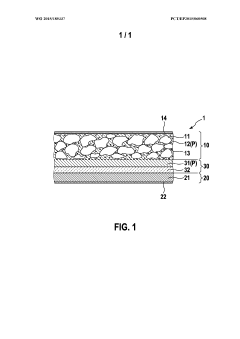
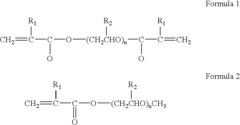
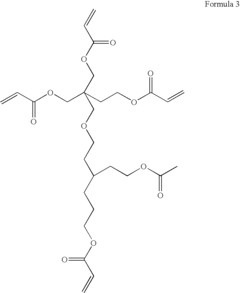
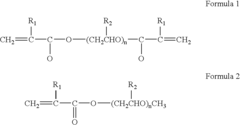
Polymer electrolytes for safe lithium-sulfur battery operation
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Electrolytes Development and Li-S Battery Goals
Polymer electrolytes have emerged as a critical component in the evolution of lithium-sulfur (Li-S) battery technology, addressing fundamental safety and performance challenges. The development trajectory of polymer electrolytes began in the 1970s with the discovery of ionic conductivity in polymer-salt complexes, progressing significantly through the 1990s with the introduction of gel polymer electrolytes that combined liquid electrolyte benefits with solid-state safety features.
The technological evolution has been driven by the inherent limitations of conventional liquid electrolytes in Li-S systems, particularly their inability to effectively suppress polysulfide shuttling and dendrite formation. These challenges have catalyzed research into specialized polymer architectures designed specifically for Li-S chemistry, including single-ion conducting polymers and polymer-ceramic composites that demonstrate enhanced electrochemical stability.
Current development goals for polymer electrolytes in Li-S batteries focus on achieving room temperature ionic conductivity exceeding 10^-3 S/cm while maintaining mechanical stability sufficient to prevent lithium dendrite penetration. Additionally, these materials must demonstrate chemical compatibility with sulfur cathodes and effectively contain polysulfide intermediates within the cathode compartment to prevent capacity fade.
The industry has established ambitious performance targets for Li-S batteries enabled by advanced polymer electrolytes, including energy densities surpassing 500 Wh/kg at the cell level, cycle life exceeding 1000 cycles with less than 20% capacity degradation, and operation across wide temperature ranges from -40°C to 60°C. These metrics represent significant improvements over current lithium-ion technology and align with requirements for next-generation energy storage applications.
Safety enhancement remains a paramount objective, with polymer electrolytes expected to eliminate thermal runaway risks associated with conventional liquid electrolytes. This includes passing nail penetration tests, crush tests, and thermal abuse scenarios without catastrophic failure or combustion, addressing critical concerns for automotive and aerospace applications.
Cost-effectiveness constitutes another crucial development goal, with targets to reduce electrolyte production costs below $10/kg through scalable manufacturing processes and readily available precursors. This economic consideration is essential for commercial viability, particularly in price-sensitive markets like electric vehicles and grid storage.
The technological roadmap extends to 2030, by which time fully integrated polymer electrolyte systems are expected to enable Li-S batteries with twice the energy density of current lithium-ion technologies while maintaining comparable cycle life and significantly improved safety profiles.
The technological evolution has been driven by the inherent limitations of conventional liquid electrolytes in Li-S systems, particularly their inability to effectively suppress polysulfide shuttling and dendrite formation. These challenges have catalyzed research into specialized polymer architectures designed specifically for Li-S chemistry, including single-ion conducting polymers and polymer-ceramic composites that demonstrate enhanced electrochemical stability.
Current development goals for polymer electrolytes in Li-S batteries focus on achieving room temperature ionic conductivity exceeding 10^-3 S/cm while maintaining mechanical stability sufficient to prevent lithium dendrite penetration. Additionally, these materials must demonstrate chemical compatibility with sulfur cathodes and effectively contain polysulfide intermediates within the cathode compartment to prevent capacity fade.
