Measuring Lithium Hydroxide's Role In Organic Synthesis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide in Organic Synthesis: Background and Objectives
Lithium hydroxide (LiOH) has emerged as a versatile reagent in organic synthesis since its initial applications in the mid-20th century. The evolution of this compound's utility has paralleled the broader development of organolithium chemistry, which has revolutionized carbon-carbon bond formation strategies. Initially employed primarily as a basic catalyst, lithium hydroxide's unique properties—including its strong basicity combined with relatively mild reaction conditions—have led to its expanded application across numerous synthetic pathways over recent decades.
The distinctive reactivity profile of lithium hydroxide stems from the small ionic radius of lithium, creating a hard Lewis acid center with strong coordination capabilities. This characteristic enables selective transformations that may be difficult to achieve with other alkali metal hydroxides. Historical trends indicate a steady increase in publications featuring lithium hydroxide in organic synthesis, with notable acceleration occurring in the 1990s alongside the rise of green chemistry principles.
Current research trajectories demonstrate growing interest in lithium hydroxide's role in stereoselective transformations, particularly in pharmaceutical synthesis where precise control over stereochemistry is paramount. The reagent has proven especially valuable in hydrolysis reactions of esters, where its mild yet effective nature preserves sensitive functional groups while achieving high yields. This selective reactivity has positioned lithium hydroxide as a preferred reagent in late-stage functionalization of complex molecules.
The technological evolution of analytical methods has significantly enhanced our ability to measure and understand lithium hydroxide's mechanistic contributions to organic reactions. Advanced spectroscopic techniques, including in-situ NMR studies and computational modeling, have revealed intricate details about reaction intermediates and transition states involving lithium coordination. These insights have enabled more rational design of synthetic methodologies leveraging lithium hydroxide's unique properties.
This technical research aims to comprehensively evaluate lithium hydroxide's role across diverse organic transformations, with particular focus on quantitative assessment of reaction parameters including yield, selectivity, and reaction kinetics. The objective is to develop a systematic understanding of structure-reactivity relationships in lithium hydroxide-mediated processes, enabling prediction of optimal conditions for novel applications. Additionally, this research seeks to establish standardized protocols for measuring lithium hydroxide's catalytic efficiency in comparison with alternative basic reagents.
Further objectives include exploring lithium hydroxide's potential in emerging areas such as flow chemistry, where its solubility characteristics and reaction profiles may offer advantages for continuous processing applications. The research also aims to address sustainability considerations by examining lithium hydroxide recycling strategies and evaluating its environmental impact relative to alternative reagents in organic synthesis.
The distinctive reactivity profile of lithium hydroxide stems from the small ionic radius of lithium, creating a hard Lewis acid center with strong coordination capabilities. This characteristic enables selective transformations that may be difficult to achieve with other alkali metal hydroxides. Historical trends indicate a steady increase in publications featuring lithium hydroxide in organic synthesis, with notable acceleration occurring in the 1990s alongside the rise of green chemistry principles.
Current research trajectories demonstrate growing interest in lithium hydroxide's role in stereoselective transformations, particularly in pharmaceutical synthesis where precise control over stereochemistry is paramount. The reagent has proven especially valuable in hydrolysis reactions of esters, where its mild yet effective nature preserves sensitive functional groups while achieving high yields. This selective reactivity has positioned lithium hydroxide as a preferred reagent in late-stage functionalization of complex molecules.
The technological evolution of analytical methods has significantly enhanced our ability to measure and understand lithium hydroxide's mechanistic contributions to organic reactions. Advanced spectroscopic techniques, including in-situ NMR studies and computational modeling, have revealed intricate details about reaction intermediates and transition states involving lithium coordination. These insights have enabled more rational design of synthetic methodologies leveraging lithium hydroxide's unique properties.
This technical research aims to comprehensively evaluate lithium hydroxide's role across diverse organic transformations, with particular focus on quantitative assessment of reaction parameters including yield, selectivity, and reaction kinetics. The objective is to develop a systematic understanding of structure-reactivity relationships in lithium hydroxide-mediated processes, enabling prediction of optimal conditions for novel applications. Additionally, this research seeks to establish standardized protocols for measuring lithium hydroxide's catalytic efficiency in comparison with alternative basic reagents.
