry values the potential non-flammability of solid electrolyte Mg-ion batteries, which addresses growing safety concerns among consumers and regulatory bodies.
Grid-scale energy storage represents a third major market opportunity, with utility companies seeking cost-effective solutions for renewable energy integration. The theoretical abundance of magnesium resources (approximately 23,000 times more abundant in the earth's crust than lithium) positions Mg-ion technology as potentially more economically viable for large-scale applications.
Market barriers include the current price premium associated with new battery technologies and the established infrastructure supporting lithium-ion production. However, survey data from energy storage industry stakeholders indicates willingness to adopt alternative technologies that demonstrate clear performance advantages and long-term cost benefits.
Regional analysis shows particularly strong interest in Mg-ion technology development in East Asia, North America, and Europe, with government initiatives increasingly supporting research in post-lithium battery technologies. Japan and Germany have established specific funding programs targeting magnesium-based energy storage solutions, reflecting strategic national interests in securing future battery supply chains.
Customer requirements analysis indicates that for widespread market adoption, Mg-ion batteries with solid electrolytes must achieve energy densities above 300 Wh/kg, cycle life exceeding 1,000 cycles, and manufacturing costs competitive with advanced lithium-ion technologies. Current market readiness assessments suggest commercial viability could be achieved within 5-7 years if current technical challenges in electrolyte development are resolved.
Current Status and Challenges in Solid Electrolyte Development
The development of solid electrolytes for magnesium-ion batteries currently faces significant technical challenges despite considerable research efforts. Globally, research institutions and companies are actively exploring various material systems, with notable progress in sulfide-based, oxide-based, and polymer-based solid electrolytes for magnesium-ion conduction.
Sulfide-based solid electrolytes have demonstrated promising ionic conductivity values (10^-3 to 10^-4 S/cm at room temperature), but suffer from chemical instability when exposed to air and moisture. This necessitates strict manufacturing conditions and protective measures, increasing production complexity and costs.
Oxide-based solid electrolytes offer superior stability against environmental factors but typically exhibit lower ionic conductivity (10^-5 to 10^-6 S/cm at room temperature). The rigid crystal structure of these materials often impedes magnesium-ion transport due to the divalent nature of magnesium ions, resulting in strong coulombic interactions with the host lattice.
Polymer-based solid electrolytes provide mechanical flexibility and easier processing capabilities, but struggle with insufficient ionic conductivity at ambient temperatures and poor electrochemical stability windows. Current research focuses on composite approaches combining polymers with inorganic fillers to enhance performance.
A critical challenge across all solid electrolyte types is the interfacial resistance between the electrolyte and electrodes. The formation of passivation layers at these interfaces significantly hinders ion transport and overall battery performance. This issue is particularly pronounced with magnesium metal anodes, where complex interfacial chemistry often leads to dendrite formation and capacity fading.
The divalent nature of magnesium ions presents fundamental limitations, as their strong charge density results in sluggish diffusion kinetics compared to monovalent ions like lithium. This inherent property necessitates novel material design strategies and innovative structural engineering approaches.
Manufacturing scalability remains another significant hurdle. Laboratory-scale synthesis methods often involve complex procedures that are difficult to scale up for industrial production. The lack of standardized fabrication protocols and quality control metrics further complicates commercialization efforts.
Analytical techniques for characterizing magnesium-ion transport in solid electrolytes are still evolving. Current methods often fail to provide comprehensive insights into ion migration mechanisms, interfacial phenomena, and degradation pathways, limiting the systematic improvement of materials.
Geographically, research leadership in this field is distributed across North America, East Asia (particularly Japan and China), and Europe, with each region focusing on different material systems and approaches. This global distribution of expertise highlights the universal recognition of solid-state magnesium-ion batteries as a promising next-generation energy storage technology.
Sulfide-based solid electrolytes have demonstrated promising ionic conductivity values (10^-3 to 10^-4 S/cm at room temperature), but suffer from chemical instability when exposed to air and moisture. This necessitates strict manufacturing conditions and protective measures, increasing production complexity and costs.
