Microchannel Cooling Systems Optimized for Pharmaceutical Manufacturing
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microchannel Cooling Technology Evolution and Objectives
Microchannel cooling technology emerged in the late 1980s as a revolutionary approach to thermal management, initially developed for aerospace and electronics applications. The fundamental principle involves the use of small channels (typically 10-500 micrometers in diameter) to facilitate enhanced heat transfer through increased surface area-to-volume ratios. This technology has evolved significantly over the past three decades, transitioning from theoretical concepts to practical implementations across various industries.
The evolution of microchannel cooling systems has been marked by several key technological advancements. Early systems faced challenges related to manufacturing precision, material compatibility, and flow distribution. By the early 2000s, improvements in microfabrication techniques enabled more precise channel geometries, while computational fluid dynamics (CFD) modeling allowed for optimized flow patterns and reduced pressure drops.
In the pharmaceutical manufacturing context, microchannel cooling represents a paradigm shift from conventional jacketed vessels and heat exchangers. Traditional pharmaceutical cooling systems often struggle with temperature uniformity, precise control, and scalability—issues that directly impact product quality, consistency, and production efficiency. The pharmaceutical industry's stringent requirements for contamination control, material compatibility, and validation have historically limited innovation in cooling technologies.
The integration of microchannel cooling into pharmaceutical manufacturing processes aims to address several critical objectives. Primary among these is achieving unprecedented temperature uniformity and control precision, which directly impacts reaction kinetics, crystallization processes, and product quality attributes. Enhanced heat transfer efficiency represents another key objective, potentially reducing energy consumption by 30-50% compared to conventional systems while enabling more rapid heating and cooling cycles.
Process intensification constitutes a third major objective, with microchannel systems supporting continuous manufacturing paradigms that align with the FDA's Quality by Design (QbD) initiatives. The compact nature of microchannel technology also addresses space constraints in existing facilities, offering modular solutions that can be scaled horizontally rather than vertically.
Looking forward, the technological trajectory points toward smart, integrated microchannel systems incorporating real-time monitoring capabilities, adaptive control algorithms, and predictive maintenance features. Material innovations focusing on pharmaceutical-grade surfaces with enhanced fouling resistance and cleanability represent another frontier. The ultimate objective is to develop a new generation of cooling systems that not only enhance thermal performance but fundamentally transform pharmaceutical manufacturing toward more efficient, flexible, and sustainable operations.
The evolution of microchannel cooling systems has been marked by several key technological advancements. Early systems faced challenges related to manufacturing precision, material compatibility, and flow distribution. By the early 2000s, improvements in microfabrication techniques enabled more precise channel geometries, while computational fluid dynamics (CFD) modeling allowed for optimized flow patterns and reduced pressure drops.
In the pharmaceutical manufacturing context, microchannel cooling represents a paradigm shift from conventional jacketed vessels and heat exchangers. Traditional pharmaceutical cooling systems often struggle with temperature uniformity, precise control, and scalability—issues that directly impact product quality, consistency, and production efficiency. The pharmaceutical industry's stringent requirements for contamination control, material compatibility, and validation have historically limited innovation in cooling technologies.
The integration of microchannel cooling into pharmaceutical manufacturing processes aims to address several critical objectives. Primary among these is achieving unprecedented temperature uniformity and control precision, which directly impacts reaction kinetics, crystallization processes, and product quality attributes. Enhanced heat transfer efficiency represents another key objective, potentially reducing energy consumption by 30-50% compared to conventional systems while enabling more rapid heating and cooling cycles.
Process intensification constitutes a third major objective, with microchannel systems supporting continuous manufacturing paradigms that align with the FDA's Quality by Design (QbD) initiatives. The compact nature of microchannel technology also addresses space constraints in existing facilities, offering modular solutions that can be scaled horizontally rather than vertically.
Looking forward, the technological trajectory points toward smart, integrated microchannel systems incorporating real-time monitoring capabilities, adaptive control algorithms, and predictive maintenance features. Material innovations focusing on pharmaceutical-grade surfaces with enhanced fouling resistance and cleanability represent another frontier. The ultimate objective is to develop a new generation of cooling systems that not only enhance thermal performance but fundamentally transform pharmaceutical manufacturing toward more efficient, flexible, and sustainable operations.
