Comparative study of magnesium-ion battery vs calcium-ion battery mechanisms
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Ca Ion Battery Evolution and Research Objectives
The evolution of energy storage technologies has witnessed significant advancements over the past decades, with lithium-ion batteries dominating the commercial landscape. However, concerns regarding lithium's limited abundance, high cost, and safety issues have prompted researchers to explore alternative battery chemistries. Magnesium and calcium, as earth-abundant elements, have emerged as promising candidates for next-generation energy storage systems.
Magnesium-ion battery research began gaining momentum in the early 2000s, following Aurbach's pioneering work demonstrating reversible magnesium electrodeposition. The bivalent nature of magnesium ions (Mg²⁺) theoretically offers higher volumetric capacity compared to monovalent lithium ions. Similarly, calcium-ion battery research, though initiated later around 2015, has attracted attention due to calcium's abundance, low cost, and favorable redox potential.
The evolution of these technologies has been marked by distinct phases. Initially, researchers focused on fundamental electrochemistry and proof-of-concept demonstrations. This was followed by intensive electrolyte development to address the challenging issue of divalent ion transport. The current phase involves concurrent development of compatible electrode materials and electrolyte systems to achieve practical energy densities and cycling stability.
Both technologies face similar challenges stemming from the divalent nature of their charge carriers. The stronger electrostatic interactions between Mg²⁺/Ca²⁺ and host lattices result in sluggish diffusion kinetics. Additionally, the high charge density leads to strong coordination with solvent molecules, creating significant desolvation energy barriers at electrode interfaces.
The primary research objectives in this field include developing electrolytes with wide electrochemical stability windows, identifying electrode materials with reversible intercalation capabilities, and understanding the fundamental interfacial phenomena governing battery performance. Specifically, researchers aim to achieve electrolytes that enable efficient ion transport while maintaining stability against both anode and cathode materials.
Comparative studies between magnesium-ion and calcium-ion systems are particularly valuable as they provide insights into how ionic radius, charge density, and coordination chemistry influence battery performance. Magnesium offers higher volumetric capacity but suffers from stronger electrostatic interactions, while calcium's larger ionic radius potentially facilitates faster diffusion but presents challenges in terms of metallic anode stability.
The ultimate goal of this research direction is to develop practical energy storage systems that can deliver energy densities comparable to lithium-ion batteries while offering advantages in terms of cost, safety, and environmental impact. This requires interdisciplinary approaches combining computational modeling, advanced characterization techniques, and innovative materials design strategies to overcome the inherent limitations of divalent ion chemistries.
Magnesium-ion battery research began gaining momentum in the early 2000s, following Aurbach's pioneering work demonstrating reversible magnesium electrodeposition. The bivalent nature of magnesium ions (Mg²⁺) theoretically offers higher volumetric capacity compared to monovalent lithium ions. Similarly, calcium-ion battery research, though initiated later around 2015, has attracted attention due to calcium's abundance, low cost, and favorable redox potential.
The evolution of these technologies has been marked by distinct phases. Initially, researchers focused on fundamental electrochemistry and proof-of-concept demonstrations. This was followed by intensive electrolyte development to address the challenging issue of divalent ion transport. The current phase involves concurrent development of compatible electrode materials and electrolyte systems to achieve practical energy densities and cycling stability.
Both technologies face similar challenges stemming from the divalent nature of their charge carriers. The stronger electrostatic interactions between Mg²⁺/Ca²⁺ and host lattices result in sluggish diffusion kinetics. Additionally, the high charge density leads to strong coordination with solvent molecules, creating significant desolvation energy barriers at electrode interfaces.
The primary research objectives in this field include developing electrolytes with wide electrochemical stability windows, identifying electrode materials with reversible intercalation capabilities, and understanding the fundamental interfacial phenomena governing battery performance. Specifically, researchers aim to achieve electrolytes that enable efficient ion transport while maintaining stability against both anode and cathode materials.
Comparative studies between magnesium-ion and calcium-ion systems are particularly valuable as they provide insights into how ionic radius, charge density, and coordination chemistry influence battery performance. Magnesium offers higher volumetric capacity but suffers from stronger electrostatic interactions, while calcium's larger ionic radius potentially facilitates faster diffusion but presents challenges in terms of metallic anode stability.