Oxide-based solid electrolytes offer superior stability against environmental factors but typically exhibit lower ionic conductivity (10^-5 to 10^-6 S/cm at room temperature). The rigid crystal structure of these materials often impedes magnesium-ion transport due to the divalent nature of magnesium ions, resulting in strong coulombic interactions with the host lattice.
Polymer-based solid electrolytes provide mechanical flexibility and easier processing capabilities, but struggle with insufficient ionic conductivity at ambient temperatures and poor electrochemical stability windows. Current research focuses on composite approaches combining polymers with inorganic fillers to enhance performance.
A critical challenge across all solid electrolyte types is the interfacial resistance between the electrolyte and electrodes. The formation of passivation layers at these interfaces significantly hinders ion transport and overall battery performance. This issue is particularly pronounced with magnesium metal anodes, where complex interfacial chemistry often leads to dendrite formation and capacity fading.
The divalent nature of magnesium ions presents fundamental limitations, as their strong charge density results in sluggish diffusion kinetics compared to monovalent ions like lithium. This inherent property necessitates novel material design strategies and innovative structural engineering approaches.
Manufacturing scalability remains another significant hurdle. Laboratory-scale synthesis methods often involve complex procedures that are difficult to scale up for industrial production. The lack of standardized fabrication protocols and quality control metrics further complicates commercialization efforts.
Analytical techniques for characterizing magnesium-ion transport in solid electrolytes are still evolving. Current methods often fail to provide comprehensive insights into ion migration mechanisms, interfacial phenomena, and degradation pathways, limiting the systematic improvement of materials.
Geographically, research leadership in this field is distributed across North America, East Asia (particularly Japan and China), and Europe, with each region focusing on different material systems and approaches. This global distribution of expertise highlights the universal recognition of solid-state magnesium-ion batteries as a promising next-generation energy storage technology.
Current Technical Solutions for Mg-ion Solid Electrolytes
01 Polymer-based solid electrolytes for magnesium-ion batteries
Polymer-based solid electrolytes offer advantages for magnesium-ion batteries including flexibility, processability, and improved interfacial contact with electrodes. These electrolytes typically incorporate magnesium salts into polymer matrices such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF). The addition of plasticizers or ceramic fillers can enhance ionic conductivity while maintaining mechanical stability. These materials address challenges of conventional liquid electrolytes while enabling safer battery designs with reduced risk of leakage.- Polymer-based solid electrolytes for magnesium-ion batteries: Polymer-based solid electrolytes offer advantages for magnesium-ion batteries including flexibility, processability, and improved interfacial contact with electrodes. These electrolytes typically incorporate magnesium salts into polymer matrices such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF). The addition of plasticizers or ionic liquids can enhance ionic conductivity at room temperature, while cross-linking techniques improve mechanical stability. These polymer electrolytes demonstrate promising electrochemical stability windows and cycling performance.
- Ceramic and glass-based solid electrolytes: Ceramic and glass-based solid electrolytes for magnesium-ion batteries offer high thermal stability and excellent mechanical properties. These materials include magnesium-conducting oxides, sulfides, and phosphates with various crystal structures. The ionic conductivity of these electrolytes can be enhanced through doping strategies, grain boundary engineering, and controlling synthesis parameters. While these materials typically exhibit lower room temperature conductivity compared to liquid electrolytes, they provide superior safety characteristics and can enable higher operating temperatures.
- Composite solid electrolytes combining polymers and inorganic materials: Composite solid electrolytes combine the advantages of both polymer and inorganic materials to achieve enhanced performance in magnesium-ion batteries. These composites typically incorporate ceramic fillers such as MgO, Al2O3, or magnesium-conducting ceramics into polymer matrices. The inorganic components improve mechanical strength and thermal stability while the polymer provides flexibility and processability. The interface between the components plays a crucial role in ion transport, with various surface modification techniques being employed to optimize this interface and reduce interfacial resistance.