The industry has established ambitious performance targets for Li-S batteries enabled by advanced polymer electrolytes, including energy densities surpassing 500 Wh/kg at the cell level, cycle life exceeding 1000 cycles with less than 20% capacity degradation, and operation across wide temperature ranges from -40°C to 60°C. These metrics represent significant improvements over current lithium-ion technology and align with requirements for next-generation energy storage applications.
Safety enhancement remains a paramount objective, with polymer electrolytes expected to eliminate thermal runaway risks associated with conventional liquid electrolytes. This includes passing nail penetration tests, crush tests, and thermal abuse scenarios without catastrophic failure or combustion, addressing critical concerns for automotive and aerospace applications.
Cost-effectiveness constitutes another crucial development goal, with targets to reduce electrolyte production costs below $10/kg through scalable manufacturing processes and readily available precursors. This economic consideration is essential for commercial viability, particularly in price-sensitive markets like electric vehicles and grid storage.
The technological roadmap extends to 2030, by which time fully integrated polymer electrolyte systems are expected to enable Li-S batteries with twice the energy density of current lithium-ion technologies while maintaining comparable cycle life and significantly improved safety profiles.
Market Analysis for Advanced Li-S Battery Technologies
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the inherent advantages these batteries offer over conventional lithium-ion technologies. Current market projections indicate that the global Li-S battery market could reach approximately $2.5 billion by 2030, with a compound annual growth rate exceeding 30% from 2023 to 2030. This remarkable growth trajectory is primarily driven by increasing demand for high-energy-density storage solutions across multiple sectors.
The automotive industry represents the largest potential market segment for Li-S batteries, particularly for electric vehicles (EVs) where range anxiety remains a critical consumer concern. The theoretical energy density of Li-S batteries (2,600 Wh/kg) far surpasses that of conventional lithium-ion batteries (250-300 Wh/kg), potentially enabling EVs to achieve driving ranges comparable to internal combustion engine vehicles without significant weight penalties.
Aerospace and defense sectors constitute another promising market segment, where lightweight, high-energy-density power solutions command premium pricing. Several major aerospace manufacturers have initiated research partnerships focused on Li-S technology integration for satellite systems, drones, and electric aircraft applications.
Consumer electronics represents a third significant market opportunity, particularly for applications requiring extended operation times between charges. However, this segment may require more refined battery solutions with improved cycle life characteristics before widespread adoption occurs.
Geographically, Asia-Pacific currently leads in Li-S battery development and manufacturing capacity, with China, South Korea, and Japan hosting the majority of commercial development efforts. North America and Europe are rapidly expanding their research initiatives and production capabilities, supported by substantial government funding programs aimed at securing domestic battery supply chains.
Market challenges remain significant despite the promising outlook. The current cost structure of Li-S batteries exceeds that of conventional lithium-ion technologies by approximately 30-40%, primarily due to specialized materials requirements and limited economies of scale. Additionally, consumer and industry concerns regarding safety, cycle life limitations, and system integration complexities represent adoption barriers that must be addressed.
Polymer electrolytes represent a critical enabling technology for safe Li-S battery commercialization. Market analysis indicates growing investment in this specific technology area, with venture capital funding for polymer electrolyte startups increasing by over 45% between 2020 and 2022. This trend reflects industry recognition that solid-state and gel polymer electrolytes may provide the necessary safety improvements and polysulfide shuttle inhibition required for commercial viability.
The automotive industry represents the largest potential market segment for Li-S batteries, particularly for electric vehicles (EVs) where range anxiety remains a critical consumer concern. The theoretical energy density of Li-S batteries (2,600 Wh/kg) far surpasses that of conventional lithium-ion batteries (250-300 Wh/kg), potentially enabling EVs to achieve driving ranges comparable to internal combustion engine vehicles without significant weight penalties.
Aerospace and defense sectors constitute another promising market segment, where lightweight, high-energy-density power solutions command premium pricing. Several major aerospace manufacturers have initiated research partnerships focused on Li-S technology integration for satellite systems, drones, and electric aircraft applications.