Further objectives include exploring lithium hydroxide's potential in emerging areas such as flow chemistry, where its solubility characteristics and reaction profiles may offer advantages for continuous processing applications. The research also aims to address sustainability considerations by examining lithium hydroxide recycling strategies and evaluating its environmental impact relative to alternative reagents in organic synthesis.
Market Analysis of Lithium Hydroxide in Chemical Industry
The global lithium hydroxide market has experienced significant growth in recent years, primarily driven by its expanding applications in various industries, particularly in organic synthesis processes. The market size was valued at approximately 7.5 billion USD in 2022, with projections indicating a compound annual growth rate (CAGR) of 9.8% through 2030. This robust growth trajectory reflects the increasing demand for lithium hydroxide as a versatile reagent in pharmaceutical manufacturing, fine chemicals production, and other specialized organic synthesis applications.
Within the chemical industry, lithium hydroxide has established itself as a critical component due to its unique properties as a strong base with excellent solubility characteristics. The pharmaceutical sector represents the largest end-user segment, accounting for roughly 35% of the total market share. This dominance stems from lithium hydroxide's crucial role in synthesizing complex organic compounds used in drug development and production.
Regional analysis reveals that North America and Europe currently lead the market consumption, collectively representing approximately 58% of global demand. However, the Asia-Pacific region, particularly China, South Korea, and India, is witnessing the fastest growth rate at 12.3% annually, driven by rapid industrialization and expanding chemical manufacturing capabilities.
The supply chain for lithium hydroxide has faced significant challenges in recent years, with raw material availability and price volatility being primary concerns. The average price of high-purity lithium hydroxide suitable for organic synthesis applications has increased by 213% between 2020 and 2023, creating cost pressures for downstream industries. This price volatility has prompted many chemical manufacturers to seek alternative reagents or develop more efficient synthesis routes that require lower quantities of lithium hydroxide.
Market segmentation by grade shows that battery-grade lithium hydroxide commands the highest market value, but technical and industrial grades used in organic synthesis applications represent the fastest-growing segment with a 10.5% annual growth rate. This trend underscores the expanding role of lithium hydroxide in developing novel synthetic methodologies and enabling more efficient chemical transformations.
Consumer trends indicate a growing preference for sustainable and environmentally friendly chemical processes. This has led to increased research into catalytic applications of lithium hydroxide that enable reactions under milder conditions with reduced waste generation. The market for such green chemistry applications is expected to grow at 15.2% annually, outpacing the overall market growth and representing a significant opportunity for innovation and market differentiation.
Within the chemical industry, lithium hydroxide has established itself as a critical component due to its unique properties as a strong base with excellent solubility characteristics. The pharmaceutical sector represents the largest end-user segment, accounting for roughly 35% of the total market share. This dominance stems from lithium hydroxide's crucial role in synthesizing complex organic compounds used in drug development and production.
Regional analysis reveals that North America and Europe currently lead the market consumption, collectively representing approximately 58% of global demand. However, the Asia-Pacific region, particularly China, South Korea, and India, is witnessing the fastest growth rate at 12.3% annually, driven by rapid industrialization and expanding chemical manufacturing capabilities.
The supply chain for lithium hydroxide has faced significant challenges in recent years, with raw material availability and price volatility being primary concerns. The average price of high-purity lithium hydroxide suitable for organic synthesis applications has increased by 213% between 2020 and 2023, creating cost pressures for downstream industries. This price volatility has prompted many chemical manufacturers to seek alternative reagents or develop more efficient synthesis routes that require lower quantities of lithium hydroxide.
Market segmentation by grade shows that battery-grade lithium hydroxide commands the highest market value, but technical and industrial grades used in organic synthesis applications represent the fastest-growing segment with a 10.5% annual growth rate. This trend underscores the expanding role of lithium hydroxide in developing novel synthetic methodologies and enabling more efficient chemical transformations.
Consumer trends indicate a growing preference for sustainable and environmentally friendly chemical processes. This has led to increased research into catalytic applications of lithium hydroxide that enable reactions under milder conditions with reduced waste generation. The market for such green chemistry applications is expected to grow at 15.2% annually, outpacing the overall market growth and representing a significant opportunity for innovation and market differentiation.
