Minimizing Contamination in ICP-MS Sample Preparation
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ICP-MS Contamination Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, becoming an essential analytical technique for trace element analysis across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and geological research. The technique's exceptional sensitivity, capable of detecting elements at parts per trillion (ppt) levels, makes it particularly vulnerable to contamination issues that can compromise analytical results.
The historical development of ICP-MS has been marked by continuous improvements in instrumentation sensitivity, which paradoxically has increased the significance of contamination control. Early systems in the 1980s and 1990s operated at detection limits in the parts per billion (ppb) range, where certain contamination sources could be tolerated. Modern systems, however, routinely achieve ppt or even parts per quadrillion (ppq) detection capabilities, making even minute contamination sources potentially significant.
Contamination in ICP-MS sample preparation represents a persistent challenge that has evolved alongside technological advancements. Sources of contamination have become increasingly subtle and diverse, ranging from laboratory environments and reagents to sampling tools and storage containers. The industry has witnessed a parallel evolution in contamination control methodologies, moving from basic clean room practices to sophisticated protocols involving specialized materials and handling techniques.
Current technological trends in the field focus on developing integrated sample preparation systems that minimize human intervention, utilizing automated clean technologies and novel materials resistant to elemental leaching. The emergence of microfluidic and lab-on-chip technologies represents promising directions for contamination reduction through minimized sample handling and reduced reagent volumes.
The primary objective of this technical research is to comprehensively evaluate current contamination sources in ICP-MS sample preparation and identify innovative approaches to minimize their impact. Specifically, we aim to: (1) characterize the most significant contamination pathways in modern ICP-MS applications; (2) assess the effectiveness of current contamination control strategies across different industry sectors; (3) identify emerging technologies and methodologies with potential to further reduce contamination; and (4) develop a strategic roadmap for implementing optimal contamination minimization protocols tailored to different analytical scenarios.
This research recognizes that as detection limits continue to improve, contamination control will remain a moving target requiring continuous innovation. By establishing a thorough understanding of contamination mechanisms and mitigation strategies, we seek to enhance the reliability and accuracy of ultra-trace elemental analysis across critical applications in environmental monitoring, pharmaceutical quality control, and advanced materials development.
The historical development of ICP-MS has been marked by continuous improvements in instrumentation sensitivity, which paradoxically has increased the significance of contamination control. Early systems in the 1980s and 1990s operated at detection limits in the parts per billion (ppb) range, where certain contamination sources could be tolerated. Modern systems, however, routinely achieve ppt or even parts per quadrillion (ppq) detection capabilities, making even minute contamination sources potentially significant.
Contamination in ICP-MS sample preparation represents a persistent challenge that has evolved alongside technological advancements. Sources of contamination have become increasingly subtle and diverse, ranging from laboratory environments and reagents to sampling tools and storage containers. The industry has witnessed a parallel evolution in contamination control methodologies, moving from basic clean room practices to sophisticated protocols involving specialized materials and handling techniques.
Current technological trends in the field focus on developing integrated sample preparation systems that minimize human intervention, utilizing automated clean technologies and novel materials resistant to elemental leaching. The emergence of microfluidic and lab-on-chip technologies represents promising directions for contamination reduction through minimized sample handling and reduced reagent volumes.
The primary objective of this technical research is to comprehensively evaluate current contamination sources in ICP-MS sample preparation and identify innovative approaches to minimize their impact. Specifically, we aim to: (1) characterize the most significant contamination pathways in modern ICP-MS applications; (2) assess the effectiveness of current contamination control strategies across different industry sectors; (3) identify emerging technologies and methodologies with potential to further reduce contamination; and (4) develop a strategic roadmap for implementing optimal contamination minimization protocols tailored to different analytical scenarios.
This research recognizes that as detection limits continue to improve, contamination control will remain a moving target requiring continuous innovation. By establishing a thorough understanding of contamination mechanisms and mitigation strategies, we seek to enhance the reliability and accuracy of ultra-trace elemental analysis across critical applications in environmental monitoring, pharmaceutical quality control, and advanced materials development.
Market Analysis for High-Purity Analytical Solutions
The global market for high-purity analytical solutions specifically designed for ICP-MS sample preparation has experienced significant growth in recent years, driven by increasing demands for accurate trace element analysis across multiple industries. The market size for specialized contamination-free sample preparation products was valued at approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate of 6.8% through 2028.
The pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 35% of the total market share. These industries require ultra-pure sample preparation environments to ensure accurate analysis of drug formulations and biological samples. Environmental testing laboratories constitute the second-largest segment at 28%, where precise detection of trace contaminants in water, soil, and air samples is critical for regulatory compliance and public safety.
Regional analysis reveals North America as the dominant market with 42% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to expanding industrial activities, increasing environmental regulations, and growing investments in analytical infrastructure.
Key market drivers include increasingly stringent regulatory requirements for product quality and safety across industries, technological advancements in analytical instrumentation demanding higher purity standards, and growing awareness of contamination issues affecting analytical results. The semiconductor industry represents an emerging high-growth segment, where even sub-ppt level contaminants can significantly impact production yields.
Customer demand patterns indicate a strong preference for integrated contamination control solutions rather than individual products. End-users increasingly seek comprehensive packages that include specialized labware, ultra-pure reagents, and validated protocols specifically designed for ICP-MS applications.
Price sensitivity varies significantly by market segment, with research institutions showing higher price sensitivity compared to pharmaceutical and semiconductor industries, where analytical accuracy directly impacts product quality and regulatory compliance. The average annual expenditure on contamination control products per laboratory ranges from $15,000 to $75,000, depending on sample throughput and application requirements.
Market challenges include high competition among established players, price pressure from generic laboratory supply companies, and the need for continuous innovation to address evolving contamination sources. Additionally, the market faces educational barriers, as many laboratories lack awareness about proper contamination control practices specific to ICP-MS sample preparation.
The pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 35% of the total market share. These industries require ultra-pure sample preparation environments to ensure accurate analysis of drug formulations and biological samples. Environmental testing laboratories constitute the second-largest segment at 28%, where precise detection of trace contaminants in water, soil, and air samples is critical for regulatory compliance and public safety.
Regional analysis reveals North America as the dominant market with 42% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to expanding industrial activities, increasing environmental regulations, and growing investments in analytical infrastructure.
Key market drivers include increasingly stringent regulatory requirements for product quality and safety across industries, technological advancements in analytical instrumentation demanding higher purity standards, and growing awareness of contamination issues affecting analytical results. The semiconductor industry represents an emerging high-growth segment, where even sub-ppt level contaminants can significantly impact production yields.
Customer demand patterns indicate a strong preference for integrated contamination control solutions rather than individual products. End-users increasingly seek comprehensive packages that include specialized labware, ultra-pure reagents, and validated protocols specifically designed for ICP-MS applications.
Price sensitivity varies significantly by market segment, with research institutions showing higher price sensitivity compared to pharmaceutical and semiconductor industries, where analytical accuracy directly impacts product quality and regulatory compliance. The average annual expenditure on contamination control products per laboratory ranges from $15,000 to $75,000, depending on sample throughput and application requirements.
Market challenges include high competition among established players, price pressure from generic laboratory supply companies, and the need for continuous innovation to address evolving contamination sources. Additionally, the market faces educational barriers, as many laboratories lack awareness about proper contamination control practices specific to ICP-MS sample preparation.
Current Challenges in ICP-MS Sample Preparation
ICP-MS (Inductively Coupled Plasma Mass Spectrometry) sample preparation faces significant contamination challenges that can compromise analytical accuracy and reliability. The ultra-sensitive nature of ICP-MS, capable of detecting elements at parts-per-trillion levels, makes it particularly vulnerable to even minute contamination sources. This sensitivity creates a paradoxical situation where the technique's greatest strength also represents its most significant vulnerability.
Laboratory environments present numerous contamination vectors, including airborne particulates, reagent impurities, and equipment surfaces. HEPA-filtered clean rooms, while effective, remain prohibitively expensive for many laboratories, creating a technological barrier to consistent high-quality analysis. Even when available, personnel movement and material transfer into these environments can introduce contaminants, requiring strict protocols that are often difficult to maintain consistently.