Pharmaceutical Manufacturing Cooling Requirements Analysis
Pharmaceutical manufacturing processes require precise temperature control to ensure product quality, stability, and compliance with regulatory standards. The cooling requirements in pharmaceutical production are significantly more stringent than in many other industries due to the sensitive nature of active pharmaceutical ingredients (APIs) and the critical impact of temperature on reaction kinetics, crystallization processes, and final product attributes.
Temperature control precision typically needs to maintain within ±0.5°C for many pharmaceutical processes, with some requiring even tighter tolerances of ±0.1°C. This level of precision is essential for ensuring batch-to-batch consistency and meeting quality standards established by regulatory bodies such as the FDA and EMA.
Heat removal requirements vary considerably across different pharmaceutical manufacturing operations. Fermentation processes can generate substantial metabolic heat, requiring cooling capacities of 10-50 kW/m³. Chemical synthesis reactions, particularly exothermic ones, may demand rapid cooling to prevent runaway reactions and maintain selectivity, with cooling loads potentially reaching 5-15 kW/m² of reactor surface area.
Crystallization processes present unique cooling challenges, requiring carefully controlled cooling rates (typically 0.1-1°C/minute) to achieve desired crystal size distribution, morphology, and polymorphic form. Inadequate cooling control during crystallization can lead to product quality issues and manufacturing inefficiencies.
Cleanability and material compatibility represent critical considerations for pharmaceutical cooling systems. All surfaces must be designed to prevent product contamination, biofilm formation, and material degradation. Cooling systems must comply with Good Manufacturing Practice (GMP) standards, utilizing materials that resist corrosion from cleaning agents and process chemicals.
Energy efficiency has become increasingly important in pharmaceutical manufacturing, with cooling systems typically accounting for 15-30% of a facility's energy consumption. Modern pharmaceutical operations seek cooling solutions that minimize environmental impact while maintaining process performance.
Scale-up considerations present significant challenges when transitioning from laboratory to production scale. Heat transfer characteristics change substantially with increasing vessel size, requiring sophisticated modeling and control strategies to maintain consistent cooling performance across different scales of operation.
Regulatory compliance necessitates comprehensive documentation of cooling system performance, including validation protocols, calibration records, and maintenance procedures. Systems must demonstrate consistent performance within validated parameters and include appropriate redundancy to prevent production disruptions.
Temperature control precision typically needs to maintain within ±0.5°C for many pharmaceutical processes, with some requiring even tighter tolerances of ±0.1°C. This level of precision is essential for ensuring batch-to-batch consistency and meeting quality standards established by regulatory bodies such as the FDA and EMA.
Heat removal requirements vary considerably across different pharmaceutical manufacturing operations. Fermentation processes can generate substantial metabolic heat, requiring cooling capacities of 10-50 kW/m³. Chemical synthesis reactions, particularly exothermic ones, may demand rapid cooling to prevent runaway reactions and maintain selectivity, with cooling loads potentially reaching 5-15 kW/m² of reactor surface area.
Crystallization processes present unique cooling challenges, requiring carefully controlled cooling rates (typically 0.1-1°C/minute) to achieve desired crystal size distribution, morphology, and polymorphic form. Inadequate cooling control during crystallization can lead to product quality issues and manufacturing inefficiencies.
Cleanability and material compatibility represent critical considerations for pharmaceutical cooling systems. All surfaces must be designed to prevent product contamination, biofilm formation, and material degradation. Cooling systems must comply with Good Manufacturing Practice (GMP) standards, utilizing materials that resist corrosion from cleaning agents and process chemicals.
Energy efficiency has become increasingly important in pharmaceutical manufacturing, with cooling systems typically accounting for 15-30% of a facility's energy consumption. Modern pharmaceutical operations seek cooling solutions that minimize environmental impact while maintaining process performance.
Scale-up considerations present significant challenges when transitioning from laboratory to production scale. Heat transfer characteristics change substantially with increasing vessel size, requiring sophisticated modeling and control strategies to maintain consistent cooling performance across different scales of operation.
Regulatory compliance necessitates comprehensive documentation of cooling system performance, including validation protocols, calibration records, and maintenance procedures. Systems must demonstrate consistent performance within validated parameters and include appropriate redundancy to prevent production disruptions.
