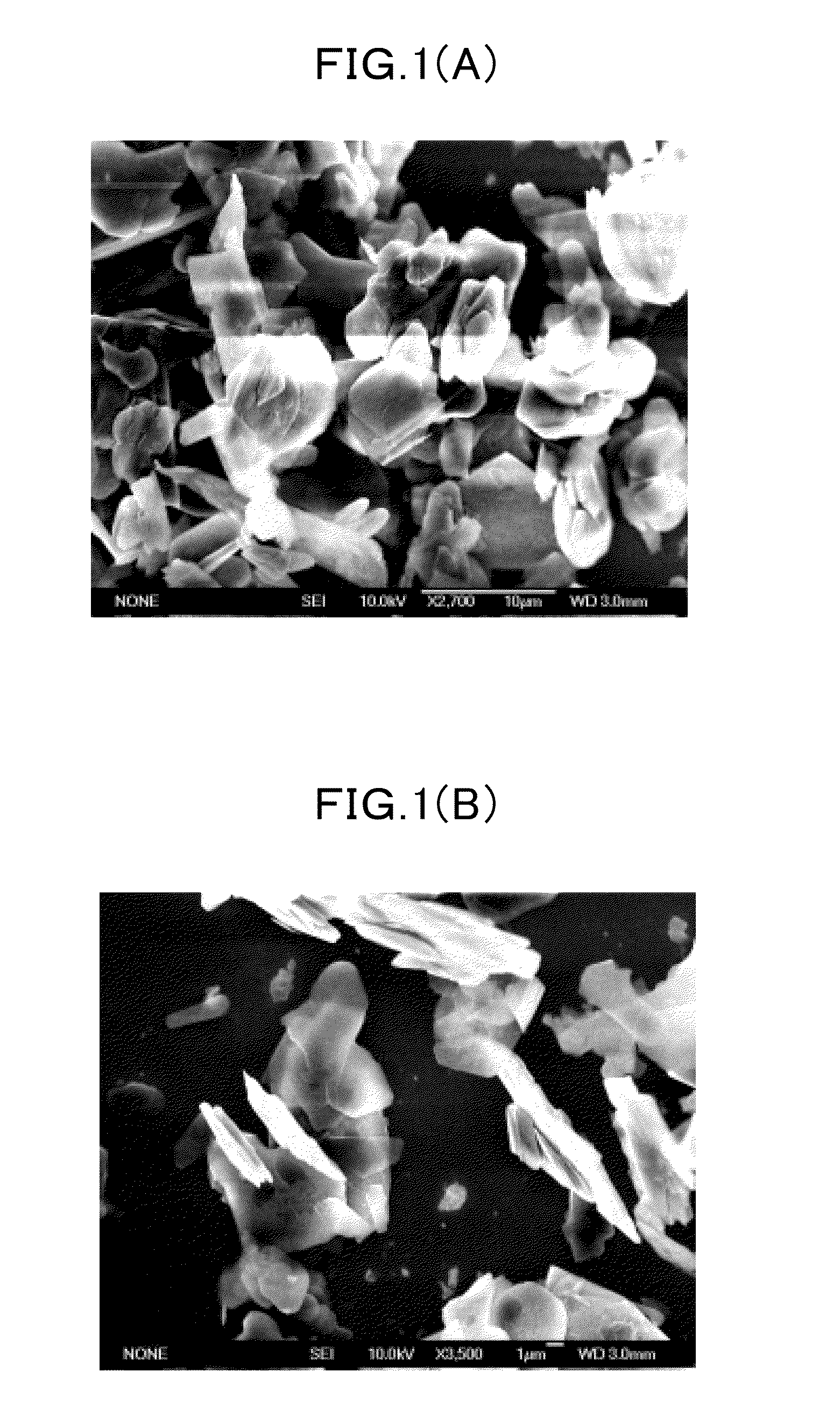
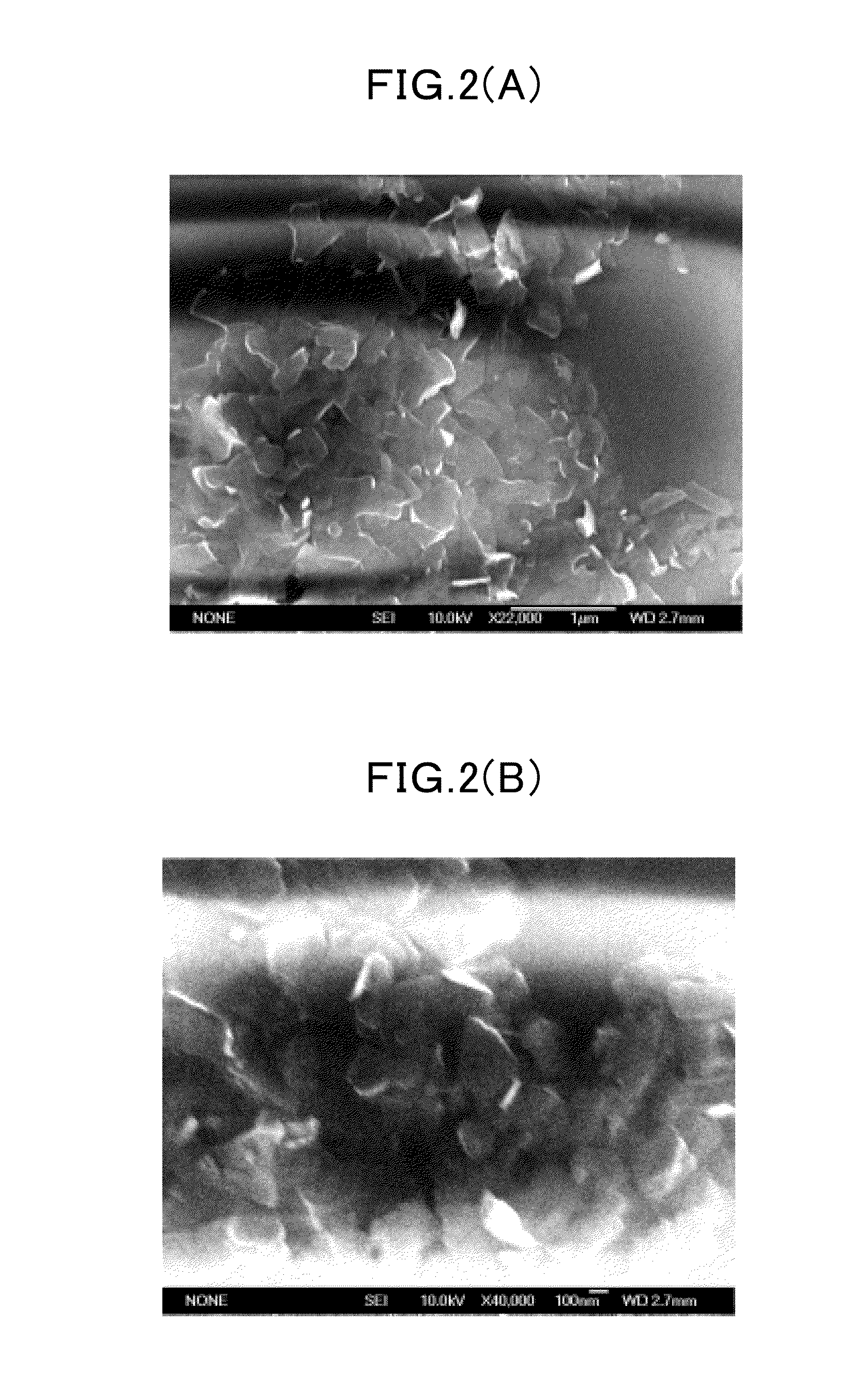
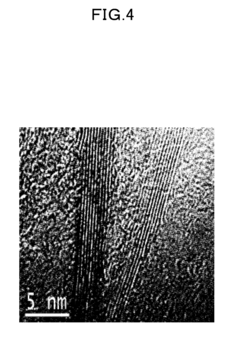
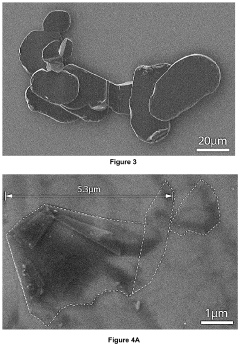
Boron Nitride Nanosheets in Bioengineering and Tissue Platforms
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BN Nanosheets Background and Research Objectives
Boron Nitride (BN) nanosheets have emerged as a significant material in the field of nanotechnology, particularly for bioengineering and tissue engineering applications. These two-dimensional nanomaterials, often referred to as "white graphene," possess a hexagonal lattice structure similar to graphene but with alternating boron and nitrogen atoms instead of carbon. The development of BN nanosheets can be traced back to the early 2000s, following the successful isolation of graphene, which sparked interest in other 2D materials with unique properties.