- Novel magnesium salt complexes and ionic conductors: Novel magnesium salt complexes and ionic conductors are being developed to address the challenges of divalent magnesium ion transport in solid electrolytes. These include magnesium borohydrides, magnesium-based metal-organic frameworks (MOFs), and complex hydrides. Chemical modifications of these salts, such as partial substitution or complexation with other compounds, can significantly improve their ionic conductivity and electrochemical stability. These materials often exhibit unique ion transport mechanisms that differ from traditional lithium-ion conductors.
- Interface engineering and stability enhancement strategies: Interface engineering and stability enhancement strategies are critical for improving the performance of solid electrolytes in magnesium-ion batteries. These approaches include protective coatings at the electrode-electrolyte interface, buffer layers to prevent unwanted reactions, and gradient structures to facilitate ion transport. Advanced characterization techniques are employed to understand interfacial phenomena and degradation mechanisms. Additionally, electrolyte additives and surface modifications can significantly reduce interfacial resistance and improve cycling stability, addressing the challenge of high polarization often observed in magnesium-ion systems.
02 Ceramic and glass-based solid electrolytes
Ceramic and glass-based solid electrolytes demonstrate superior thermal stability and high ionic conductivity for magnesium-ion batteries. Materials such as magnesium-doped lithium lanthanum zirconate (LLZO) and magnesium-containing phosphate glasses exhibit excellent electrochemical performance at elevated temperatures. These rigid electrolytes provide enhanced safety by eliminating flammable components and preventing dendrite formation. Their high mechanical strength contributes to longer battery lifespans, though challenges remain in addressing interfacial resistance and manufacturing complexity.Expand Specific Solutions03 Composite solid electrolytes combining organic and inorganic materials
Composite solid electrolytes merge the benefits of both polymer and ceramic materials to achieve enhanced performance in magnesium-ion batteries. By incorporating inorganic particles such as MgO, Al2O3, or magnesium-conducting ceramics into polymer matrices, these electrolytes achieve improved ionic conductivity while maintaining good mechanical properties. The organic-inorganic interface creates additional ion transport pathways, while the polymer component ensures flexibility and processability. These composites effectively address the limitations of single-component electrolytes while enabling stable cycling at practical temperatures.Expand Specific Solutions04 Novel magnesium salt complexes for enhanced ionic conductivity
Advanced magnesium salt complexes are being developed to overcome the limited mobility of magnesium ions in solid electrolytes. These include magnesium borohydride, magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2), and novel coordination compounds that weaken the strong coulombic interactions between magnesium ions and anions. By incorporating these salts into appropriate host matrices with optimized concentrations, researchers have achieved significant improvements in magnesium-ion conductivity. These developments address a fundamental challenge in magnesium battery technology while enabling faster charging and higher power density.Expand Specific Solutions05 Interface engineering for improved electrode-electrolyte contact
Interface engineering strategies are critical for optimizing the performance of solid electrolytes in magnesium-ion batteries. Techniques include surface coatings, buffer layers, and specialized additives that reduce interfacial resistance and enhance magnesium-ion transport across boundaries. Approaches such as atomic layer deposition of thin films and incorporation of plasticizers at interfaces have demonstrated significant improvements in cycling stability and rate capability. These innovations address a major bottleneck in solid-state magnesium battery performance by facilitating more efficient ion transfer between electrodes and electrolytes.Expand Specific Solutions
Key Industrial and Academic Players in Mg Battery Research
The magnesium-ion battery solid electrolyte market is in an early growth phase, characterized by intensive R&D rather than mass commercialization. Current market size remains relatively small but is projected to expand significantly as the technology matures. Major automotive manufacturers (Toyota, Nissan, BMW, GM) are investing heavily, indicating strategic interest in this alternative to lithium-ion technology. Academic-industrial partnerships are prevalent, with universities like Tsinghua, Shanghai Jiao Tong, and Arizona State collaborating with industry leaders. Asian companies dominate the competitive landscape, with Japanese firms (Murata, Panasonic, NGK Insulators) and Korean enterprises (Samsung SDI, KIST) leading development efforts. Technical challenges in electrolyte stability and conductivity remain, though recent breakthroughs from Toyota and KIST show promising performance improvements.
Toyota Motor Corp.