Consumer electronics represents a third significant market opportunity, particularly for applications requiring extended operation times between charges. However, this segment may require more refined battery solutions with improved cycle life characteristics before widespread adoption occurs.
Geographically, Asia-Pacific currently leads in Li-S battery development and manufacturing capacity, with China, South Korea, and Japan hosting the majority of commercial development efforts. North America and Europe are rapidly expanding their research initiatives and production capabilities, supported by substantial government funding programs aimed at securing domestic battery supply chains.
Market challenges remain significant despite the promising outlook. The current cost structure of Li-S batteries exceeds that of conventional lithium-ion technologies by approximately 30-40%, primarily due to specialized materials requirements and limited economies of scale. Additionally, consumer and industry concerns regarding safety, cycle life limitations, and system integration complexities represent adoption barriers that must be addressed.
Polymer electrolytes represent a critical enabling technology for safe Li-S battery commercialization. Market analysis indicates growing investment in this specific technology area, with venture capital funding for polymer electrolyte startups increasing by over 45% between 2020 and 2022. This trend reflects industry recognition that solid-state and gel polymer electrolytes may provide the necessary safety improvements and polysulfide shuttle inhibition required for commercial viability.
Current Challenges in Polymer Electrolytes for Li-S Batteries
Despite significant advancements in lithium-sulfur (Li-S) battery technology, polymer electrolytes face several critical challenges that hinder their widespread commercial adoption. The primary obstacle remains the polysulfide shuttle effect, where soluble lithium polysulfides migrate between electrodes during cycling, causing capacity fading and reduced battery lifespan. Current polymer electrolyte systems struggle to effectively suppress this phenomenon while maintaining adequate ionic conductivity.
Mechanical stability presents another significant challenge, as polymer electrolytes must withstand volume changes of up to 80% that occur in sulfur cathodes during cycling. Most existing polymer systems lack the necessary mechanical resilience, leading to interfacial delamination and increased internal resistance over multiple charge-discharge cycles.
The inherent trade-off between ionic conductivity and mechanical strength continues to plague polymer electrolyte development. Systems with excellent mechanical properties often exhibit poor room-temperature conductivity (<10^-5 S/cm), while those with superior conductivity typically lack adequate mechanical integrity. This fundamental contradiction has yet to be satisfactorily resolved in a single polymer electrolyte system.
Interfacial compatibility between polymer electrolytes and electrodes remains problematic. Poor wetting characteristics and high interfacial resistance at the polymer-electrode interface lead to increased polarization and decreased rate capability. Additionally, many polymer electrolytes demonstrate limited electrochemical stability windows that fail to accommodate the wide voltage range required for Li-S battery operation.
The lithium dendrite growth issue persists as a critical safety concern. While polymer electrolytes theoretically offer better resistance to dendrite penetration than liquid counterparts, real-world performance has been inconsistent. Many polymer systems still allow dendrite propagation after extended cycling, creating potential short-circuit risks.
Manufacturing scalability presents significant industrial challenges. Current production methods for high-performance polymer electrolytes often involve complex synthesis procedures, expensive materials, or processing conditions incompatible with existing battery manufacturing infrastructure. The transition from laboratory-scale production to industrial manufacturing remains largely unresolved.
Environmental stability is another crucial concern, as many polymer electrolytes demonstrate sensitivity to moisture and oxygen, necessitating stringent manufacturing conditions. Additionally, the long-term thermal stability of polymer electrolytes under various operating conditions requires further investigation to ensure safe battery operation across a wide temperature range.
Addressing these interconnected challenges requires innovative approaches that transcend traditional polymer chemistry, potentially incorporating hybrid systems, novel polymer architectures, or functional additives that can simultaneously address multiple limitations without compromising the inherent safety advantages that make polymer electrolytes attractive for Li-S battery applications.
Mechanical stability presents another significant challenge, as polymer electrolytes must withstand volume changes of up to 80% that occur in sulfur cathodes during cycling. Most existing polymer systems lack the necessary mechanical resilience, leading to interfacial delamination and increased internal resistance over multiple charge-discharge cycles.