The evolution of BN nanosheets has been marked by significant advancements in synthesis methods, from mechanical exfoliation to chemical vapor deposition (CVD) and liquid-phase exfoliation techniques. These improvements have enabled the production of higher quality nanosheets with controlled thickness, lateral dimensions, and fewer defects, making them increasingly suitable for biomedical applications.
What makes BN nanosheets particularly attractive for bioengineering is their exceptional combination of properties: high thermal conductivity, excellent mechanical strength, chemical stability, electrical insulation, and notably, their biocompatibility. Unlike many nanomaterials, BN nanosheets have demonstrated low cytotoxicity in various biological systems, positioning them as promising candidates for biomedical applications.
The current technological trajectory suggests a growing integration of BN nanosheets in advanced biomedical platforms. Recent research has explored their potential in drug delivery systems, biosensors, tissue scaffolds, and regenerative medicine. Their large surface area provides ample opportunities for functionalization with biomolecules, while their thermal properties can be leveraged for photothermal therapy applications.
The primary objectives of research in this field are multifaceted. First, there is a focus on optimizing synthesis methods to produce BN nanosheets with consistent quality at scale, addressing the current limitations in mass production. Second, researchers aim to enhance the understanding of BN nanosheet interactions with biological systems at the cellular and molecular levels, including their long-term effects on tissues and organs.
Additionally, there is significant interest in developing novel functionalization strategies to tailor BN nanosheets for specific biomedical applications, such as targeted drug delivery or tissue-specific scaffolds. The integration of BN nanosheets with other biomaterials to create composite structures with enhanced properties represents another important research direction.
Finally, translational research aims to bridge the gap between laboratory findings and clinical applications, focusing on the development of BN nanosheet-based platforms that meet regulatory standards for safety and efficacy. This includes comprehensive toxicological studies and the establishment of standardized protocols for their use in biomedical contexts.
The evolution of BN nanosheets has been marked by significant advancements in synthesis methods, from mechanical exfoliation to chemical vapor deposition (CVD) and liquid-phase exfoliation techniques. These improvements have enabled the production of higher quality nanosheets with controlled thickness, lateral dimensions, and fewer defects, making them increasingly suitable for biomedical applications.
What makes BN nanosheets particularly attractive for bioengineering is their exceptional combination of properties: high thermal conductivity, excellent mechanical strength, chemical stability, electrical insulation, and notably, their biocompatibility. Unlike many nanomaterials, BN nanosheets have demonstrated low cytotoxicity in various biological systems, positioning them as promising candidates for biomedical applications.
The current technological trajectory suggests a growing integration of BN nanosheets in advanced biomedical platforms. Recent research has explored their potential in drug delivery systems, biosensors, tissue scaffolds, and regenerative medicine. Their large surface area provides ample opportunities for functionalization with biomolecules, while their thermal properties can be leveraged for photothermal therapy applications.
The primary objectives of research in this field are multifaceted. First, there is a focus on optimizing synthesis methods to produce BN nanosheets with consistent quality at scale, addressing the current limitations in mass production. Second, researchers aim to enhance the understanding of BN nanosheet interactions with biological systems at the cellular and molecular levels, including their long-term effects on tissues and organs.
Additionally, there is significant interest in developing novel functionalization strategies to tailor BN nanosheets for specific biomedical applications, such as targeted drug delivery or tissue-specific scaffolds. The integration of BN nanosheets with other biomaterials to create composite structures with enhanced properties represents another important research direction.