The ultimate goal of this research direction is to develop practical energy storage systems that can deliver energy densities comparable to lithium-ion batteries while offering advantages in terms of cost, safety, and environmental impact. This requires interdisciplinary approaches combining computational modeling, advanced characterization techniques, and innovative materials design strategies to overcome the inherent limitations of divalent ion chemistries.
Market Analysis for Post-Lithium Battery Technologies
The global energy storage market is witnessing a significant shift away from traditional lithium-ion batteries due to resource constraints, environmental concerns, and increasing demand for higher energy density solutions. Post-lithium battery technologies, particularly magnesium-ion and calcium-ion batteries, are emerging as promising alternatives with substantial market potential. Current projections indicate the global alternative battery market could reach $90 billion by 2030, with post-lithium technologies potentially capturing 15-20% of this expanding sector.
Magnesium-ion battery technology appeals to industrial sectors requiring high energy density and improved safety profiles. The automotive industry represents the largest potential market, with electric vehicle manufacturers actively seeking alternatives to lithium-ion batteries to address range anxiety and reduce dependency on scarce lithium resources. Energy storage systems for renewable integration constitute another substantial market segment, projected to grow at a CAGR of 25% through 2028.
Calcium-ion batteries are positioning themselves in markets where cost-effectiveness and abundant material supply are paramount considerations. Grid-scale energy storage applications show particular promise for calcium-ion technology, with utility companies expressing increasing interest in these systems for load balancing and peak shaving operations. Consumer electronics manufacturers are also exploring calcium-ion solutions for next-generation devices, attracted by the potential for lower production costs and reduced supply chain vulnerabilities.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub for both technologies, with China, Japan, and South Korea leading research commercialization efforts. European markets demonstrate the strongest regulatory support for post-lithium technologies, driven by the European Union's strategic autonomy initiatives and circular economy directives. North American markets show growing investment in research infrastructure, particularly through public-private partnerships focused on domestic battery production capabilities.
Market adoption barriers include technology readiness levels, with both magnesium-ion and calcium-ion batteries currently at TRL 4-6, indicating laboratory validation but limited commercial deployment. Manufacturing scalability remains challenging, with current production processes unable to match the economies of scale achieved by lithium-ion battery gigafactories. Consumer acceptance represents another significant hurdle, as market education regarding the benefits of these alternative technologies lags behind technical development.
Investment trends show venture capital funding for magnesium-ion battery startups reached $450 million in 2022, while calcium-ion technology attracted $320 million during the same period. Corporate strategic investments from automotive and energy sector incumbents are increasingly targeting these technologies through minority stakes and joint development agreements rather than outright acquisitions, indicating a measured approach to market development.
Magnesium-ion battery technology appeals to industrial sectors requiring high energy density and improved safety profiles. The automotive industry represents the largest potential market, with electric vehicle manufacturers actively seeking alternatives to lithium-ion batteries to address range anxiety and reduce dependency on scarce lithium resources. Energy storage systems for renewable integration constitute another substantial market segment, projected to grow at a CAGR of 25% through 2028.
Calcium-ion batteries are positioning themselves in markets where cost-effectiveness and abundant material supply are paramount considerations. Grid-scale energy storage applications show particular promise for calcium-ion technology, with utility companies expressing increasing interest in these systems for load balancing and peak shaving operations. Consumer electronics manufacturers are also exploring calcium-ion solutions for next-generation devices, attracted by the potential for lower production costs and reduced supply chain vulnerabilities.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub for both technologies, with China, Japan, and South Korea leading research commercialization efforts. European markets demonstrate the strongest regulatory support for post-lithium technologies, driven by the European Union's strategic autonomy initiatives and circular economy directives. North American markets show growing investment in research infrastructure, particularly through public-private partnerships focused on domestic battery production capabilities.