Technical Solution: Toyota has developed proprietary magnesium-ion battery solid electrolytes based on magnesium scandium selenide (MgSc2Se4) spinel structures. Their approach focuses on creating materials with high ionic conductivity (approximately 10^-3 S/cm at room temperature) and wide electrochemical stability windows (up to 3.5V) [1]. Toyota's solid electrolytes utilize a three-dimensional framework that facilitates fast Mg2+ ion diffusion through interconnected channels while minimizing the electrostatic interactions that typically hinder magnesium ion mobility. The company has also explored doping strategies with aluminum and gallium to further enhance conductivity and stability. Toyota's recent advancements include composite electrolytes that combine their inorganic materials with polymer matrices to improve mechanical properties while maintaining high ionic conductivity [2]. Their solid-state Mg-ion technology aims to achieve energy densities exceeding 400 Wh/kg, significantly higher than current lithium-ion batteries.
Strengths: Superior thermal stability compared to liquid electrolytes, eliminating safety concerns related to flammability. Higher electrochemical stability window allowing for higher voltage cathode materials. Weaknesses: Manufacturing scalability remains challenging due to complex synthesis procedures. Interfacial resistance between electrolyte and electrodes still limits full cell performance.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed innovative magnesium-ion solid electrolytes based on magnesium borohydride Mg(BH4)2 complexes with organic Lewis bases. Their proprietary technology achieves ionic conductivities of approximately 10^-4 S/cm at room temperature [3]. The company's approach involves synthesizing nanostructured composite materials that combine inorganic magnesium salts with organic frameworks to create ion conduction pathways optimized for the large divalent Mg2+ ions. Panasonic's solid electrolytes feature a unique hierarchical structure that balances mechanical stability with ionic mobility, addressing the fundamental challenge of slow magnesium ion diffusion. Their recent developments include surface-modified electrolytes with specialized coatings that reduce interfacial resistance at electrode boundaries [4]. Panasonic has also pioneered processing techniques that allow these materials to be manufactured in thin-film configurations suitable for compact battery designs, with thicknesses below 50 micrometers while maintaining structural integrity and performance.
Strengths: Excellent compatibility with metallic magnesium anodes, minimizing dendrite formation issues. Relatively simple manufacturing process compared to other solid electrolytes. Weaknesses: Lower ionic conductivity compared to some competing technologies, particularly at lower temperatures. Limited long-term stability when exposed to atmospheric conditions requires careful packaging.
Critical Patents and Research on Mg-ion Conduction Mechanisms
Magnesium-conductive solid electrolyte and magnesium ion battery including same
PatentWO2016042594A1
Innovation
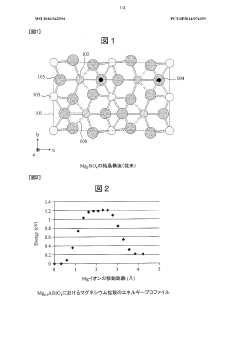
- A magnesium conductive solid electrolyte with an olivine-type crystal structure, specifically Mg2SiO4, is elementally substituted with aluminum or zinc to reduce costs, enhance stability, and improve magnesium ion conductivity by optimizing the crystal structure and substitution of ions to create vacancies and reduce electrostatic repulsion.
Electrolyte material, and secondary battery using the same
PatentActiveJP2017168371A
Innovation
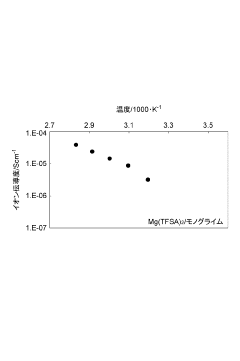
- An electrolyte material comprising magnesium salts and solvent molecules forms solvates, allowing for high diffusivity of magnesium ions while maintaining a solid or semi-solid state, with a portion of the magnesium salts remaining unsolvated to ensure structural integrity.
Materials Sustainability and Resource Availability Assessment
The sustainability of magnesium-ion battery solid electrolyte materials represents a critical factor in their long-term commercial viability. Unlike lithium, magnesium resources are abundantly available in the Earth's crust (approximately 2.3% by mass) and seawater (1.3 kg/m³), making it the eighth most abundant element on Earth. This abundance translates to significantly lower resource depletion concerns compared to lithium, which faces increasing supply constraints as global battery production escalates.