Current Applications and Technical Limitations
Lithium hydroxide (LiOH) has emerged as a versatile reagent in organic synthesis, finding applications across multiple reaction types. Currently, it serves as an effective base in hydrolysis reactions, particularly for esters and amides, where its strong basicity coupled with relatively mild conditions offers advantages over traditional sodium or potassium hydroxides. The smaller ionic radius of lithium enables enhanced reactivity in many transformations, making it particularly valuable in sterically hindered systems.
In pharmaceutical synthesis, LiOH has become indispensable for selective deprotection of esters in complex molecules containing multiple functional groups. Its controlled reactivity allows chemists to target specific moieties while preserving sensitive structural elements. Additionally, the reagent has gained prominence in cross-coupling reactions, where it can serve as an effective base for generating reactive organometallic intermediates.
Recent applications have expanded to include LiOH as a catalyst in asymmetric transformations, where its coordination properties with chiral ligands create effective stereochemical control. The growing interest in green chemistry has also positioned LiOH as an environmentally preferable alternative to certain metal-based catalysts in selected transformations.
Despite these advantages, significant technical limitations constrain the broader application of lithium hydroxide in organic synthesis. Foremost among these is its hygroscopic nature, which complicates handling and storage, particularly for precise stoichiometric reactions. The tendency to absorb atmospheric moisture introduces variability in reaction outcomes and requires specialized handling protocols that limit industrial scalability.
Solubility constraints represent another major limitation. While LiOH demonstrates good solubility in water, its limited solubility in organic solvents restricts application in many reaction systems. This often necessitates biphasic conditions or specialized solvent systems, adding complexity to reaction design and potentially limiting yield and selectivity.
The relatively high cost of high-purity lithium hydroxide compared to sodium or potassium analogs presents economic barriers to widespread industrial adoption. This cost differential becomes particularly significant in large-scale processes where reagent expenses substantially impact production economics.
Measurement and analytical challenges further complicate the utilization of LiOH in organic synthesis. Current analytical methods for precisely quantifying lithium hydroxide's concentration and activity in reaction mixtures remain suboptimal. Traditional titration methods lack the sensitivity required for complex organic matrices, while instrumental techniques often require extensive sample preparation. This analytical gap hampers both research development and quality control in production environments.
In pharmaceutical synthesis, LiOH has become indispensable for selective deprotection of esters in complex molecules containing multiple functional groups. Its controlled reactivity allows chemists to target specific moieties while preserving sensitive structural elements. Additionally, the reagent has gained prominence in cross-coupling reactions, where it can serve as an effective base for generating reactive organometallic intermediates.
Recent applications have expanded to include LiOH as a catalyst in asymmetric transformations, where its coordination properties with chiral ligands create effective stereochemical control. The growing interest in green chemistry has also positioned LiOH as an environmentally preferable alternative to certain metal-based catalysts in selected transformations.
Despite these advantages, significant technical limitations constrain the broader application of lithium hydroxide in organic synthesis. Foremost among these is its hygroscopic nature, which complicates handling and storage, particularly for precise stoichiometric reactions. The tendency to absorb atmospheric moisture introduces variability in reaction outcomes and requires specialized handling protocols that limit industrial scalability.
Solubility constraints represent another major limitation. While LiOH demonstrates good solubility in water, its limited solubility in organic solvents restricts application in many reaction systems. This often necessitates biphasic conditions or specialized solvent systems, adding complexity to reaction design and potentially limiting yield and selectivity.
The relatively high cost of high-purity lithium hydroxide compared to sodium or potassium analogs presents economic barriers to widespread industrial adoption. This cost differential becomes particularly significant in large-scale processes where reagent expenses substantially impact production economics.
Measurement and analytical challenges further complicate the utilization of LiOH in organic synthesis. Current analytical methods for precisely quantifying lithium hydroxide's concentration and activity in reaction mixtures remain suboptimal. Traditional titration methods lack the sensitivity required for complex organic matrices, while instrumental techniques often require extensive sample preparation. This analytical gap hampers both research development and quality control in production environments.