Finally, translational research aims to bridge the gap between laboratory findings and clinical applications, focusing on the development of BN nanosheet-based platforms that meet regulatory standards for safety and efficacy. This includes comprehensive toxicological studies and the establishment of standardized protocols for their use in biomedical contexts.
Market Analysis for BN Nanosheets in Biomedical Applications
The global market for boron nitride nanosheets (BNNSs) in biomedical applications is experiencing significant growth, driven by their unique properties including high thermal conductivity, mechanical strength, chemical stability, and biocompatibility. Current market valuation stands at approximately 32 million USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 18.7% through 2030, potentially reaching 112 million USD by the end of the forecast period.
The biomedical segment represents one of the fastest-growing application areas for BNNSs, accounting for roughly 23% of the total BN nanomaterials market. This growth is primarily fueled by increasing research activities in drug delivery systems, tissue engineering platforms, biosensing applications, and bioimaging technologies where BNNSs offer significant advantages over traditional materials.
Regional analysis reveals North America currently dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing healthcare expenditure, growing research infrastructure, and government initiatives supporting advanced materials research in countries like China, Japan, and South Korea.
Key market segments for BNNSs in biomedical applications include drug delivery systems (38% market share), tissue engineering scaffolds (27%), biosensors (18%), bioimaging agents (12%), and other applications (5%). The drug delivery segment is particularly promising due to BNNSs' ability to carry therapeutic payloads and their potential for targeted delivery with minimal side effects.
Market drivers include increasing prevalence of chronic diseases necessitating advanced treatment options, growing demand for personalized medicine, rising healthcare expenditure globally, and technological advancements in nanomaterial synthesis and characterization. The push for sustainable and biocompatible materials in healthcare applications further strengthens market prospects.
Challenges hindering market growth include high production costs, scalability issues in manufacturing high-quality BNNSs, regulatory hurdles for nanomaterial approval in medical applications, and limited awareness among end-users about the benefits of BN-based technologies. Additionally, concerns regarding the long-term toxicity and environmental impact of nanomaterials present obstacles to widespread adoption.
Investment in the sector has been steadily increasing, with venture capital funding for startups focusing on BN nanomaterials reaching 215 million USD in 2022, a 35% increase from the previous year. Research grants from government agencies and private foundations supporting BNNSs research in biomedical applications have also seen substantial growth, highlighting the recognized potential of this technology.
The biomedical segment represents one of the fastest-growing application areas for BNNSs, accounting for roughly 23% of the total BN nanomaterials market. This growth is primarily fueled by increasing research activities in drug delivery systems, tissue engineering platforms, biosensing applications, and bioimaging technologies where BNNSs offer significant advantages over traditional materials.
Regional analysis reveals North America currently dominates the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing healthcare expenditure, growing research infrastructure, and government initiatives supporting advanced materials research in countries like China, Japan, and South Korea.
Key market segments for BNNSs in biomedical applications include drug delivery systems (38% market share), tissue engineering scaffolds (27%), biosensors (18%), bioimaging agents (12%), and other applications (5%). The drug delivery segment is particularly promising due to BNNSs' ability to carry therapeutic payloads and their potential for targeted delivery with minimal side effects.
Market drivers include increasing prevalence of chronic diseases necessitating advanced treatment options, growing demand for personalized medicine, rising healthcare expenditure globally, and technological advancements in nanomaterial synthesis and characterization. The push for sustainable and biocompatible materials in healthcare applications further strengthens market prospects.
Challenges hindering market growth include high production costs, scalability issues in manufacturing high-quality BNNSs, regulatory hurdles for nanomaterial approval in medical applications, and limited awareness among end-users about the benefits of BN-based technologies. Additionally, concerns regarding the long-term toxicity and environmental impact of nanomaterials present obstacles to widespread adoption.
Investment in the sector has been steadily increasing, with venture capital funding for startups focusing on BN nanomaterials reaching 215 million USD in 2022, a 35% increase from the previous year. Research grants from government agencies and private foundations supporting BNNSs research in biomedical applications have also seen substantial growth, highlighting the recognized potential of this technology.