Reagent purity represents another critical challenge, with even "ultra-pure" commercial acids and solvents potentially containing trace elements at levels significant enough to affect analytical results. The economic implications are substantial, as higher-purity reagents command premium prices, forcing laboratories to balance analytical requirements against budgetary constraints. Additionally, reagent handling and storage introduce further contamination risks, as containers may leach elements or allow atmospheric contamination over time.
Sample collection and transport procedures present particularly difficult challenges, as these often occur outside controlled laboratory environments. Field sampling introduces variables difficult to standardize, while transport containers may contribute contaminants through leaching or inadequate sealing. The time delay between collection and analysis creates additional opportunities for sample degradation or contamination.
Cross-contamination between samples represents a persistent challenge, particularly in high-throughput environments. Carryover effects from sample introduction systems, including nebulizers, spray chambers, and tubing, can transfer elements between sequential analyses. Automated systems, while improving throughput, may exacerbate these issues through reduced manual oversight of cleaning procedures between samples.
Digestion procedures necessary for solid samples introduce additional contamination risks through heating processes, vessel materials, and increased handling steps. Microwave digestion systems, while efficient, utilize vessels that may contribute contaminants, particularly as they age and develop micro-fissures. Open-vessel digestion methods face even greater challenges from environmental exposure during processing.
Personnel training and protocol adherence remain significant human factors affecting contamination control. The specialized knowledge required for ultra-trace analysis is not universally available, creating inconsistencies in practice between laboratories and even between analysts within the same facility. Documentation and validation of contamination control measures often lack standardization across the industry, complicating inter-laboratory comparisons and method transfers.
Laboratory environments present numerous contamination vectors, including airborne particulates, reagent impurities, and equipment surfaces. HEPA-filtered clean rooms, while effective, remain prohibitively expensive for many laboratories, creating a technological barrier to consistent high-quality analysis. Even when available, personnel movement and material transfer into these environments can introduce contaminants, requiring strict protocols that are often difficult to maintain consistently.
Reagent purity represents another critical challenge, with even "ultra-pure" commercial acids and solvents potentially containing trace elements at levels significant enough to affect analytical results. The economic implications are substantial, as higher-purity reagents command premium prices, forcing laboratories to balance analytical requirements against budgetary constraints. Additionally, reagent handling and storage introduce further contamination risks, as containers may leach elements or allow atmospheric contamination over time.
Sample collection and transport procedures present particularly difficult challenges, as these often occur outside controlled laboratory environments. Field sampling introduces variables difficult to standardize, while transport containers may contribute contaminants through leaching or inadequate sealing. The time delay between collection and analysis creates additional opportunities for sample degradation or contamination.
Cross-contamination between samples represents a persistent challenge, particularly in high-throughput environments. Carryover effects from sample introduction systems, including nebulizers, spray chambers, and tubing, can transfer elements between sequential analyses. Automated systems, while improving throughput, may exacerbate these issues through reduced manual oversight of cleaning procedures between samples.
Digestion procedures necessary for solid samples introduce additional contamination risks through heating processes, vessel materials, and increased handling steps. Microwave digestion systems, while efficient, utilize vessels that may contribute contaminants, particularly as they age and develop micro-fissures. Open-vessel digestion methods face even greater challenges from environmental exposure during processing.
Personnel training and protocol adherence remain significant human factors affecting contamination control. The specialized knowledge required for ultra-trace analysis is not universally available, creating inconsistencies in practice between laboratories and even between analysts within the same facility. Documentation and validation of contamination control measures often lack standardization across the industry, complicating inter-laboratory comparisons and method transfers.
Established Contamination Mitigation Protocols
01 Clean room environments for ICP-MS sample preparation
To minimize contamination during ICP-MS sample preparation, specialized clean room environments are utilized. These controlled spaces feature filtered air systems, specialized materials, and strict protocols to prevent introduction of external contaminants. Clean rooms maintain positive pressure and employ laminar flow systems to ensure particulate-free environments, which is critical for trace element analysis where even minimal contamination can significantly impact results.- Clean room environments for ICP-MS sample preparation: To minimize contamination during ICP-MS sample preparation, specialized clean room environments are utilized. These controlled environments feature filtered air systems, specialized materials, and strict protocols to prevent introduction of external contaminants. Clean rooms maintain positive pressure and employ HEPA filtration to ensure dust-free conditions, which is critical for trace element analysis where even minimal contamination can significantly impact results.