Current Microchannel Cooling Challenges in Pharma Applications
Microchannel cooling systems in pharmaceutical manufacturing face several significant technical challenges that limit their widespread adoption and optimal performance. The primary obstacle remains the complex design requirements for microchannels that must balance heat transfer efficiency with pressure drop considerations. Current systems struggle to achieve uniform cooling across pharmaceutical processing equipment, leading to temperature gradients that can compromise product quality, especially in heat-sensitive biologics production.
Material compatibility presents another substantial hurdle, as pharmaceutical-grade requirements demand materials that are corrosion-resistant, non-reactive, and compliant with regulatory standards. Conventional microchannel materials like copper, while thermally efficient, may introduce contamination risks in pharmaceutical applications, necessitating the use of less thermally conductive alternatives such as stainless steel or specialized polymers.
Fouling and clogging of microchannels represent persistent operational challenges in pharmaceutical environments. The small dimensions of microchannels (typically 10-500 micrometers) make them particularly susceptible to blockage from particulates, precipitates, or biofilm formation. Current cleaning and maintenance protocols often prove inadequate for ensuring long-term reliability without system disassembly, which increases production downtime.
Scale-up difficulties continue to plague the industry, as laboratory-scale microchannel cooling solutions frequently fail to maintain performance when implemented in full production environments. The transition from small-scale to industrial-scale systems introduces flow distribution problems and manufacturing complexities that current design methodologies struggle to address effectively.
Integration with existing pharmaceutical manufacturing equipment presents significant compatibility issues. Retrofitting legacy systems with microchannel cooling technology requires custom engineering solutions that are often cost-prohibitive and technically challenging. The lack of standardized interfaces between microchannel cooling systems and pharmaceutical processing equipment creates implementation barriers.
Control system limitations further complicate microchannel cooling applications in pharmaceutical manufacturing. Current control algorithms struggle to respond rapidly enough to the dynamic thermal loads characteristic of batch pharmaceutical processes. The high thermal responsiveness of microchannel systems requires more sophisticated control strategies than those typically employed in conventional cooling systems.
Regulatory compliance and validation challenges remain significant barriers to adoption. Documentation requirements for cleaning validation, materials compatibility, and system performance qualification create substantial overhead for pharmaceutical manufacturers considering microchannel cooling technology. The conservative nature of pharmaceutical manufacturing, driven by regulatory scrutiny, slows the implementation of novel cooling technologies despite their potential benefits.
Material compatibility presents another substantial hurdle, as pharmaceutical-grade requirements demand materials that are corrosion-resistant, non-reactive, and compliant with regulatory standards. Conventional microchannel materials like copper, while thermally efficient, may introduce contamination risks in pharmaceutical applications, necessitating the use of less thermally conductive alternatives such as stainless steel or specialized polymers.
Fouling and clogging of microchannels represent persistent operational challenges in pharmaceutical environments. The small dimensions of microchannels (typically 10-500 micrometers) make them particularly susceptible to blockage from particulates, precipitates, or biofilm formation. Current cleaning and maintenance protocols often prove inadequate for ensuring long-term reliability without system disassembly, which increases production downtime.
Scale-up difficulties continue to plague the industry, as laboratory-scale microchannel cooling solutions frequently fail to maintain performance when implemented in full production environments. The transition from small-scale to industrial-scale systems introduces flow distribution problems and manufacturing complexities that current design methodologies struggle to address effectively.
Integration with existing pharmaceutical manufacturing equipment presents significant compatibility issues. Retrofitting legacy systems with microchannel cooling technology requires custom engineering solutions that are often cost-prohibitive and technically challenging. The lack of standardized interfaces between microchannel cooling systems and pharmaceutical processing equipment creates implementation barriers.
Control system limitations further complicate microchannel cooling applications in pharmaceutical manufacturing. Current control algorithms struggle to respond rapidly enough to the dynamic thermal loads characteristic of batch pharmaceutical processes. The high thermal responsiveness of microchannel systems requires more sophisticated control strategies than those typically employed in conventional cooling systems.
Regulatory compliance and validation challenges remain significant barriers to adoption. Documentation requirements for cleaning validation, materials compatibility, and system performance qualification create substantial overhead for pharmaceutical manufacturers considering microchannel cooling technology. The conservative nature of pharmaceutical manufacturing, driven by regulatory scrutiny, slows the implementation of novel cooling technologies despite their potential benefits.