Market adoption barriers include technology readiness levels, with both magnesium-ion and calcium-ion batteries currently at TRL 4-6, indicating laboratory validation but limited commercial deployment. Manufacturing scalability remains challenging, with current production processes unable to match the economies of scale achieved by lithium-ion battery gigafactories. Consumer acceptance represents another significant hurdle, as market education regarding the benefits of these alternative technologies lags behind technical development.
Investment trends show venture capital funding for magnesium-ion battery startups reached $450 million in 2022, while calcium-ion technology attracted $320 million during the same period. Corporate strategic investments from automotive and energy sector incumbents are increasingly targeting these technologies through minority stakes and joint development agreements rather than outright acquisitions, indicating a measured approach to market development.
Technical Barriers in Mg-Ca Ion Battery Development
Despite significant advancements in battery technology, both magnesium-ion and calcium-ion batteries face substantial technical barriers that hinder their commercial viability. The primary challenge for magnesium-ion batteries lies in the development of suitable electrolytes. Conventional electrolytes used in lithium-ion systems are incompatible due to the formation of passivation layers on magnesium metal anodes, which block ion transport. Current magnesium electrolytes, such as Grignard reagents and magnesium aluminum chloride complexes, suffer from narrow electrochemical stability windows and poor compatibility with cathode materials.
For calcium-ion batteries, electrolyte challenges are even more pronounced. The development of electrolytes that enable reversible calcium plating and stripping remains elusive. Most calcium salts have limited solubility in conventional organic solvents, and those that do dissolve often lead to irreversible calcium deposition or parasitic reactions with solvent molecules.
Cathode materials present another significant barrier for both systems. The divalent nature of Mg²⁺ and Ca²⁺ ions results in strong electrostatic interactions with host lattices, leading to sluggish diffusion kinetics. This manifests as poor rate capability and rapid capacity fading during cycling. While several cathode materials have been proposed, including Chevrel phases for magnesium and various transition metal oxides for both systems, none have demonstrated the combination of high capacity, good rate capability, and cycling stability required for practical applications.
The anode interface chemistry poses unique challenges. Unlike lithium-ion batteries that benefit from stable solid electrolyte interphase (SEI) formation, magnesium and calcium metal anodes typically form non-conductive surface films that impede ion transport. This phenomenon is particularly problematic for calcium, where the higher reactivity leads to more aggressive passivation layer formation.
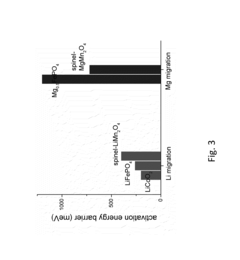
Fundamental understanding of ion transport mechanisms in these systems remains incomplete. The larger ionic radii of Mg²⁺ (0.72 Å) and Ca²⁺ (1.00 Å) compared to Li⁺ (0.76 Å) affect intercalation kinetics in complex ways that are not fully characterized. Additionally, the higher charge density of these divalent ions creates stronger coordination environments in both the electrolyte solution and at electrode interfaces.
Manufacturing challenges further complicate development efforts. Both systems require strict moisture and oxygen exclusion during cell assembly, more stringent than lithium-ion batteries. The reactive nature of calcium metal presents particular handling difficulties, as it can spontaneously ignite in air. Additionally, the lack of established supply chains for high-purity calcium and magnesium battery materials increases costs and limits research progress.
For calcium-ion batteries, electrolyte challenges are even more pronounced. The development of electrolytes that enable reversible calcium plating and stripping remains elusive. Most calcium salts have limited solubility in conventional organic solvents, and those that do dissolve often lead to irreversible calcium deposition or parasitic reactions with solvent molecules.
Cathode materials present another significant barrier for both systems. The divalent nature of Mg²⁺ and Ca²⁺ ions results in strong electrostatic interactions with host lattices, leading to sluggish diffusion kinetics. This manifests as poor rate capability and rapid capacity fading during cycling. While several cathode materials have been proposed, including Chevrel phases for magnesium and various transition metal oxides for both systems, none have demonstrated the combination of high capacity, good rate capability, and cycling stability required for practical applications.