- Specialized sample preparation equipment and containers: Dedicated equipment and containers designed specifically for ICP-MS sample preparation help minimize contamination risks. These include specialized digestion vessels, sample introduction systems, and storage containers made from inert materials like PTFE, quartz, or high-purity polymers. Such materials prevent leaching of elements into samples and resist chemical degradation from acids and other reagents used in sample preparation processes.
- Ultra-pure reagents and dilution techniques: The use of ultra-pure reagents and careful dilution techniques is essential for preventing contamination in ICP-MS analysis. High-purity acids, water, and other chemicals specially manufactured for trace element analysis help maintain sample integrity. Sequential dilution methods and careful handling protocols minimize introduction of contaminants during sample processing, ensuring accurate quantification of trace elements at parts-per-billion or parts-per-trillion levels.
- Automated sample preparation systems: Automated systems for ICP-MS sample preparation reduce human handling and associated contamination risks. These systems perform tasks such as digestion, dilution, and introduction of samples with minimal human intervention. Automation ensures consistency in preparation protocols and creates a controlled environment that limits exposure to external contaminants, improving reproducibility and accuracy of analytical results.
- Contamination monitoring and quality control procedures: Comprehensive quality control procedures and contamination monitoring are implemented throughout the ICP-MS sample preparation process. These include the use of method blanks, certified reference materials, and internal standards to identify and quantify potential contamination sources. Regular system cleaning protocols, performance verification tests, and documentation of preparation conditions help maintain analytical integrity and ensure reliable results in trace element analysis.
02 Specialized equipment and materials to prevent contamination
Specialized equipment and materials are essential for preventing contamination during ICP-MS sample preparation. This includes using high-purity reagents, PTFE or PFA containers, acid-cleaned labware, and dedicated tools that minimize metal leaching. Automated sample handling systems can reduce human contact with samples, while specialized digestion vessels made from contamination-resistant materials help maintain sample integrity throughout the preparation process.Expand Specific Solutions03 Sample digestion and dissolution techniques
Proper sample digestion and dissolution techniques are crucial for minimizing contamination in ICP-MS analysis. Methods include microwave-assisted digestion, high-pressure digestion systems, and temperature-controlled dissolution processes that efficiently break down samples while minimizing the introduction of contaminants. These techniques often employ ultra-pure acids and closed vessel systems to prevent environmental contamination during the digestion process.Expand Specific Solutions04 Contamination monitoring and quality control procedures
Effective contamination monitoring and quality control procedures are essential for reliable ICP-MS analysis. These include regular analysis of procedural blanks, certified reference materials, and duplicate samples to identify and quantify potential contamination sources. Statistical methods are employed to establish detection limits and quantify uncertainty, while systematic documentation of sample handling procedures helps identify and eliminate contamination sources.Expand Specific Solutions05 Sample collection and preservation methods
Proper sample collection and preservation methods are fundamental to preventing contamination before ICP-MS analysis. This includes using appropriate sampling containers made of contamination-free materials, implementing field filtration techniques, adding stabilizing agents to prevent analyte loss, and maintaining proper storage conditions. Protocols for field blanks and transportation controls help ensure sample integrity from collection site to laboratory analysis.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The ICP-MS sample preparation contamination minimization market is in a growth phase, with increasing demand driven by stringent analytical requirements across pharmaceutical, environmental, and semiconductor industries. The global market size for high-purity laboratory consumables and clean room technologies is expanding at approximately 6-8% annually. Leading players like Thermo Fisher Scientific and Agilent Technologies dominate with comprehensive contamination control solutions, while specialized companies such as DuPont and Air Liquide America provide complementary technologies. Academic institutions including China University of Geosciences and Sun Yat-Sen University contribute significant research advancements. The technology maturity varies across applications, with semiconductor-focused solutions (from companies like SMIC and Winbond Electronics) being most advanced, while environmental monitoring applications (supported by Hangzhou Puyu Technology) continue to evolve with emerging clean chemistry protocols.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed comprehensive contamination control strategies for ICP-MS sample preparation, including their CleanTech™ protocols. Their approach integrates specialized clean room facilities with automated sample handling systems that minimize human contact. The company's PrepFAST automated sample preparation system incorporates in-line dilution and internal standard addition, reducing manual handling steps that often introduce contaminants. Their specialized acid purification systems produce ultra-pure reagents on-demand, eliminating contamination from commercial reagents. Thermo Fisher's consumables feature specialized polymers resistant to acid leaching and particle shedding, while their sample introduction systems include specialized nebulizers and spray chambers designed to reduce memory effects and cross-contamination between samples. The company's integrated workflow approach addresses contamination at every stage from sampling to analysis, with validation protocols that include procedural blanks and certified reference materials to quantify and correct for any residual contamination.