The inherent trade-off between ionic conductivity and mechanical strength continues to plague polymer electrolyte development. Systems with excellent mechanical properties often exhibit poor room-temperature conductivity (<10^-5 S/cm), while those with superior conductivity typically lack adequate mechanical integrity. This fundamental contradiction has yet to be satisfactorily resolved in a single polymer electrolyte system.
Interfacial compatibility between polymer electrolytes and electrodes remains problematic. Poor wetting characteristics and high interfacial resistance at the polymer-electrode interface lead to increased polarization and decreased rate capability. Additionally, many polymer electrolytes demonstrate limited electrochemical stability windows that fail to accommodate the wide voltage range required for Li-S battery operation.
The lithium dendrite growth issue persists as a critical safety concern. While polymer electrolytes theoretically offer better resistance to dendrite penetration than liquid counterparts, real-world performance has been inconsistent. Many polymer systems still allow dendrite propagation after extended cycling, creating potential short-circuit risks.
Manufacturing scalability presents significant industrial challenges. Current production methods for high-performance polymer electrolytes often involve complex synthesis procedures, expensive materials, or processing conditions incompatible with existing battery manufacturing infrastructure. The transition from laboratory-scale production to industrial manufacturing remains largely unresolved.
Environmental stability is another crucial concern, as many polymer electrolytes demonstrate sensitivity to moisture and oxygen, necessitating stringent manufacturing conditions. Additionally, the long-term thermal stability of polymer electrolytes under various operating conditions requires further investigation to ensure safe battery operation across a wide temperature range.
Addressing these interconnected challenges requires innovative approaches that transcend traditional polymer chemistry, potentially incorporating hybrid systems, novel polymer architectures, or functional additives that can simultaneously address multiple limitations without compromising the inherent safety advantages that make polymer electrolytes attractive for Li-S battery applications.
Current Polymer Electrolyte Solutions for Li-S Batteries
01 Solid polymer electrolytes for enhanced safety
Solid polymer electrolytes provide improved safety for lithium-sulfur batteries by eliminating the risk of leakage and reducing flammability compared to liquid electrolytes. These solid electrolytes create a physical barrier that prevents the formation of lithium dendrites, which can cause short circuits. Additionally, they help contain the polysulfide shuttle effect, which is a common issue in lithium-sulfur batteries that can lead to capacity fading and safety concerns.- Solid polymer electrolytes for enhanced safety: Solid polymer electrolytes provide enhanced safety for lithium-sulfur batteries by eliminating the risk of leakage and reducing flammability compared to liquid electrolytes. These solid-state systems create a physical barrier that prevents dendrite growth and polysulfide shuttling, which are common safety concerns in lithium-sulfur batteries. The mechanical stability of solid polymer electrolytes also improves the overall structural integrity of the battery under various operating conditions.
- Flame-retardant polymer electrolyte additives: Incorporating flame-retardant additives into polymer electrolytes significantly enhances the safety profile of lithium-sulfur batteries. These additives can include phosphorus-containing compounds, fluorinated polymers, or ceramic particles that suppress combustion and reduce heat generation during thermal runaway events. The flame-retardant components work by interrupting the combustion process or by forming a protective char layer when exposed to high temperatures, thereby preventing catastrophic battery failure.
- Gel polymer electrolytes with safety features: Gel polymer electrolytes combine the high ionic conductivity of liquid electrolytes with the improved safety characteristics of solid polymers. These hybrid systems incorporate safety features such as shutdown mechanisms that activate at elevated temperatures, preventing further electrochemical reactions during overheating. The gel structure also helps contain electrolyte components and limit their interaction with electrodes under abuse conditions, reducing the risk of thermal runaway and improving overall battery safety.
- Polymer electrolytes with polysulfide trapping mechanisms: Specialized polymer electrolytes designed with polysulfide trapping mechanisms enhance safety by preventing the shuttle effect in lithium-sulfur batteries. These electrolytes contain functional groups that chemically bind with dissolved polysulfides, preventing their migration between electrodes. By controlling polysulfide dissolution and migration, these polymer systems reduce the risk of internal short circuits, capacity fading, and thermal instability that could lead to safety hazards during battery operation.