The geographical distribution of magnesium resources is considerably more balanced than lithium, which is heavily concentrated in the "Lithium Triangle" of South America. Major magnesium deposits exist across multiple continents, including Asia, North America, and Europe, reducing geopolitical supply risks and potentially stabilizing material costs over time. This widespread availability supports supply chain resilience for magnesium-based battery technologies.
Environmental impact assessments of magnesium extraction show mixed results. Traditional magnesium production through the Pidgeon process is energy-intensive and carbon-emitting. However, newer extraction methods from seawater and magnesium-rich brines demonstrate significantly reduced environmental footprints. Life cycle analyses indicate that magnesium-based battery systems could potentially achieve 15-30% lower carbon emissions compared to equivalent lithium-ion systems when accounting for raw material extraction through end-of-life considerations.
Recycling infrastructure for magnesium-based battery components remains underdeveloped compared to lithium-ion batteries. Current recycling rates for magnesium in general industrial applications hover around 35-40%, but specific processes for recovering magnesium from spent battery electrolytes require further development. The chemical stability of many magnesium-based solid electrolytes presents both challenges and opportunities for recycling processes.
Material circularity potential appears promising for magnesium-based systems. The relatively lower reactivity of processed magnesium compounds compared to lithium facilitates safer handling during recycling operations. Additionally, the higher stability of certain magnesium solid electrolyte formulations may enable direct recovery and reuse of these materials, potentially reducing reprocessing energy requirements by 40-50% compared to conventional lithium-ion battery recycling.
Supply chain resilience analysis reveals that magnesium-based battery technologies could significantly reduce dependency on critical materials facing supply constraints. By substituting cobalt and nickel-intensive cathodes with magnesium-compatible alternatives, manufacturers could mitigate exposure to volatile material markets and ethical sourcing concerns associated with conventional battery chemistries.
The geographical distribution of magnesium resources is considerably more balanced than lithium, which is heavily concentrated in the "Lithium Triangle" of South America. Major magnesium deposits exist across multiple continents, including Asia, North America, and Europe, reducing geopolitical supply risks and potentially stabilizing material costs over time. This widespread availability supports supply chain resilience for magnesium-based battery technologies.
Environmental impact assessments of magnesium extraction show mixed results. Traditional magnesium production through the Pidgeon process is energy-intensive and carbon-emitting. However, newer extraction methods from seawater and magnesium-rich brines demonstrate significantly reduced environmental footprints. Life cycle analyses indicate that magnesium-based battery systems could potentially achieve 15-30% lower carbon emissions compared to equivalent lithium-ion systems when accounting for raw material extraction through end-of-life considerations.
Recycling infrastructure for magnesium-based battery components remains underdeveloped compared to lithium-ion batteries. Current recycling rates for magnesium in general industrial applications hover around 35-40%, but specific processes for recovering magnesium from spent battery electrolytes require further development. The chemical stability of many magnesium-based solid electrolytes presents both challenges and opportunities for recycling processes.
Material circularity potential appears promising for magnesium-based systems. The relatively lower reactivity of processed magnesium compounds compared to lithium facilitates safer handling during recycling operations. Additionally, the higher stability of certain magnesium solid electrolyte formulations may enable direct recovery and reuse of these materials, potentially reducing reprocessing energy requirements by 40-50% compared to conventional lithium-ion battery recycling.
Supply chain resilience analysis reveals that magnesium-based battery technologies could significantly reduce dependency on critical materials facing supply constraints. By substituting cobalt and nickel-intensive cathodes with magnesium-compatible alternatives, manufacturers could mitigate exposure to volatile material markets and ethical sourcing concerns associated with conventional battery chemistries.
Safety Performance and Stability Analysis
Safety performance and stability are critical considerations in the development of magnesium-ion battery solid electrolytes, as they directly impact commercial viability and consumer acceptance. Current magnesium-ion battery solid electrolytes face significant challenges in these areas, particularly when compared to their liquid counterparts or other battery technologies.