Current Microchannel Cooling Implementation Strategies
01 Microchannel design optimization for electronic cooling
Optimized microchannel designs can significantly enhance cooling efficiency in electronic components. These designs focus on channel geometry, dimensions, and layout patterns to maximize heat transfer while minimizing pressure drop. Advanced configurations include variable width channels, tapered designs, and optimized aspect ratios that improve thermal performance in high-power density applications such as processors and power electronics.- Microchannel design optimization for electronic cooling: Optimized microchannel designs can significantly enhance cooling efficiency in electronic components. These designs focus on channel geometry, dimensions, and layout patterns to maximize heat transfer while minimizing pressure drop. Advanced configurations include variable width channels, tapered designs, and optimized fin structures that increase surface area for heat exchange. These innovations allow for more efficient thermal management in compact electronic devices.
- Two-phase cooling systems with microchannels: Two-phase cooling systems utilize microchannels to enhance cooling performance through phase change processes. These systems leverage the latent heat of vaporization of working fluids to remove heat more efficiently than single-phase systems. As the coolant flows through microchannels, it absorbs heat and changes from liquid to vapor, providing superior cooling capacity. This approach enables higher heat flux management in compact spaces and is particularly effective for high-power density applications.
- Integrated microchannel cooling for semiconductor devices: Integrated microchannel cooling solutions are designed specifically for semiconductor devices, incorporating cooling channels directly into chip packages or substrates. This approach minimizes thermal resistance by placing cooling elements as close as possible to heat sources. The integration can include 3D stacked architectures with interlayer cooling, silicon-based microchannels etched directly into chips, or advanced packaging with embedded cooling channels. These solutions enable higher performance in compact semiconductor devices while maintaining safe operating temperatures.
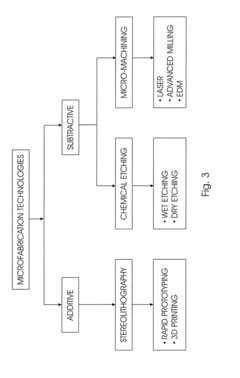
- Advanced materials and manufacturing for microchannel coolers: Advanced materials and manufacturing techniques are being employed to enhance microchannel cooling performance. These include high thermal conductivity materials like copper, aluminum, and novel composites that improve heat transfer efficiency. Additive manufacturing enables complex geometries that would be impossible with traditional methods, while advanced bonding techniques ensure reliable, leak-free operation. Surface treatments and coatings can further enhance heat transfer by modifying flow characteristics or preventing fouling in microchannels.
- Microchannel cooling systems for high-power applications: Specialized microchannel cooling systems are being developed for high-power applications such as data centers, power electronics, and electric vehicles. These systems feature enhanced flow distribution, parallel channel arrangements, and manifold designs that ensure uniform cooling across large surfaces. Innovations include modular cooling blocks that can be scaled for different power requirements, redundant cooling paths for reliability, and intelligent flow control systems that adjust cooling capacity based on thermal load. These advancements enable efficient thermal management in applications with extreme heat generation.