- Composite polymer electrolytes with thermal stability enhancers: Composite polymer electrolytes incorporating inorganic fillers and thermal stability enhancers improve the safety of lithium-sulfur batteries at elevated temperatures. These composites typically contain ceramic particles, metal oxides, or other thermally stable materials that maintain structural integrity and prevent electrolyte degradation under thermal stress. The enhanced thermal stability prevents electrolyte decomposition at high temperatures, reducing gas generation and pressure build-up that could lead to battery rupture or explosion.
02 Flame-retardant polymer electrolyte additives
Incorporating flame-retardant additives into polymer electrolytes significantly enhances the safety profile of lithium-sulfur batteries. These additives can include phosphorus-containing compounds, fluorinated polymers, or ceramic particles that suppress combustion in case of thermal runaway. The flame-retardant components work by interrupting the combustion process, releasing fire-suppressing gases, or forming a protective char layer when exposed to high temperatures, thereby preventing catastrophic battery failure.Expand Specific Solutions03 Gel polymer electrolytes with safety features
Gel polymer electrolytes combine the high ionic conductivity of liquid electrolytes with the improved safety characteristics of solid polymers. These hybrid systems typically consist of a polymer matrix swollen with a liquid electrolyte, providing mechanical stability while maintaining good ion transport properties. The gel structure helps contain electrolyte leakage in case of battery damage and reduces volatility, while specialized additives can provide thermal shutdown mechanisms that activate at elevated temperatures to prevent thermal runaway.Expand Specific Solutions04 Polymer electrolytes with polysulfide trapping mechanisms
Advanced polymer electrolytes incorporate specific functional groups or nanostructures designed to trap polysulfide intermediates, which are formed during the charge-discharge cycles of lithium-sulfur batteries. By immobilizing these polysulfides, the electrolytes prevent their migration to the lithium anode, reducing the risk of side reactions that can lead to thermal instability and safety hazards. These trapping mechanisms also improve the cycle life and performance stability of the batteries while enhancing their overall safety profile.Expand Specific Solutions05 Composite polymer electrolytes with ceramic reinforcements
Composite polymer electrolytes incorporating ceramic particles or fibers offer enhanced mechanical strength and thermal stability for lithium-sulfur batteries. These ceramic components, such as aluminum oxide, silicon dioxide, or lithium-containing ceramics, create a more robust electrolyte structure that can withstand physical stress and prevent internal short circuits. The ceramic reinforcements also help dissipate heat more effectively during battery operation, reducing hotspot formation and the risk of thermal runaway, while maintaining sufficient ionic conductivity for battery performance.Expand Specific Solutions
Key Industry Players in Li-S Battery Electrolyte Development
The lithium-sulfur battery market is currently in its early growth phase, characterized by intensive R&D activities and limited commercial deployment. With a projected market size of $1-2 billion by 2025, this technology offers significant potential due to its higher theoretical energy density compared to conventional lithium-ion batteries. The polymer electrolyte segment represents a critical innovation area for addressing safety challenges. Leading companies like SAMSUNG SDI, LG Energy Solution, and Robert Bosch are investing heavily in polymer electrolyte development, while specialized players such as Oxis Energy and Global Graphene Group focus on niche applications. Academic institutions including Zhejiang University and CNRS are advancing fundamental research. The technology is approaching commercial readiness, with major battery manufacturers establishing pilot production lines, though widespread adoption requires further improvements in cycle life and manufacturing scalability.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed advanced polymer electrolytes for lithium-sulfur batteries featuring a dual-functional polymer matrix system. Their approach combines a mechanically robust polymer framework with polysulfide-trapping functional groups to address the shuttle effect. The technology incorporates fluorinated polymers with high electrochemical stability and specialized additives that form a stable solid electrolyte interphase (SEI). Samsung's polymer electrolytes demonstrate ionic conductivities exceeding 1 mS/cm at room temperature while maintaining excellent mechanical properties. Their multi-layer design includes a protective coating on the lithium anode to prevent dendrite formation and a specialized polymer layer at the cathode interface to trap dissolved polysulfides. This comprehensive approach has enabled Samsung to achieve lithium-sulfur cells with energy densities approaching 400 Wh/kg with significantly improved cycle life compared to conventional liquid electrolyte systems.