The anode interface chemistry poses unique challenges. Unlike lithium-ion batteries that benefit from stable solid electrolyte interphase (SEI) formation, magnesium and calcium metal anodes typically form non-conductive surface films that impede ion transport. This phenomenon is particularly problematic for calcium, where the higher reactivity leads to more aggressive passivation layer formation.
Fundamental understanding of ion transport mechanisms in these systems remains incomplete. The larger ionic radii of Mg²⁺ (0.72 Å) and Ca²⁺ (1.00 Å) compared to Li⁺ (0.76 Å) affect intercalation kinetics in complex ways that are not fully characterized. Additionally, the higher charge density of these divalent ions creates stronger coordination environments in both the electrolyte solution and at electrode interfaces.
Manufacturing challenges further complicate development efforts. Both systems require strict moisture and oxygen exclusion during cell assembly, more stringent than lithium-ion batteries. The reactive nature of calcium metal presents particular handling difficulties, as it can spontaneously ignite in air. Additionally, the lack of established supply chains for high-purity calcium and magnesium battery materials increases costs and limits research progress.
Current Electrode and Electrolyte Solutions Comparison
01 Electrode materials for magnesium-ion batteries
Various electrode materials can be used in magnesium-ion batteries to enhance performance. These include specialized cathode materials that allow for efficient magnesium ion intercalation and extraction, as well as anode materials designed to store and release magnesium ions. The selection of appropriate electrode materials is crucial for addressing challenges such as slow diffusion kinetics and improving the overall energy density and cycle life of magnesium-ion batteries.- Electrode materials for magnesium-ion batteries: Various materials can be used as electrodes in magnesium-ion batteries to enhance performance. These include specific cathode materials that allow efficient magnesium ion intercalation and extraction, and anode materials with high magnesium storage capacity. The electrode materials are designed to accommodate the divalent nature of magnesium ions and overcome challenges related to slow diffusion kinetics. Advanced electrode structures and compositions help improve cycling stability and rate capability of magnesium-ion batteries.
- Electrolyte systems for calcium-ion batteries: Specialized electrolyte formulations are crucial for calcium-ion battery operation. These electrolytes must facilitate calcium ion transport while preventing unwanted side reactions at the electrode surfaces. Researchers have developed various electrolyte compositions including organic solvents with calcium salts and ionic liquids that enable reversible calcium deposition and dissolution. The electrolyte systems address challenges related to the formation of passivation layers and the high charge density of calcium ions, which can otherwise hinder ion mobility and battery performance.
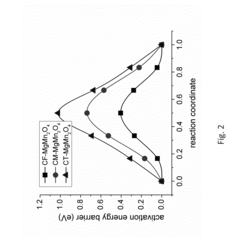
- Comparative mechanisms between magnesium and calcium-ion batteries: Magnesium and calcium-ion batteries operate on similar principles but with distinct characteristics due to differences in ion properties. Both utilize divalent cations (Mg²⁺ or Ca²⁺) that shuttle between electrodes during charge/discharge cycles. Calcium ions offer higher theoretical energy density due to their lower standard reduction potential compared to magnesium, but face greater challenges with ion mobility. The larger ionic radius of calcium compared to magnesium affects diffusion kinetics and intercalation mechanisms, resulting in different performance characteristics and design requirements for each battery type.
- Novel cathode designs for multivalent ion batteries: Advanced cathode designs are being developed specifically for multivalent ion batteries including magnesium and calcium systems. These designs incorporate layered structures, spinel-type materials, and organic compounds that provide channels for ion diffusion while maintaining structural stability during repeated cycling. Researchers are exploring nanostructured cathodes and composite materials to enhance ion insertion/extraction kinetics. These novel cathode architectures aim to overcome the sluggish diffusion of divalent ions and improve the overall energy density and power capability of multivalent ion batteries.
- Interface phenomena and stability issues in magnesium and calcium-ion batteries: The electrode-electrolyte interfaces in magnesium and calcium-ion batteries present significant challenges for battery performance and longevity. These interfaces often develop passivation layers that can block ion transport, especially with conventional electrolytes. Research focuses on understanding and controlling interfacial reactions to prevent dendrite formation and ensure stable cycling. Various strategies are being explored, including protective coatings, electrolyte additives, and surface modifications to improve the compatibility between electrodes and electrolytes, thereby enhancing the overall stability and cycle life of these battery systems.