Strengths: Comprehensive end-to-end contamination control strategy with specialized equipment and consumables designed specifically for ultra-trace analysis. Their integrated systems approach addresses multiple contamination sources simultaneously. Weaknesses: Higher initial investment costs compared to basic laboratory setups, and requires specialized training for optimal performance. Some proprietary consumables may create vendor lock-in.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has pioneered the PFA (perfluoroalkoxy) microflow nebulizer technology specifically designed to minimize contamination in ICP-MS sample preparation. Their approach focuses on reducing sample contact surfaces and utilizing ultra-clean materials throughout the analytical pathway. Agilent's Ultra High-Purity (UHP) sample introduction system incorporates specialized components manufactured from high-purity fluoropolymers that resist acid leaching and particle generation. Their Clean Room Sample Preparation Protocol includes specialized acid cleaning procedures for all labware using sub-boiling distillation techniques. Agilent's automated sample preparation systems feature enclosed environments with HEPA filtration to prevent airborne contamination. The company has developed specialized ICP-MS autosamplers with enclosures that protect samples from laboratory air and incorporate wash protocols that effectively eliminate memory effects between samples. Their integrated software monitors quality control parameters including procedural blanks to detect contamination events in real-time, allowing immediate corrective action before compromising entire analytical batches.
Strengths: Industry-leading expertise in ultra-trace analysis with specialized components designed specifically for contamination-sensitive applications. Their systems demonstrate exceptional stability for long analytical runs with minimal drift. Weaknesses: Specialized consumables can be costly and may require more frequent replacement than standard components. Some automated systems have limited flexibility for non-standard sample types.
Critical Technologies for Ultra-Trace Analysis
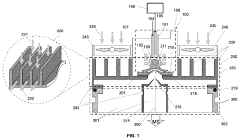
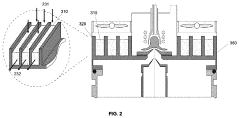
Air-cooled interface for inductively coupled plasma mass spectrometer (ICP-MS)
PatentActiveUS11864303B2
Innovation
- An air-cooled interface for ICP-MS systems using fins, open-cell metal foams, compact heat exchangers, or heat pipes to manage heat dissipation, with adjustable thermal resistors to direct heat away from sensitive components and prevent recombination, utilizing natural or forced convection to enhance cooling efficiency.
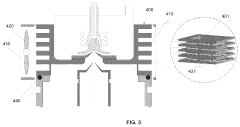
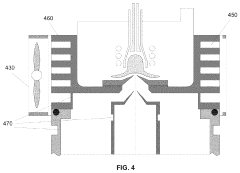
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
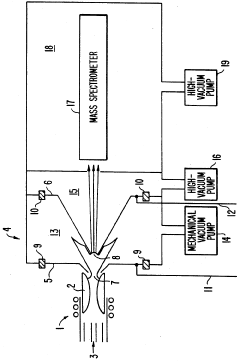
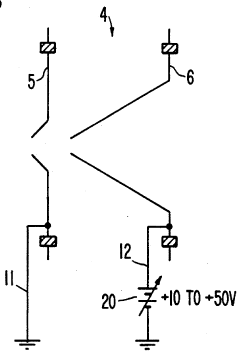
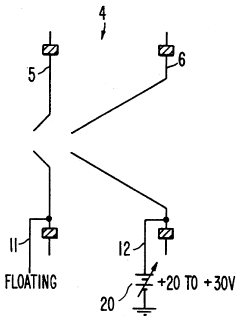
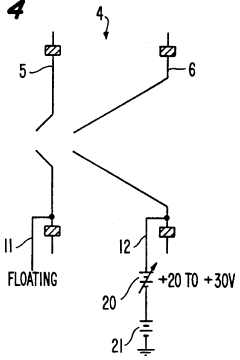
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Clean Laboratory Design and Infrastructure
Clean laboratory design represents a critical foundation for minimizing contamination in ICP-MS sample preparation. The infrastructure must be meticulously planned to create an environment that systematically reduces potential sources of contamination. Modern clean laboratories typically employ a hierarchical design with progressively cleaner zones, from Class 1000 (ISO 6) outer areas to Class 10 (ISO 4) or cleaner core processing spaces where the most sensitive sample preparation occurs.