Strengths: Samsung's polymer electrolytes offer excellent mechanical stability while maintaining high ionic conductivity, effectively suppressing the polysulfide shuttle effect. Their multi-layer approach provides comprehensive protection for both electrodes. Weaknesses: The complex manufacturing process may increase production costs, and the technology still faces challenges with long-term cycling stability beyond 500 cycles.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has pioneered a composite polymer electrolyte system for lithium-sulfur batteries that combines the benefits of solid polymer matrices with ionic liquid components. Their proprietary technology features a cross-linked polymer network based on modified polyethylene oxide (PEO) with ceramic nanofillers that enhance mechanical properties and ionic conductivity. The electrolyte incorporates lithium salt complexes specifically designed to promote fast lithium-ion transport while inhibiting polysulfide migration. LG's approach includes a gradient polymer structure with varying chemical compositions across the electrolyte thickness to optimize interfaces with both electrodes. Their polymer electrolytes demonstrate thermal stability up to 150°C, making them particularly safe for high-energy applications. Recent developments include self-healing polymer components that can repair microcracks formed during cycling, extending battery lifespan. LG has reported lithium-sulfur cells using these electrolytes achieving over 600 Wh/kg in laboratory settings with retention of 80% capacity after 200 cycles.
Strengths: LG's composite polymer electrolytes offer exceptional thermal stability and safety characteristics while providing sufficient ionic conductivity for practical applications. The self-healing properties represent a significant innovation for long-term durability. Weaknesses: The system still shows limitations in low-temperature performance, and the complex formulation may present challenges for mass production and quality control.
Critical Patents and Innovations in Polymer Electrolyte Design
Polymer electrlyte for a lithium sulfur cell
PatentWO2015185337A2
Innovation
- A polymer electrolyte with specific repeating units, such as those containing uncharged or charged groups attached to the polymer backbone, enhances lithium ion conductivity and reduces polysulfide solubility, improving the performance of lithium-sulfur cells by acting as a binder or separator in sulfur-carbon composites.
Polymer electrolyte for lithium-sulfur battery and lithium-sulfur battery comprising same
PatentInactiveUS20040029016A1
Innovation
- A polymer electrolyte comprising a methacrylate monomer, initiator, organic solvent, and lithium salt is used in lithium-sulfur batteries, forming a gel polymer electrolyte that does not react with lithium metal, thereby enhancing cycle life characteristics by preventing dendrite formation and improving the stability of the sulfur electrode.
Safety Standards and Testing Protocols for Li-S Batteries
The development of lithium-sulfur (Li-S) batteries with polymer electrolytes necessitates comprehensive safety standards and testing protocols to ensure their reliable operation in various applications. Currently, the safety assessment framework for Li-S batteries remains less standardized compared to conventional lithium-ion technologies, creating challenges for industry-wide adoption.
International organizations including IEC, ISO, and UL have begun adapting existing lithium battery standards to address the unique characteristics of Li-S systems. These adaptations focus particularly on the chemical interactions between sulfur cathodes and polymer electrolytes, which present distinct safety considerations compared to traditional battery chemistries.
Thermal stability testing represents a critical component of Li-S battery safety protocols. Tests typically include thermal runaway assessment, with polymer electrolyte Li-S cells demonstrating different failure modes than liquid electrolyte systems. Differential Scanning Calorimetry (DSC) and Accelerating Rate Calorimetry (ARC) have emerged as essential analytical techniques for evaluating the thermal behavior of polymer electrolytes in contact with sulfur species.