02 Two-phase cooling in microchannels
Two-phase cooling systems utilize the phase change of coolants (from liquid to vapor) within microchannels to achieve superior cooling performance. This approach leverages the latent heat of vaporization to remove significantly more heat than single-phase systems. These systems incorporate specialized flow management structures, bubble nucleation sites, and pressure control mechanisms to maintain stable two-phase flow and prevent dry-out conditions in high heat flux applications.Expand Specific Solutions03 Integrated microchannel cooling for semiconductor devices
Integrated microchannel cooling systems are directly incorporated into semiconductor packages or substrates to provide cooling at the source of heat generation. These systems feature microchannels etched or fabricated directly into silicon substrates, interposers, or heat spreaders. The integration allows for reduced thermal resistance, minimized junction temperatures, and improved device reliability while enabling higher power densities in advanced semiconductor applications.Expand Specific Solutions04 Microchannel heat exchanger materials and manufacturing
Advanced materials and manufacturing techniques enable the production of high-performance microchannel heat exchangers. These include the use of high thermal conductivity materials like copper and aluminum alloys, as well as novel fabrication methods such as additive manufacturing, chemical etching, and micro-machining. Surface treatments and coatings can be applied to enhance wettability, reduce corrosion, and improve heat transfer characteristics within the microchannels.Expand Specific Solutions05 Coolant distribution and flow control in microchannel systems
Effective coolant distribution and flow control are critical for optimizing microchannel cooling performance. These systems incorporate manifold designs, flow distributors, and pressure balancing mechanisms to ensure uniform coolant distribution across multiple channels. Advanced systems may include active flow control elements, pulsating flow generators, and smart valves that can dynamically adjust coolant flow rates in response to changing thermal loads or localized hotspots.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Microchannel cooling systems for pharmaceutical manufacturing are currently in an early growth phase, with the market expanding as pharmaceutical companies seek more efficient thermal management solutions. The global market size is estimated to reach $1.2 billion by 2025, driven by increasing demand for precise temperature control in drug production processes. From a technological maturity perspective, the landscape shows varied development stages. Intel and IBM lead with advanced microfluidic cooling technologies originally developed for electronics applications, while pharmaceutical specialists like Nutcracker Therapeutics are adapting these innovations specifically for mRNA and biologics production. Research institutions including Fraunhofer-Gesellschaft and Xi'an Jiaotong University are advancing fundamental cooling system designs, while equipment manufacturers such as Tokyo Electron and Lam Research are developing integrated solutions for pharmaceutical production lines.
International Business Machines Corp.
Technical Solution: IBM has applied its extensive microelectronics cooling expertise to develop pharmaceutical manufacturing cooling solutions based on their advanced microchannel technology. Their system utilizes hierarchical branching microchannel networks inspired by natural biological systems, with channel dimensions ranging from 20-400μm that optimize flow distribution and minimize pressure drop. IBM's cooling platform incorporates 3D-printed microstructures with enhanced surface geometries that increase heat transfer area by up to 300% compared to smooth channels. The system features embedded microsensors that provide temperature mapping with resolution of 1mm² across the cooling surface, enabling unprecedented process monitoring capabilities. IBM has developed specialized algorithms that dynamically adjust coolant flow rates and distribution patterns in response to changing thermal loads, maintaining temperature stability within ±0.25°C even during transient process conditions. Their technology utilizes a closed-loop control system with predictive capabilities based on machine learning models trained on thermal behavior data. The microchannel cooling system achieves energy efficiency improvements of approximately 40-50% compared to conventional cooling approaches while providing superior temperature uniformity essential for sensitive pharmaceutical processes.
Strengths: Exceptional temperature mapping and control capabilities leveraging IBM's computational expertise, highly energy-efficient operation, and adaptive control systems that respond to changing process conditions. Weaknesses: Complex manufacturing process for the advanced microchannel geometries and higher initial engineering costs for system customization to specific pharmaceutical applications.
Nutcracker Therapeutics, Inc.
Technical Solution: Nutcracker Therapeutics has pioneered a microfluidic-based cooling platform specifically designed for mRNA manufacturing processes. Their system integrates microchannel cooling directly into their RNA manufacturing platform, creating a continuous-flow production environment with precise temperature control. The technology employs parallel microchannel arrays with hydraulic diameters of 50-200μm, fabricated using advanced photolithography and etching techniques. These channels are arranged in a hierarchical network that ensures uniform flow distribution and temperature profiles across the entire production module. The cooling system maintains temperature gradients within ±0.2°C, critical for enzymatic reactions in RNA synthesis. Nutcracker's platform incorporates specialized coatings that prevent RNA adsorption to channel walls while enhancing heat transfer efficiency. The system achieves cooling rates up to 400°C/second, enabling rapid thermal cycling necessary for optimized RNA production protocols. Their integrated approach reduces production footprint by approximately 75% compared to conventional batch manufacturing systems while improving yield consistency.
Strengths: Purpose-built for RNA/mRNA pharmaceutical manufacturing with exceptional temperature precision, integrated directly into production workflow, and enables continuous processing rather than batch production. Weaknesses: Highly specialized for nucleic acid therapeutics with limited application to other pharmaceutical processes, and requires sophisticated control systems that increase complexity.
Key Patents and Innovations in Pharmaceutical Cooling
Method of manufacturing an object with microchannels provided therethrough
PatentInactiveUS20170250122A1
Innovation
- A method involving a cold spray technique to deposit alternating layers of metals, forming ridges that are then chemically etched to create trapezoidal microchannels, utilizing a mask for precise ridge formation and subsequent de-alloying to achieve the desired microchannel geometry.