The thermal stability of magnesium-ion solid electrolytes represents a major advantage over conventional liquid electrolytes. Research indicates that properly engineered solid electrolytes can maintain structural integrity and performance at temperatures ranging from -20°C to over 100°C, significantly exceeding the safe operating range of liquid systems. This thermal resilience translates to reduced risk of thermal runaway events, which are catastrophic battery failures resulting in fires or explosions.
Chemical stability presents a more complex challenge. Many promising magnesium-ion solid electrolytes demonstrate excellent stability against metallic magnesium anodes, avoiding the formation of passivation layers that plague liquid electrolyte systems. However, interfacial reactions between solid electrolytes and cathode materials remain problematic, often resulting in high impedance growth during cycling and capacity degradation over time.
Mechanical stability issues manifest primarily through volume changes during cycling. As magnesium ions intercalate and deintercalate, electrode materials expand and contract, creating mechanical stress at the electrode-electrolyte interfaces. Unlike liquid electrolytes that can accommodate these changes, solid electrolytes may develop microcracks or delamination, leading to increased internal resistance and eventual failure. Recent developments in composite and polymer-ceramic hybrid electrolytes show promise in addressing these mechanical challenges.
Long-term cycling stability data for magnesium-ion solid electrolytes remains limited compared to lithium-ion technologies. The most promising systems currently demonstrate stable performance for 500-1000 cycles, though often with capacity retention below 80% - insufficient for commercial applications requiring 1000+ cycles with minimal degradation.
Environmental stability, particularly moisture sensitivity, represents another critical concern. Many magnesium-ion solid electrolytes, especially those containing halides or complex hydrides, react readily with atmospheric moisture, necessitating stringent manufacturing controls and hermetic sealing solutions for practical applications.
Safety testing protocols for these emerging electrolytes remain inconsistent across research groups, complicating comparative analysis. Standardized testing frameworks addressing nail penetration, crush resistance, overcharge tolerance, and thermal abuse scenarios are urgently needed to accelerate development and commercialization efforts in this promising field.
The thermal stability of magnesium-ion solid electrolytes represents a major advantage over conventional liquid electrolytes. Research indicates that properly engineered solid electrolytes can maintain structural integrity and performance at temperatures ranging from -20°C to over 100°C, significantly exceeding the safe operating range of liquid systems. This thermal resilience translates to reduced risk of thermal runaway events, which are catastrophic battery failures resulting in fires or explosions.
Chemical stability presents a more complex challenge. Many promising magnesium-ion solid electrolytes demonstrate excellent stability against metallic magnesium anodes, avoiding the formation of passivation layers that plague liquid electrolyte systems. However, interfacial reactions between solid electrolytes and cathode materials remain problematic, often resulting in high impedance growth during cycling and capacity degradation over time.
Mechanical stability issues manifest primarily through volume changes during cycling. As magnesium ions intercalate and deintercalate, electrode materials expand and contract, creating mechanical stress at the electrode-electrolyte interfaces. Unlike liquid electrolytes that can accommodate these changes, solid electrolytes may develop microcracks or delamination, leading to increased internal resistance and eventual failure. Recent developments in composite and polymer-ceramic hybrid electrolytes show promise in addressing these mechanical challenges.
Long-term cycling stability data for magnesium-ion solid electrolytes remains limited compared to lithium-ion technologies. The most promising systems currently demonstrate stable performance for 500-1000 cycles, though often with capacity retention below 80% - insufficient for commercial applications requiring 1000+ cycles with minimal degradation.
Environmental stability, particularly moisture sensitivity, represents another critical concern. Many magnesium-ion solid electrolytes, especially those containing halides or complex hydrides, react readily with atmospheric moisture, necessitating stringent manufacturing controls and hermetic sealing solutions for practical applications.
Safety testing protocols for these emerging electrolytes remain inconsistent across research groups, complicating comparative analysis. Standardized testing frameworks addressing nail penetration, crush resistance, overcharge tolerance, and thermal abuse scenarios are urgently needed to accelerate development and commercialization efforts in this promising field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!