Mechanical integrity testing protocols have been established to evaluate polymer electrolyte resilience against dendrite penetration—a significant advantage of solid polymer systems over liquid alternatives. These tests include puncture resistance, compression tolerance, and vibration endurance, with specialized parameters developed for the unique mechanical properties of polymer electrolytes in Li-S configurations.
Electrical safety testing standards address the polysulfide shuttle effect specific to Li-S chemistry. Protocols include overcharge/overdischarge tolerance, short-circuit response, and voltage fluctuation tests under various operational conditions. The polymer electrolyte's ability to suppress shuttle mechanisms represents a key safety parameter measured in these assessments.
Environmental safety considerations have gained prominence in recent testing frameworks, with protocols evaluating gas emission profiles, environmental degradation pathways, and end-of-life recycling compatibility. Polymer electrolytes typically demonstrate favorable environmental safety profiles compared to liquid alternatives containing volatile organic compounds.
Aging and cycle life testing protocols have been specifically modified for Li-S systems to account for the gradual changes in polymer electrolyte properties over extended cycling. These tests evaluate conductivity retention, interfacial stability, and mechanical integrity preservation throughout the battery's operational lifetime.
The harmonization of these testing protocols across different regulatory jurisdictions remains an ongoing challenge, with efforts underway to establish globally recognized safety certification pathways for polymer electrolyte Li-S batteries. This standardization will be crucial for facilitating commercial deployment across diverse application environments.
International organizations including IEC, ISO, and UL have begun adapting existing lithium battery standards to address the unique characteristics of Li-S systems. These adaptations focus particularly on the chemical interactions between sulfur cathodes and polymer electrolytes, which present distinct safety considerations compared to traditional battery chemistries.
Thermal stability testing represents a critical component of Li-S battery safety protocols. Tests typically include thermal runaway assessment, with polymer electrolyte Li-S cells demonstrating different failure modes than liquid electrolyte systems. Differential Scanning Calorimetry (DSC) and Accelerating Rate Calorimetry (ARC) have emerged as essential analytical techniques for evaluating the thermal behavior of polymer electrolytes in contact with sulfur species.
Mechanical integrity testing protocols have been established to evaluate polymer electrolyte resilience against dendrite penetration—a significant advantage of solid polymer systems over liquid alternatives. These tests include puncture resistance, compression tolerance, and vibration endurance, with specialized parameters developed for the unique mechanical properties of polymer electrolytes in Li-S configurations.
Electrical safety testing standards address the polysulfide shuttle effect specific to Li-S chemistry. Protocols include overcharge/overdischarge tolerance, short-circuit response, and voltage fluctuation tests under various operational conditions. The polymer electrolyte's ability to suppress shuttle mechanisms represents a key safety parameter measured in these assessments.
Environmental safety considerations have gained prominence in recent testing frameworks, with protocols evaluating gas emission profiles, environmental degradation pathways, and end-of-life recycling compatibility. Polymer electrolytes typically demonstrate favorable environmental safety profiles compared to liquid alternatives containing volatile organic compounds.
Aging and cycle life testing protocols have been specifically modified for Li-S systems to account for the gradual changes in polymer electrolyte properties over extended cycling. These tests evaluate conductivity retention, interfacial stability, and mechanical integrity preservation throughout the battery's operational lifetime.
The harmonization of these testing protocols across different regulatory jurisdictions remains an ongoing challenge, with efforts underway to establish globally recognized safety certification pathways for polymer electrolyte Li-S batteries. This standardization will be crucial for facilitating commercial deployment across diverse application environments.
Environmental Impact and Sustainability of Polymer Electrolytes
The environmental impact of polymer electrolytes in lithium-sulfur batteries represents a critical consideration in the broader context of sustainable energy storage solutions. Traditional lithium-ion batteries often contain toxic and flammable liquid electrolytes that pose significant environmental hazards throughout their lifecycle. Polymer electrolytes offer a promising alternative with potentially reduced environmental footprint.
The production of polymer electrolytes generally requires fewer toxic solvents compared to conventional liquid electrolytes. Many polymer systems utilize water-based processing methods, significantly reducing the emission of volatile organic compounds (VOCs) during manufacturing. This translates to lower air pollution and reduced health risks for workers in production facilities.