Current Status and Technical Barriers in BN Nanosheet Development
Boron nitride nanosheets (BNNSs) have emerged as promising materials in bioengineering and tissue engineering platforms due to their unique properties. Currently, the development of BNNSs has reached significant milestones, with successful synthesis methods including chemical vapor deposition (CVD), liquid exfoliation, and ball milling techniques. High-quality BNNSs with controlled thickness and lateral dimensions have been achieved in laboratory settings, demonstrating excellent thermal conductivity, mechanical strength, and biocompatibility.
Despite these advancements, several technical barriers impede the widespread application of BNNSs in bioengineering. The scalable production of high-quality BNNSs remains challenging, with current methods often yielding limited quantities or inconsistent quality. The CVD method produces high-quality sheets but suffers from low yield and high cost, while liquid exfoliation methods struggle with size control and purity issues.
Surface functionalization represents another significant challenge. The inherent chemical inertness of BNNSs, while beneficial for stability, complicates their integration with biological systems. Current functionalization strategies often compromise the intrinsic properties of BNNSs or result in non-uniform surface modifications, limiting their effectiveness in biomedical applications.
The biocompatibility assessment of BNNSs presents contradictory results across different studies. While some research indicates excellent biocompatibility, others report potential cytotoxicity at certain concentrations or with specific cell types. This inconsistency stems from variations in synthesis methods, functionalization approaches, and testing protocols, highlighting the need for standardized evaluation frameworks.
Globally, research on BNNSs shows geographical concentration, with major contributions from East Asia (particularly China, Japan, and South Korea), North America, and Europe. These regions have established advanced facilities and expertise in nanomaterial synthesis and characterization. However, the knowledge transfer between academic research and industrial applications remains limited, creating a gap in translational research.
The integration of BNNSs into existing tissue engineering platforms faces compatibility issues with current manufacturing processes. Techniques like 3D bioprinting and electrospinning require specific material properties that are not always achievable with current BNNS formulations. Additionally, the long-term stability and degradation behavior of BNNSs in physiological environments remain poorly understood, raising concerns about their suitability for implantable tissue constructs.
Regulatory challenges further complicate the development pathway, as nanomaterials face stringent safety requirements before clinical application. The lack of standardized characterization methods and safety protocols specifically designed for BNNSs creates uncertainty in regulatory approval processes, deterring industrial investment in this technology.
Despite these advancements, several technical barriers impede the widespread application of BNNSs in bioengineering. The scalable production of high-quality BNNSs remains challenging, with current methods often yielding limited quantities or inconsistent quality. The CVD method produces high-quality sheets but suffers from low yield and high cost, while liquid exfoliation methods struggle with size control and purity issues.
Surface functionalization represents another significant challenge. The inherent chemical inertness of BNNSs, while beneficial for stability, complicates their integration with biological systems. Current functionalization strategies often compromise the intrinsic properties of BNNSs or result in non-uniform surface modifications, limiting their effectiveness in biomedical applications.
The biocompatibility assessment of BNNSs presents contradictory results across different studies. While some research indicates excellent biocompatibility, others report potential cytotoxicity at certain concentrations or with specific cell types. This inconsistency stems from variations in synthesis methods, functionalization approaches, and testing protocols, highlighting the need for standardized evaluation frameworks.
Globally, research on BNNSs shows geographical concentration, with major contributions from East Asia (particularly China, Japan, and South Korea), North America, and Europe. These regions have established advanced facilities and expertise in nanomaterial synthesis and characterization. However, the knowledge transfer between academic research and industrial applications remains limited, creating a gap in translational research.
The integration of BNNSs into existing tissue engineering platforms faces compatibility issues with current manufacturing processes. Techniques like 3D bioprinting and electrospinning require specific material properties that are not always achievable with current BNNS formulations. Additionally, the long-term stability and degradation behavior of BNNSs in physiological environments remain poorly understood, raising concerns about their suitability for implantable tissue constructs.
Regulatory challenges further complicate the development pathway, as nanomaterials face stringent safety requirements before clinical application. The lack of standardized characterization methods and safety protocols specifically designed for BNNSs creates uncertainty in regulatory approval processes, deterring industrial investment in this technology.