02 Electrolyte compositions for divalent ion batteries
Electrolyte formulations play a critical role in both magnesium and calcium-ion batteries. Due to the divalent nature of these ions, specialized electrolytes are required to facilitate ion transport while preventing unwanted side reactions. These electrolytes often contain specific salts and solvents that enable reversible deposition and dissolution of magnesium or calcium, while maintaining compatibility with electrode materials and providing sufficient ionic conductivity for practical battery operation.Expand Specific Solutions03 Calcium-ion battery mechanisms and components
Calcium-ion batteries operate through the reversible insertion and extraction of calcium ions between electrodes. The mechanism involves calcium ion transport through an electrolyte and intercalation into host structures. Due to calcium's abundance and potentially higher energy density compared to lithium, these batteries are being developed as alternative energy storage systems. Research focuses on addressing challenges such as finding suitable electrode materials that can accommodate the larger size of calcium ions and developing electrolytes that enable efficient calcium ion transport.Expand Specific Solutions04 Comparative analysis of multivalent ion battery systems
Comparative studies between different multivalent ion battery systems, including magnesium and calcium-ion batteries, reveal their respective advantages and limitations. Magnesium-ion batteries offer benefits such as higher theoretical volumetric capacity and reduced dendrite formation compared to lithium-ion batteries. Calcium-ion batteries potentially provide higher voltage and energy density than magnesium systems. Both face challenges related to slow diffusion kinetics and electrolyte compatibility, but offer promising alternatives to conventional lithium-ion technology due to the greater abundance and lower cost of these elements.Expand Specific Solutions05 Novel battery architectures and manufacturing methods
Innovative battery designs and manufacturing techniques are being developed to optimize the performance of magnesium and calcium-ion batteries. These include specialized cell configurations, electrode preparation methods, and assembly processes tailored to the unique characteristics of these battery chemistries. Advanced manufacturing approaches aim to address challenges such as interfacial stability, ion transport limitations, and cycle life, while enabling scalable production of these next-generation energy storage systems.Expand Specific Solutions
Leading Research Institutions and Industrial Stakeholders
The magnesium-ion and calcium-ion battery technology landscape is currently in an early development stage, with significant research momentum but limited commercial deployment. The market remains relatively small compared to lithium-ion batteries but shows promising growth potential due to the abundance and lower cost of magnesium and calcium resources. Technologically, these battery systems are still maturing, with challenges in electrolyte stability and electrode materials. Leading research institutions like Toyota Motor Corp., Shenzhen Institutes of Advanced Technology, and Naval Research Laboratory are advancing fundamental mechanisms, while companies including Sony Group, Samsung Electronics, and Murata Manufacturing focus on practical applications. Academic institutions such as Jilin University, Arizona State University, and Tohoku University contribute significantly to theoretical understanding and materials development, creating a collaborative ecosystem spanning Asia, North America, and Europe.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has established comprehensive research programs comparing magnesium-ion and calcium-ion battery technologies as potential alternatives to lithium-ion batteries for automotive applications. Their magnesium-ion battery research focuses on developing high-voltage spinel cathodes compatible with non-corrosive electrolytes, demonstrating stable cycling at voltages exceeding 3V. For calcium-ion batteries, Toyota has pioneered work on calcium-graphite intercalation compounds as anode materials and calcium cobalt oxide structures as cathodes. Their comparative studies have revealed that while magnesium-ion batteries offer potentially higher volumetric energy density due to magnesium's divalent nature, calcium-ion systems may provide better power performance due to potentially faster ion diffusion kinetics. Toyota has also developed innovative electrolyte formulations for both systems, including boron-cluster based electrolytes for magnesium and calcium tetrafluoroborate solutions that show promising electrochemical stability windows approaching 4V.
Strengths: Strong integration of fundamental research with practical automotive applications; extensive testing capabilities for battery performance under real-world conditions; significant resources for scaling promising technologies. Weaknesses: Some materials show promising initial performance but suffer from capacity fading during extended cycling; challenges remain in achieving energy densities competitive with advanced lithium-ion systems.