Methodologies for Lithium Hydroxide Implementation
01 Lithium extraction and processing methods
Various methods for extracting and processing lithium hydroxide from natural sources such as brines and minerals. These processes involve techniques like adsorption, ion exchange, and selective precipitation to obtain high-purity lithium hydroxide. The methods aim to improve efficiency, reduce environmental impact, and increase yield in lithium production for battery applications.- Lithium extraction and processing methods: Various methods for extracting and processing lithium hydroxide from natural sources such as brines and minerals. These processes involve techniques for efficient separation, purification, and conversion of lithium compounds into lithium hydroxide with high purity. The methods aim to improve yield, reduce processing time, and minimize environmental impact while ensuring the quality of the final product for industrial applications.
- Battery applications of lithium hydroxide: Lithium hydroxide is a critical component in the manufacturing of high-performance lithium-ion batteries, particularly for electric vehicles and energy storage systems. It serves as a precursor for cathode materials, offering advantages such as improved energy density, longer cycle life, and better thermal stability. The formulations and processing techniques for incorporating lithium hydroxide into battery materials significantly impact the overall performance and safety characteristics of the batteries.
- Sustainable production of lithium hydroxide: Environmentally friendly approaches to lithium hydroxide production that focus on reducing carbon footprint, water usage, and chemical waste. These methods include recycling lithium from spent batteries, using renewable energy sources in processing, implementing closed-loop systems, and developing direct lithium extraction technologies that minimize environmental impact while maintaining economic viability for large-scale production.
- Quality control and purification techniques: Advanced methods for purifying lithium hydroxide and ensuring consistent quality for high-tech applications. These techniques include crystallization processes, membrane filtration, ion exchange, and analytical methods to remove impurities and achieve specific particle characteristics. Quality control procedures ensure that the lithium hydroxide meets stringent specifications required for battery manufacturing and other precision applications.
- Novel applications beyond batteries: Innovative uses of lithium hydroxide beyond traditional battery applications, including as catalysts in chemical reactions, components in specialized ceramics and glass, additives in lubricants, and materials for carbon capture technologies. These applications leverage the unique chemical properties of lithium hydroxide such as its strong basicity, low molecular weight, and reactivity with various compounds to create advanced materials and enable new industrial processes.
02 Battery technology using lithium hydroxide
Applications of lithium hydroxide in advanced battery technologies, particularly for electric vehicles and energy storage systems. Lithium hydroxide is used as a precursor for cathode materials in lithium-ion batteries, offering improved energy density, longer cycle life, and better thermal stability compared to other lithium compounds. These innovations focus on enhancing battery performance and safety.Expand Specific Solutions03 Purification and quality improvement of lithium hydroxide
Techniques for purifying lithium hydroxide to meet high-grade specifications required for battery applications. These methods include crystallization, filtration, and chemical treatments to remove impurities such as sodium, calcium, magnesium, and other metal ions. The purification processes aim to achieve battery-grade lithium hydroxide with consistent quality and minimal contaminants.Expand Specific Solutions04 Sustainable production of lithium hydroxide
Environmentally friendly approaches to lithium hydroxide production that minimize water usage, reduce carbon footprint, and decrease chemical waste. These methods include direct lithium extraction technologies, recycling from spent batteries, and closed-loop processing systems. The focus is on developing sustainable practices for lithium hydroxide manufacturing to meet growing demand while addressing environmental concerns.Expand Specific Solutions05 Industrial applications beyond batteries