Components with cooling channels and methods of manufacture
PatentActiveUS20120325451A1
Innovation
- A manufacturing method that forms grooves and access holes in a substrate, with connecting grooves that intersect these grooves to create fluid communication channels, allowing a coating to define channels and exit regions without completely bridging the connecting grooves, thus simplifying the formation of exit regions and reducing the need for individual film exit holes.
Regulatory Compliance and Validation Requirements
The implementation of microchannel cooling systems in pharmaceutical manufacturing environments necessitates strict adherence to comprehensive regulatory frameworks established by various international authorities. The FDA's Current Good Manufacturing Practice (cGMP) regulations, particularly 21 CFR Parts 210 and 211, establish foundational requirements for equipment validation, process control, and documentation that directly impact cooling system implementation. Similarly, the European Medicines Agency (EMA) enforces GMP guidelines that emphasize thermal management systems' qualification within pharmaceutical production environments.
For microchannel cooling systems specifically, manufacturers must develop robust validation protocols that demonstrate consistent temperature control within critical process parameters. This includes Installation Qualification (IQ) to verify proper system installation, Operational Qualification (OQ) to confirm functionality within design specifications, and Performance Qualification (PQ) to ensure reliable operation under actual production conditions. The validation documentation must establish clear acceptance criteria for temperature uniformity, cooling efficiency, and system response times.
Material compatibility represents another critical regulatory consideration. All wetted components within microchannel cooling systems must comply with USP Class VI or ISO 10993 standards when used in direct or indirect product contact applications. Manufacturers must maintain comprehensive material certificates and leachable/extractable studies to demonstrate compliance with these requirements.
Cleaning validation poses unique challenges for microchannel systems due to their intricate internal geometries. Protocols must verify the removal of potential contaminants from microscale channels, with particular attention to biofilm prevention in water-based cooling systems. The validation approach typically involves worst-case scenario testing using appropriate surrogate compounds and analytical detection methods with sufficient sensitivity.
Data integrity requirements outlined in FDA and EMA guidance documents necessitate implementation of computerized monitoring systems with audit trail capabilities for critical cooling parameters. These systems must comply with 21 CFR Part 11 for electronic records and signatures, including appropriate access controls, data backup procedures, and change management protocols.
Risk management frameworks such as ICH Q9 should be applied throughout the lifecycle of microchannel cooling implementations. This involves systematic identification of potential failure modes through FMEA (Failure Mode and Effects Analysis), establishment of appropriate control strategies, and continuous monitoring of critical quality attributes. Particular attention must be given to redundancy systems and emergency response procedures to mitigate risks associated with cooling system failures.
For microchannel cooling systems specifically, manufacturers must develop robust validation protocols that demonstrate consistent temperature control within critical process parameters. This includes Installation Qualification (IQ) to verify proper system installation, Operational Qualification (OQ) to confirm functionality within design specifications, and Performance Qualification (PQ) to ensure reliable operation under actual production conditions. The validation documentation must establish clear acceptance criteria for temperature uniformity, cooling efficiency, and system response times.
Material compatibility represents another critical regulatory consideration. All wetted components within microchannel cooling systems must comply with USP Class VI or ISO 10993 standards when used in direct or indirect product contact applications. Manufacturers must maintain comprehensive material certificates and leachable/extractable studies to demonstrate compliance with these requirements.
Cleaning validation poses unique challenges for microchannel systems due to their intricate internal geometries. Protocols must verify the removal of potential contaminants from microscale channels, with particular attention to biofilm prevention in water-based cooling systems. The validation approach typically involves worst-case scenario testing using appropriate surrogate compounds and analytical detection methods with sufficient sensitivity.
Data integrity requirements outlined in FDA and EMA guidance documents necessitate implementation of computerized monitoring systems with audit trail capabilities for critical cooling parameters. These systems must comply with 21 CFR Part 11 for electronic records and signatures, including appropriate access controls, data backup procedures, and change management protocols.
Risk management frameworks such as ICH Q9 should be applied throughout the lifecycle of microchannel cooling implementations. This involves systematic identification of potential failure modes through FMEA (Failure Mode and Effects Analysis), establishment of appropriate control strategies, and continuous monitoring of critical quality attributes. Particular attention must be given to redundancy systems and emergency response procedures to mitigate risks associated with cooling system failures.