Wuhan University of Technology
Technical Solution: Wuhan University of Technology has established a comprehensive research program comparing magnesium-ion and calcium-ion battery technologies, with particular emphasis on novel electrode materials and electrolyte systems. Their magnesium-ion battery research focuses on developing high-performance cathode materials based on vanadium oxide structures with expanded interlayer spacing to facilitate magnesium ion diffusion. For calcium-ion batteries, they've pioneered work on calcium transition metal oxides with tailored crystal structures that demonstrate improved calcium ion insertion/extraction kinetics. Their comparative studies have revealed that while magnesium-ion batteries face severe challenges with electrolyte compatibility and slow diffusion kinetics, calcium-ion systems offer potentially faster kinetics but struggle with calcium metal anode stability. The university has also developed innovative electrolyte formulations for both systems, including borohydride-based electrolytes for magnesium and calcium tetrafluoroborate solutions that show promising electrochemical performance.
Strengths: Strong materials science expertise applied to both battery chemistries; innovative cathode design approaches; comprehensive comparative analysis between the two battery systems. Weaknesses: Some materials show promising initial performance but suffer from capacity fading; challenges remain in developing stable electrolytes compatible with both anode and cathode materials.
Key Patents and Scientific Breakthroughs in Mg-Ca Systems
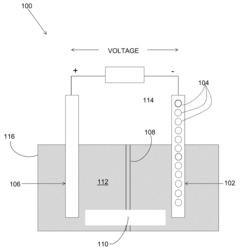
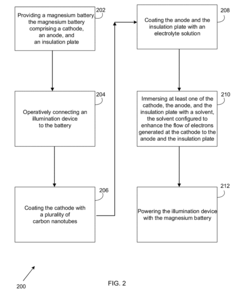
Magnesium battery and method of actuating
PatentInactiveUS20170256814A1
Innovation
- A magnesium battery design featuring a cathode coated with hydrophilic carbon nanotubes, an anode configured to absorb electrons, and an insulation plate with conductive porous material, using an electrolyte solution of sea salt, glutamine sodium, and calcium carbonate to enhance ionization and inhibit corrosion, allowing the battery to power an illumination device through immersion in a solvent.
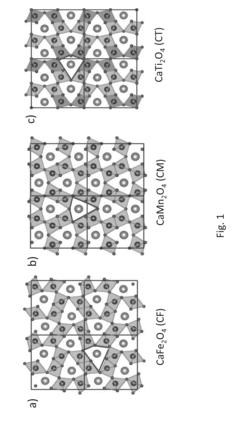
MgMn2O4 WITH A CRYSTAL STRUCTURE ANALOGUE TO CaFe2O4, CaMn2O4, OR CaTi2O4 AS RECHARGEABLE MAGNESIUM BATTERY CATHODE
PatentInactiveUS20140295280A1
Innovation
- A cathode active material with the formula MgxMn2O4, featuring a crystal structure analogous to CaFe2O4, CaMn2O4, or CaTi2O4, which creates an open channel for single-dimensional ionic diffusion, facilitating lower activation energy barriers and cooperative ion hopping, thereby enhancing magnesium ion mobility.
Material Sustainability and Resource Availability Assessment
The sustainability and resource availability of battery materials represent critical factors in determining the long-term viability of emerging battery technologies. Magnesium and calcium, as potential alternatives to lithium in next-generation batteries, offer distinct advantages from a resource perspective that warrant careful examination.
Magnesium demonstrates exceptional resource abundance, ranking eighth among elements in the Earth's crust with a concentration of approximately 2.1%. This widespread availability translates to significantly lower extraction costs compared to lithium, with global reserves estimated at over 12 billion tons. Magnesium is primarily sourced from seawater, dolomite, and magnesite, with extraction methods that generally require less water consumption than lithium extraction from brines.
Calcium presents similarly favorable resource metrics, being the fifth most abundant element in the Earth's crust (3.4%) with estimated reserves exceeding 7 billion tons. Calcium compounds are widely distributed geographically, primarily in limestone, gypsum, and fluorite deposits, reducing supply chain vulnerabilities associated with geopolitical concentration.