The physical layout should incorporate airlocks and buffer zones between areas of different cleanliness levels. These transition spaces help maintain pressure differentials that prevent contaminant migration from less clean to cleaner areas. Positive pressure gradients, typically 5-15 Pascal between adjacent zones, create an outward airflow that blocks external contaminants from entering critical preparation areas.
HVAC systems in clean laboratories require specialized design considerations, including HEPA or ULPA filtration capable of removing 99.99% of particles ≥0.3μm. Air should flow in laminar patterns from ceiling to floor at velocities of 0.3-0.5 m/s to minimize turbulence that could redistribute contaminants. Complete air exchange rates typically range from 15-20 changes per hour in Class 1000 areas to 60+ changes per hour in Class 10 environments.
Construction materials must be carefully selected to minimize particle generation and chemical outgassing. Non-shedding, non-porous surfaces like epoxy-coated concrete floors, PVC or polypropylene wall panels, and powder-coated metal fixtures are preferred. All materials should be resistant to the cleaning agents used in laboratory maintenance protocols, typically including dilute acids and specialized detergents.
Dedicated utility systems represent another crucial infrastructure component. Ultra-pure water systems (18.2 MΩ·cm resistivity) with point-of-use filtration should be installed directly in sample preparation areas. Similarly, specialized gas delivery systems with appropriate purification trains are necessary for analytical instruments requiring high-purity gases.
Equipment layout within the clean laboratory should follow workflow optimization principles while maintaining separation between potentially contaminating processes. Dedicated spaces for different preparation steps help prevent cross-contamination, with the most sensitive operations performed in the cleanest zones. Strategic placement of equipment can create natural workflow patterns that minimize sample transport distances and handling requirements.
Human factors engineering must also be incorporated into laboratory design, as personnel represent a significant contamination source. Gowning rooms with appropriate storage for clean garments, sticky mats at transitions, and ergonomic workstations that minimize unnecessary movement all contribute to contamination control effectiveness.
The physical layout should incorporate airlocks and buffer zones between areas of different cleanliness levels. These transition spaces help maintain pressure differentials that prevent contaminant migration from less clean to cleaner areas. Positive pressure gradients, typically 5-15 Pascal between adjacent zones, create an outward airflow that blocks external contaminants from entering critical preparation areas.
HVAC systems in clean laboratories require specialized design considerations, including HEPA or ULPA filtration capable of removing 99.99% of particles ≥0.3μm. Air should flow in laminar patterns from ceiling to floor at velocities of 0.3-0.5 m/s to minimize turbulence that could redistribute contaminants. Complete air exchange rates typically range from 15-20 changes per hour in Class 1000 areas to 60+ changes per hour in Class 10 environments.
Construction materials must be carefully selected to minimize particle generation and chemical outgassing. Non-shedding, non-porous surfaces like epoxy-coated concrete floors, PVC or polypropylene wall panels, and powder-coated metal fixtures are preferred. All materials should be resistant to the cleaning agents used in laboratory maintenance protocols, typically including dilute acids and specialized detergents.
Dedicated utility systems represent another crucial infrastructure component. Ultra-pure water systems (18.2 MΩ·cm resistivity) with point-of-use filtration should be installed directly in sample preparation areas. Similarly, specialized gas delivery systems with appropriate purification trains are necessary for analytical instruments requiring high-purity gases.
Equipment layout within the clean laboratory should follow workflow optimization principles while maintaining separation between potentially contaminating processes. Dedicated spaces for different preparation steps help prevent cross-contamination, with the most sensitive operations performed in the cleanest zones. Strategic placement of equipment can create natural workflow patterns that minimize sample transport distances and handling requirements.