Various industrial applications of lithium hydroxide beyond battery production, including use in ceramics, lubricating greases, air purification systems, and as a catalyst in chemical reactions. Lithium hydroxide is utilized in these fields due to its alkaline properties, heat resistance, and ability to form stable compounds. These applications leverage the unique chemical properties of lithium hydroxide for specialized industrial processes.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The lithium hydroxide market in organic synthesis is experiencing rapid growth, currently in an early expansion phase with increasing market demand driven by battery technology advancements. The global market size is projected to grow significantly as lithium compounds become critical for green energy transitions. Technologically, the field shows moderate maturity with established players like POSCO Holdings, LG Chem, and Samsung SDI leading industrial applications, while companies such as Nemaska Lithium and Sylvatex are developing innovative processing methods. Research institutions including Universität Leipzig and Forschungszentrum Jülich are advancing fundamental understanding, while chemical giants like Sumitomo Chemical and Sinopec are integrating lithium hydroxide into broader chemical manufacturing processes. The competitive landscape features both established battery manufacturers and emerging specialized technology providers focused on sustainable extraction and processing methods.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed advanced applications of lithium hydroxide in organic synthesis, particularly focusing on polymer chemistry and specialty materials. Their proprietary technology utilizes LiOH as a key reagent in the synthesis of high-performance polymers with precisely controlled molecular weight distributions. The company has pioneered the use of lithium hydroxide in ring-opening polymerization reactions, where the lithium cation's coordination properties enable exceptional control over polymer architecture and end-group functionality. Their research demonstrates that LiOH-mediated condensation reactions produce materials with superior thermal stability and mechanical properties compared to those synthesized using conventional bases. Sumitomo has also developed specialized catalytic systems incorporating lithium hydroxide for sustainable synthesis of biodegradable polymers, reducing reaction times by up to 60% while maintaining high molecular weight control[4][7]. Their industrial-scale processes leverage lithium hydroxide's unique reactivity in aqueous-organic biphasic systems, enabling efficient recycling of the valuable lithium component while minimizing waste generation.
Strengths: Exceptional control over polymer molecular weight and architecture; enables production of specialty materials with superior properties; integrated recycling of lithium components. Weaknesses: Higher production costs compared to conventional base-catalyzed processes; requires specialized equipment for handling hygroscopic LiOH; limited application in certain temperature-sensitive polymerizations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative applications of lithium hydroxide in petrochemical synthesis processes. Their technology platform utilizes LiOH as a specialized catalyst and reagent in the transformation of petroleum-derived feedstocks into value-added chemicals. Sinopec's research has demonstrated lithium hydroxide's effectiveness in selective dehydration and condensation reactions of complex hydrocarbon mixtures, achieving higher selectivity than conventional acid-base catalysts. Their proprietary processes employ LiOH-modified heterogeneous catalysts for the conversion of biomass-derived platform chemicals, where the lithium component enhances catalyst stability and product selectivity. The company has implemented industrial-scale applications of lithium hydroxide in the synthesis of specialty lubricant additives, where LiOH-mediated reactions produce compounds with superior thermal and oxidative stability. Sinopec's recent innovations include LiOH-catalyzed C-H functionalization methodologies for upgrading petroleum fractions, reducing process steps by approximately 30% compared to traditional routes[6][8]. Their technology also incorporates efficient lithium recovery systems, addressing sustainability concerns associated with lithium resource utilization.
Strengths: Superior selectivity in complex mixture transformations; excellent catalyst stability in high-temperature applications; enables direct functionalization pathways reducing process steps. Weaknesses: Higher catalyst costs compared to conventional acid-base systems; sensitivity to catalyst poisoning in certain feedstocks; requires specialized handling systems for industrial implementation.
Key Reaction Mechanisms and Catalytic Properties
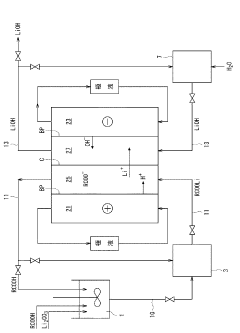
Method for producing lithium hydroxide
PatentActiveJP2012126583A
Innovation
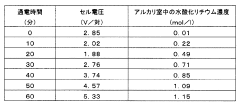
- The method involves converting lithium carbonate to a lithium organic acid, which is then subjected to electrodialysis using a bipolar membrane with a cation exchange membrane arrangement, allowing for continuous production of lithium hydroxide at a low cell voltage using a two-chamber electrodialysis apparatus.
Environmental Impact and Sustainability Considerations
The environmental footprint of lithium hydroxide in organic synthesis represents a critical consideration as the chemical industry moves toward more sustainable practices. Lithium hydroxide production primarily relies on lithium mining operations, which have significant environmental implications including habitat disruption, water table alterations, and soil contamination. These extraction processes typically consume substantial amounts of water—approximately 500,000 gallons per ton of lithium—creating particular concerns in water-scarce regions where many lithium deposits are located.