Both elements demonstrate substantially lower environmental impact profiles compared to lithium mining operations. Magnesium extraction from seawater generates fewer toxic byproducts, while calcium mining typically involves less invasive quarrying techniques. Life cycle assessments indicate potential carbon footprint reductions of 30-45% for magnesium-ion batteries and 25-40% for calcium-ion batteries compared to conventional lithium-ion technologies.
The recycling infrastructure for both elements remains underdeveloped compared to established lithium recovery processes. However, preliminary research indicates promising recyclability characteristics, with theoretical recovery rates of 85-90% for magnesium and 80-85% for calcium from spent batteries. The simpler chemistry of magnesium-ion systems may offer advantages in end-of-life processing.
Supply chain resilience represents another critical dimension, with both elements demonstrating more geographically distributed production capabilities than lithium. Current magnesium production is concentrated in China (87%), while calcium compound production shows greater distribution across North America, Europe, and Asia, potentially reducing supply vulnerabilities.
Market forecasts suggest sufficient material availability to support large-scale commercialization of either technology, with projected supply-demand equilibrium maintained even under aggressive adoption scenarios through 2040. However, scaling production infrastructure remains a significant challenge requiring substantial capital investment to meet potential future demand.
Magnesium demonstrates exceptional resource abundance, ranking eighth among elements in the Earth's crust with a concentration of approximately 2.1%. This widespread availability translates to significantly lower extraction costs compared to lithium, with global reserves estimated at over 12 billion tons. Magnesium is primarily sourced from seawater, dolomite, and magnesite, with extraction methods that generally require less water consumption than lithium extraction from brines.
Calcium presents similarly favorable resource metrics, being the fifth most abundant element in the Earth's crust (3.4%) with estimated reserves exceeding 7 billion tons. Calcium compounds are widely distributed geographically, primarily in limestone, gypsum, and fluorite deposits, reducing supply chain vulnerabilities associated with geopolitical concentration.
Both elements demonstrate substantially lower environmental impact profiles compared to lithium mining operations. Magnesium extraction from seawater generates fewer toxic byproducts, while calcium mining typically involves less invasive quarrying techniques. Life cycle assessments indicate potential carbon footprint reductions of 30-45% for magnesium-ion batteries and 25-40% for calcium-ion batteries compared to conventional lithium-ion technologies.
The recycling infrastructure for both elements remains underdeveloped compared to established lithium recovery processes. However, preliminary research indicates promising recyclability characteristics, with theoretical recovery rates of 85-90% for magnesium and 80-85% for calcium from spent batteries. The simpler chemistry of magnesium-ion systems may offer advantages in end-of-life processing.
Supply chain resilience represents another critical dimension, with both elements demonstrating more geographically distributed production capabilities than lithium. Current magnesium production is concentrated in China (87%), while calcium compound production shows greater distribution across North America, Europe, and Asia, potentially reducing supply vulnerabilities.
Market forecasts suggest sufficient material availability to support large-scale commercialization of either technology, with projected supply-demand equilibrium maintained even under aggressive adoption scenarios through 2040. However, scaling production infrastructure remains a significant challenge requiring substantial capital investment to meet potential future demand.
Safety and Performance Benchmarking Methodologies
Establishing robust safety and performance benchmarking methodologies is critical for the comparative evaluation of magnesium-ion and calcium-ion battery technologies. These methodologies must address the unique characteristics and challenges presented by each battery chemistry while maintaining standardized protocols that enable meaningful comparisons.
For safety benchmarking, thermal stability testing represents a fundamental methodology that evaluates cell behavior under extreme temperature conditions. Magnesium-ion batteries typically demonstrate superior thermal stability compared to calcium-ion batteries due to the lower reactivity of magnesium metal with electrolytes. Differential scanning calorimetry (DSC) and accelerated rate calorimetry (ARC) provide quantitative measurements of heat generation during thermal events, enabling precise comparison between these battery chemistries.