Human factors engineering must also be incorporated into laboratory design, as personnel represent a significant contamination source. Gowning rooms with appropriate storage for clean garments, sticky mats at transitions, and ergonomic workstations that minimize unnecessary movement all contribute to contamination control effectiveness.
Quality Assurance and Validation Methods
Quality assurance and validation methods are critical components in minimizing contamination during ICP-MS sample preparation. Establishing robust quality control protocols ensures reliable analytical results and maintains the integrity of trace element analysis.
Method validation represents the cornerstone of quality assurance in ICP-MS analysis. This process typically involves determining method detection limits (MDLs), quantification limits (LOQs), linear dynamic range, precision, accuracy, and measurement uncertainty. For contamination control specifically, method blanks serve as essential indicators of background contamination levels introduced during sample preparation.
Standard reference materials (SRMs) play a pivotal role in validating sample preparation procedures. By processing these certified materials alongside unknown samples, analysts can verify the effectiveness of their contamination control measures. The recovery rates of target analytes from SRMs provide quantitative evidence of method performance and highlight potential contamination issues.
Internal quality control samples should be strategically incorporated throughout analytical batches. These include method blanks, laboratory control samples, matrix spikes, and duplicates. Method blanks, prepared using the same reagents and procedures as actual samples but without sample material, are particularly valuable for detecting contamination sources during preparation steps.
Statistical process control charts offer a systematic approach to monitoring contamination levels over time. By tracking blank values and control sample results, laboratories can identify trends, shifts, or outliers that may indicate contamination problems. Establishing control limits based on historical data enables prompt identification of systematic contamination issues before they affect sample results.
Proficiency testing and interlaboratory comparisons provide external validation of contamination control measures. Participation in these programs allows laboratories to benchmark their performance against peers and identify potential blind spots in their contamination prevention strategies.
Documentation and traceability form the foundation of effective quality assurance. Detailed records of reagent preparation, equipment cleaning procedures, and environmental monitoring create an audit trail that facilitates troubleshooting when contamination issues arise. Electronic laboratory information management systems (LIMS) can streamline this documentation process while enabling trend analysis of quality control data.
Regular audits of sample preparation areas and procedures help maintain compliance with established protocols. These reviews should evaluate adherence to clean techniques, proper reagent handling, and equipment maintenance schedules. Findings from these audits drive continuous improvement in contamination control practices.
Method validation represents the cornerstone of quality assurance in ICP-MS analysis. This process typically involves determining method detection limits (MDLs), quantification limits (LOQs), linear dynamic range, precision, accuracy, and measurement uncertainty. For contamination control specifically, method blanks serve as essential indicators of background contamination levels introduced during sample preparation.
Standard reference materials (SRMs) play a pivotal role in validating sample preparation procedures. By processing these certified materials alongside unknown samples, analysts can verify the effectiveness of their contamination control measures. The recovery rates of target analytes from SRMs provide quantitative evidence of method performance and highlight potential contamination issues.
Internal quality control samples should be strategically incorporated throughout analytical batches. These include method blanks, laboratory control samples, matrix spikes, and duplicates. Method blanks, prepared using the same reagents and procedures as actual samples but without sample material, are particularly valuable for detecting contamination sources during preparation steps.
Statistical process control charts offer a systematic approach to monitoring contamination levels over time. By tracking blank values and control sample results, laboratories can identify trends, shifts, or outliers that may indicate contamination problems. Establishing control limits based on historical data enables prompt identification of systematic contamination issues before they affect sample results.
Proficiency testing and interlaboratory comparisons provide external validation of contamination control measures. Participation in these programs allows laboratories to benchmark their performance against peers and identify potential blind spots in their contamination prevention strategies.
Documentation and traceability form the foundation of effective quality assurance. Detailed records of reagent preparation, equipment cleaning procedures, and environmental monitoring create an audit trail that facilitates troubleshooting when contamination issues arise. Electronic laboratory information management systems (LIMS) can streamline this documentation process while enabling trend analysis of quality control data.
Regular audits of sample preparation areas and procedures help maintain compliance with established protocols. These reviews should evaluate adherence to clean techniques, proper reagent handling, and equipment maintenance schedules. Findings from these audits drive continuous improvement in contamination control practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!