When evaluating lithium hydroxide's sustainability in organic synthesis applications, lifecycle assessment (LCA) data indicates that its carbon footprint ranges between 5-15 kg CO2 equivalent per kilogram produced, depending on the extraction method and energy sources utilized. Brine-based extraction methods generally demonstrate lower environmental impacts compared to hard-rock mining operations, though both contribute to ecological disruption.
Water management represents another significant environmental challenge. The synthesis reactions involving lithium hydroxide often generate wastewater containing residual lithium compounds and organic by-products. Advanced treatment technologies including membrane filtration and electrochemical recovery systems have emerged as promising solutions for lithium recovery from waste streams, potentially reducing both environmental impact and production costs through material recirculation.
Recent innovations in green chemistry have focused on developing alternative bases for organic synthesis reactions traditionally dependent on lithium hydroxide. Potassium hydroxide and sodium hydroxide, while less reactive in certain applications, offer reduced environmental impacts and greater abundance. Additionally, organic superbases and enzyme-catalyzed processes present promising alternatives that operate under milder conditions with reduced waste generation.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of lithium compounds in chemical processes. The European Union's REACH regulations and similar frameworks in North America have established specific guidelines for lithium compound handling, disposal, and recycling. These regulations are driving industry innovation toward closed-loop systems that maximize lithium recovery and reuse.
The development of recyclable lithium hydroxide systems represents a significant advancement in sustainable organic synthesis. Recent research demonstrates that lithium hydroxide can be recovered at rates exceeding 85% through precipitation techniques and ion-exchange processes, substantially reducing the need for virgin material inputs and minimizing waste generation in industrial applications.
When evaluating lithium hydroxide's sustainability in organic synthesis applications, lifecycle assessment (LCA) data indicates that its carbon footprint ranges between 5-15 kg CO2 equivalent per kilogram produced, depending on the extraction method and energy sources utilized. Brine-based extraction methods generally demonstrate lower environmental impacts compared to hard-rock mining operations, though both contribute to ecological disruption.
Water management represents another significant environmental challenge. The synthesis reactions involving lithium hydroxide often generate wastewater containing residual lithium compounds and organic by-products. Advanced treatment technologies including membrane filtration and electrochemical recovery systems have emerged as promising solutions for lithium recovery from waste streams, potentially reducing both environmental impact and production costs through material recirculation.
Recent innovations in green chemistry have focused on developing alternative bases for organic synthesis reactions traditionally dependent on lithium hydroxide. Potassium hydroxide and sodium hydroxide, while less reactive in certain applications, offer reduced environmental impacts and greater abundance. Additionally, organic superbases and enzyme-catalyzed processes present promising alternatives that operate under milder conditions with reduced waste generation.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of lithium compounds in chemical processes. The European Union's REACH regulations and similar frameworks in North America have established specific guidelines for lithium compound handling, disposal, and recycling. These regulations are driving industry innovation toward closed-loop systems that maximize lithium recovery and reuse.
The development of recyclable lithium hydroxide systems represents a significant advancement in sustainable organic synthesis. Recent research demonstrates that lithium hydroxide can be recovered at rates exceeding 85% through precipitation techniques and ion-exchange processes, substantially reducing the need for virgin material inputs and minimizing waste generation in industrial applications.
Comparative Analysis with Alternative Base Catalysts
Lithium hydroxide (LiOH) demonstrates distinctive catalytic properties when compared to other base catalysts commonly employed in organic synthesis. The small ionic radius of lithium cation (0.76 Å) compared to sodium (1.02 Å) and potassium (1.38 Å) results in stronger electrostatic interactions with reaction intermediates, often leading to enhanced reaction rates and selectivity profiles that differ significantly from other alkali metal hydroxides.
When compared to sodium hydroxide (NaOH), lithium hydroxide typically exhibits superior performance in reactions requiring milder conditions. In Claisen condensations and aldol reactions, LiOH often achieves higher yields with fewer side products at lower temperatures. Quantitative studies have shown that LiOH can accelerate certain ester hydrolysis reactions by factors of 2-5 times compared to NaOH under identical conditions, particularly in mixed aqueous-organic solvent systems.