Electrochemical stability window (ESW) assessment constitutes another critical safety benchmark. This methodology measures the voltage range within which the electrolyte remains stable without decomposition. Calcium-ion systems often present narrower ESWs due to the higher reactivity of calcium metal, necessitating specialized testing protocols that account for these limitations while maintaining comparative validity with magnesium-ion systems.
Performance benchmarking methodologies must include standardized cycling protocols that evaluate capacity retention under various discharge rates and environmental conditions. The rate capability testing methodology should incorporate C-rates ranging from C/20 to 10C to fully characterize the power capabilities of both battery chemistries. Magnesium-ion batteries typically exhibit slower kinetics due to divalent ion diffusion limitations, requiring extended testing durations compared to monovalent systems.
Coulombic efficiency measurement represents a crucial performance benchmark that quantifies the reversibility of electrochemical reactions. This methodology must account for the different dendrite formation tendencies between magnesium and calcium systems, with calcium generally showing higher propensity for dendrite growth that impacts long-term efficiency metrics.
Impedance spectroscopy serves as an essential methodology for characterizing internal resistance evolution. The technique should be standardized to measure resistance changes at various state-of-charge levels and after defined cycling intervals, enabling direct comparison of the interfacial stability between magnesium and calcium systems.
Environmental testing methodologies must evaluate performance across temperature ranges from -40°C to 85°C, with particular attention to low-temperature performance where both chemistries face significant challenges due to reduced ion mobility. Calcium-ion batteries typically demonstrate more severe capacity limitations at low temperatures compared to magnesium-ion systems, necessitating specialized low-temperature protocols.
Ultimately, these benchmarking methodologies must be integrated into a comprehensive evaluation framework that weighs safety and performance metrics according to specific application requirements, enabling technology developers to make informed decisions regarding the selection and optimization of these promising post-lithium battery technologies.
For safety benchmarking, thermal stability testing represents a fundamental methodology that evaluates cell behavior under extreme temperature conditions. Magnesium-ion batteries typically demonstrate superior thermal stability compared to calcium-ion batteries due to the lower reactivity of magnesium metal with electrolytes. Differential scanning calorimetry (DSC) and accelerated rate calorimetry (ARC) provide quantitative measurements of heat generation during thermal events, enabling precise comparison between these battery chemistries.
Electrochemical stability window (ESW) assessment constitutes another critical safety benchmark. This methodology measures the voltage range within which the electrolyte remains stable without decomposition. Calcium-ion systems often present narrower ESWs due to the higher reactivity of calcium metal, necessitating specialized testing protocols that account for these limitations while maintaining comparative validity with magnesium-ion systems.
Performance benchmarking methodologies must include standardized cycling protocols that evaluate capacity retention under various discharge rates and environmental conditions. The rate capability testing methodology should incorporate C-rates ranging from C/20 to 10C to fully characterize the power capabilities of both battery chemistries. Magnesium-ion batteries typically exhibit slower kinetics due to divalent ion diffusion limitations, requiring extended testing durations compared to monovalent systems.
Coulombic efficiency measurement represents a crucial performance benchmark that quantifies the reversibility of electrochemical reactions. This methodology must account for the different dendrite formation tendencies between magnesium and calcium systems, with calcium generally showing higher propensity for dendrite growth that impacts long-term efficiency metrics.
Impedance spectroscopy serves as an essential methodology for characterizing internal resistance evolution. The technique should be standardized to measure resistance changes at various state-of-charge levels and after defined cycling intervals, enabling direct comparison of the interfacial stability between magnesium and calcium systems.
Environmental testing methodologies must evaluate performance across temperature ranges from -40°C to 85°C, with particular attention to low-temperature performance where both chemistries face significant challenges due to reduced ion mobility. Calcium-ion batteries typically demonstrate more severe capacity limitations at low temperatures compared to magnesium-ion systems, necessitating specialized low-temperature protocols.
Ultimately, these benchmarking methodologies must be integrated into a comprehensive evaluation framework that weighs safety and performance metrics according to specific application requirements, enabling technology developers to make informed decisions regarding the selection and optimization of these promising post-lithium battery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!