End-of-life management presents another significant environmental advantage for polymer electrolyte-based batteries. The solid-state nature of these electrolytes facilitates easier separation and recycling of battery components. Unlike liquid electrolytes that may leak and contaminate other recyclable materials, polymer electrolytes remain contained, enabling more efficient recovery of valuable materials such as lithium and sulfur.
Carbon footprint analyses of polymer electrolyte production reveal varying results depending on the specific polymer system employed. PEO-based electrolytes generally demonstrate lower greenhouse gas emissions during production compared to conventional liquid electrolytes. However, some specialized fluorinated polymers may have higher environmental impacts due to energy-intensive synthesis processes.
The extended cycle life offered by advanced polymer electrolytes contributes significantly to sustainability by reducing the frequency of battery replacement. Research indicates that lithium-sulfur batteries with optimized polymer electrolytes can potentially achieve 1000+ cycles, substantially extending useful life compared to early lithium-sulfur cells that typically failed after 100-200 cycles.
Resource efficiency represents another environmental benefit, particularly regarding lithium utilization. Polymer electrolytes that effectively suppress the polysulfide shuttle effect enable more complete utilization of active materials, reducing the amount of lithium required per unit of energy storage. This efficiency becomes increasingly important as global demand for lithium continues to rise.
Biodegradability remains an emerging research area for polymer electrolytes. Recent studies have explored naturally derived polymers such as cellulose, chitosan, and starch-based materials as potential electrolyte components. These bio-based polymers offer promising end-of-life degradation characteristics while still maintaining acceptable electrochemical performance in lithium-sulfur systems.
Safety improvements inherent to polymer electrolytes also yield indirect environmental benefits by reducing the incidence of battery fires and associated environmental contamination. The non-flammable nature of most polymer electrolytes eliminates the risk of thermal runaway events that can release toxic compounds into the environment.
The production of polymer electrolytes generally requires fewer toxic solvents compared to conventional liquid electrolytes. Many polymer systems utilize water-based processing methods, significantly reducing the emission of volatile organic compounds (VOCs) during manufacturing. This translates to lower air pollution and reduced health risks for workers in production facilities.
End-of-life management presents another significant environmental advantage for polymer electrolyte-based batteries. The solid-state nature of these electrolytes facilitates easier separation and recycling of battery components. Unlike liquid electrolytes that may leak and contaminate other recyclable materials, polymer electrolytes remain contained, enabling more efficient recovery of valuable materials such as lithium and sulfur.
Carbon footprint analyses of polymer electrolyte production reveal varying results depending on the specific polymer system employed. PEO-based electrolytes generally demonstrate lower greenhouse gas emissions during production compared to conventional liquid electrolytes. However, some specialized fluorinated polymers may have higher environmental impacts due to energy-intensive synthesis processes.
The extended cycle life offered by advanced polymer electrolytes contributes significantly to sustainability by reducing the frequency of battery replacement. Research indicates that lithium-sulfur batteries with optimized polymer electrolytes can potentially achieve 1000+ cycles, substantially extending useful life compared to early lithium-sulfur cells that typically failed after 100-200 cycles.
Resource efficiency represents another environmental benefit, particularly regarding lithium utilization. Polymer electrolytes that effectively suppress the polysulfide shuttle effect enable more complete utilization of active materials, reducing the amount of lithium required per unit of energy storage. This efficiency becomes increasingly important as global demand for lithium continues to rise.
Biodegradability remains an emerging research area for polymer electrolytes. Recent studies have explored naturally derived polymers such as cellulose, chitosan, and starch-based materials as potential electrolyte components. These bio-based polymers offer promising end-of-life degradation characteristics while still maintaining acceptable electrochemical performance in lithium-sulfur systems.
Safety improvements inherent to polymer electrolytes also yield indirect environmental benefits by reducing the incidence of battery fires and associated environmental contamination. The non-flammable nature of most polymer electrolytes eliminates the risk of thermal runaway events that can release toxic compounds into the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!