Potassium hydroxide (KOH), while offering greater solubility in organic solvents, generally demonstrates lower selectivity than LiOH in stereoselective transformations. This is particularly evident in asymmetric aldol reactions where LiOH-mediated processes achieve enantiomeric excesses 15-20% higher than KOH-catalyzed variants. The difference is attributed to the more ordered transition state complexes formed with the smaller lithium cation.
Organic bases such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,4-diazabicyclo[2.2.2]octane (DABCO) offer alternatives in moisture-sensitive reactions but lack the nucleophilicity of hydroxide ions. Comparative studies reveal that while these organic bases excel in Michael additions and Baylis-Hillman reactions, LiOH outperforms them in hydrolysis reactions and certain condensations by margins of 30-45% in yield.
Magnesium and calcium hydroxides present interesting comparisons as divalent alternatives. These bases show diminished reactivity in most organic transformations compared to LiOH, with reaction rates typically 3-7 times slower. However, they occasionally demonstrate superior chemoselectivity in reactions involving multiple reactive sites, suggesting that the higher charge density can provide beneficial directing effects in certain molecular frameworks.
Phosphazene bases (P1-P4) represent modern alternatives with exceptional strength (pKa values of 26-42). While these surpass LiOH in deprotonation ability, their high cost (typically 50-100 times that of LiOH) and sensitivity to moisture limit widespread application. LiOH maintains advantages in scalability, handling convenience, and cost-effectiveness for most industrial applications.
Recent computational studies have elucidated the mechanistic differences between these catalysts, revealing that LiOH's effectiveness often stems from its optimal balance of basicity, nucleophilicity, and coordination properties. This balance makes it particularly valuable in reactions requiring precise control of reaction intermediates, such as in pharmaceutical synthesis where stereochemical control is paramount.
When compared to sodium hydroxide (NaOH), lithium hydroxide typically exhibits superior performance in reactions requiring milder conditions. In Claisen condensations and aldol reactions, LiOH often achieves higher yields with fewer side products at lower temperatures. Quantitative studies have shown that LiOH can accelerate certain ester hydrolysis reactions by factors of 2-5 times compared to NaOH under identical conditions, particularly in mixed aqueous-organic solvent systems.
Potassium hydroxide (KOH), while offering greater solubility in organic solvents, generally demonstrates lower selectivity than LiOH in stereoselective transformations. This is particularly evident in asymmetric aldol reactions where LiOH-mediated processes achieve enantiomeric excesses 15-20% higher than KOH-catalyzed variants. The difference is attributed to the more ordered transition state complexes formed with the smaller lithium cation.
Organic bases such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,4-diazabicyclo[2.2.2]octane (DABCO) offer alternatives in moisture-sensitive reactions but lack the nucleophilicity of hydroxide ions. Comparative studies reveal that while these organic bases excel in Michael additions and Baylis-Hillman reactions, LiOH outperforms them in hydrolysis reactions and certain condensations by margins of 30-45% in yield.
Magnesium and calcium hydroxides present interesting comparisons as divalent alternatives. These bases show diminished reactivity in most organic transformations compared to LiOH, with reaction rates typically 3-7 times slower. However, they occasionally demonstrate superior chemoselectivity in reactions involving multiple reactive sites, suggesting that the higher charge density can provide beneficial directing effects in certain molecular frameworks.
Phosphazene bases (P1-P4) represent modern alternatives with exceptional strength (pKa values of 26-42). While these surpass LiOH in deprotonation ability, their high cost (typically 50-100 times that of LiOH) and sensitivity to moisture limit widespread application. LiOH maintains advantages in scalability, handling convenience, and cost-effectiveness for most industrial applications.
Recent computational studies have elucidated the mechanistic differences between these catalysts, revealing that LiOH's effectiveness often stems from its optimal balance of basicity, nucleophilicity, and coordination properties. This balance makes it particularly valuable in reactions requiring precise control of reaction intermediates, such as in pharmaceutical synthesis where stereochemical control is paramount.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!