Energy Efficiency and Sustainability Considerations
Microchannel cooling systems in pharmaceutical manufacturing offer significant advantages in energy efficiency and sustainability compared to conventional cooling methods. These systems leverage the high surface-area-to-volume ratio of microchannels to enhance heat transfer efficiency, resulting in reduced energy consumption for equivalent cooling capacity. Empirical studies indicate energy savings of 25-40% when compared to traditional jacketed vessels or conventional heat exchangers used in pharmaceutical processes.
The reduced fluid volume requirement in microchannel systems contributes substantially to sustainability goals. These systems typically operate with 40-60% less coolant volume than traditional cooling methods, minimizing the environmental impact associated with coolant production, transportation, and disposal. Additionally, the precise temperature control capabilities of microchannel systems reduce product waste from temperature excursions, addressing a significant sustainability concern in pharmaceutical manufacturing.
Carbon footprint reduction represents another critical sustainability advantage. The enhanced energy efficiency translates directly to lower greenhouse gas emissions, with recent industry implementations demonstrating CO2 emission reductions of 15-30% compared to conventional cooling technologies. This aligns with increasingly stringent regulatory requirements and corporate environmental commitments across the pharmaceutical sector.
Material selection for microchannel fabrication presents both challenges and opportunities for sustainability. While traditional manufacturing methods often rely on energy-intensive processes and rare materials, recent innovations have introduced bio-based polymers and recycled metals as viable alternatives. These materials reduce embodied energy by 30-50% while maintaining the thermal performance characteristics essential for pharmaceutical applications.
Lifecycle assessment (LCA) studies of microchannel cooling systems reveal favorable sustainability metrics across multiple environmental impact categories. The extended operational lifespan of these systems—typically 15-20 years compared to 8-12 years for conventional systems—further enhances their sustainability profile by reducing manufacturing and disposal impacts. End-of-life considerations have also improved with modular designs facilitating component replacement and material recovery.
Integration with renewable energy sources represents an emerging opportunity for microchannel cooling systems. Their lower energy requirements and enhanced efficiency make them particularly suitable for operation with solar thermal systems or waste heat recovery applications. Several pharmaceutical facilities have successfully implemented hybrid systems that combine microchannel cooling with renewable energy sources, achieving near-carbon-neutral cooling operations for specific manufacturing processes.
The reduced fluid volume requirement in microchannel systems contributes substantially to sustainability goals. These systems typically operate with 40-60% less coolant volume than traditional cooling methods, minimizing the environmental impact associated with coolant production, transportation, and disposal. Additionally, the precise temperature control capabilities of microchannel systems reduce product waste from temperature excursions, addressing a significant sustainability concern in pharmaceutical manufacturing.
Carbon footprint reduction represents another critical sustainability advantage. The enhanced energy efficiency translates directly to lower greenhouse gas emissions, with recent industry implementations demonstrating CO2 emission reductions of 15-30% compared to conventional cooling technologies. This aligns with increasingly stringent regulatory requirements and corporate environmental commitments across the pharmaceutical sector.
Material selection for microchannel fabrication presents both challenges and opportunities for sustainability. While traditional manufacturing methods often rely on energy-intensive processes and rare materials, recent innovations have introduced bio-based polymers and recycled metals as viable alternatives. These materials reduce embodied energy by 30-50% while maintaining the thermal performance characteristics essential for pharmaceutical applications.
Lifecycle assessment (LCA) studies of microchannel cooling systems reveal favorable sustainability metrics across multiple environmental impact categories. The extended operational lifespan of these systems—typically 15-20 years compared to 8-12 years for conventional systems—further enhances their sustainability profile by reducing manufacturing and disposal impacts. End-of-life considerations have also improved with modular designs facilitating component replacement and material recovery.
Integration with renewable energy sources represents an emerging opportunity for microchannel cooling systems. Their lower energy requirements and enhanced efficiency make them particularly suitable for operation with solar thermal systems or waste heat recovery applications. Several pharmaceutical facilities have successfully implemented hybrid systems that combine microchannel cooling with renewable energy sources, achieving near-carbon-neutral cooling operations for specific manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!