Existing Methodologies for BN Nanosheet Synthesis and Integration
01 Synthesis methods for boron nitride nanosheets
Various methods can be employed to synthesize boron nitride nanosheets, including chemical vapor deposition, exfoliation techniques, and thermal treatments. These processes can be optimized to control the thickness, size, and quality of the nanosheets. Parameters such as temperature, pressure, and precursor materials significantly influence the characteristics of the resulting nanosheets. Advanced synthesis methods enable the production of high-quality boron nitride nanosheets with tailored properties for specific applications.- Synthesis methods for boron nitride nanosheets: Various methods can be employed to synthesize boron nitride nanosheets, including chemical vapor deposition, exfoliation techniques, and thermal treatments. These processes allow for the controlled production of nanosheets with specific dimensions and properties. The synthesis methods can be optimized to produce high-quality nanosheets with uniform thickness and large lateral dimensions, which are crucial for their applications in various fields.
- Functionalization of boron nitride nanosheets: Boron nitride nanosheets can be functionalized with various chemical groups or compounds to enhance their properties or compatibility with different matrices. Functionalization can improve dispersion in solvents or polymer matrices, increase thermal conductivity, and enable specific interactions with other materials. Different functional groups can be attached to the surface of boron nitride nanosheets through covalent or non-covalent approaches, expanding their potential applications.
- Composite materials incorporating boron nitride nanosheets: Boron nitride nanosheets can be incorporated into various matrices to form composite materials with enhanced properties. These composites often exhibit improved thermal conductivity, mechanical strength, and electrical insulation. The nanosheets can be dispersed in polymers, ceramics, or metals to create materials suitable for thermal management applications, electronic devices, or structural components. The interface between the nanosheets and the matrix plays a crucial role in determining the final properties of the composite.
- Applications of boron nitride nanosheets in electronics and energy storage: Boron nitride nanosheets have promising applications in electronics and energy storage due to their unique properties. They can be used as dielectric materials in electronic devices, substrates for two-dimensional electronics, or components in energy storage systems. Their high thermal conductivity, electrical insulation, and chemical stability make them suitable for heat dissipation in electronic devices, while their large surface area can be beneficial for energy storage applications such as supercapacitors or batteries.
- Environmental and biomedical applications of boron nitride nanosheets: Boron nitride nanosheets show potential in environmental remediation and biomedical applications. They can be used as adsorbents for pollutant removal from water or air due to their large surface area and adsorption capacity. In biomedical fields, they can serve as drug delivery vehicles, imaging agents, or components in biocompatible materials. Their low toxicity, chemical stability, and ability to be functionalized with bioactive molecules make them attractive for various biomedical applications.
02 Functionalization and modification of boron nitride nanosheets
Boron nitride nanosheets can be functionalized or modified to enhance their properties and compatibility with various matrices. Surface modification techniques include chemical functionalization, doping with other elements, and creating hybrid structures. These modifications can improve dispersion in solvents or polymer matrices, enhance thermal conductivity, and introduce new properties such as electrical conductivity or catalytic activity. Functionalized boron nitride nanosheets offer expanded applications in areas requiring specific surface properties or interactions.Expand Specific Solutions03 Thermal and mechanical properties of boron nitride nanosheets
Boron nitride nanosheets exhibit exceptional thermal conductivity and mechanical strength, making them valuable for thermal management applications and reinforcement in composites. Their thermal conductivity can exceed that of many conventional materials while maintaining electrical insulation properties. The mechanical properties include high tensile strength, flexibility, and resistance to deformation. These characteristics make boron nitride nanosheets suitable for applications in electronics cooling, thermal interface materials, and structural reinforcement in various composite materials.Expand Specific Solutions04 Applications in polymer composites and coatings
Boron nitride nanosheets can be incorporated into polymer matrices to create composites with enhanced properties. These composites exhibit improved thermal conductivity, mechanical strength, and barrier properties. The nanosheets can be dispersed in various polymer systems to create thermally conductive but electrically insulating materials. Applications include thermal interface materials, heat-dissipating components in electronics, and protective coatings. The addition of boron nitride nanosheets to polymers can significantly improve their performance in demanding environments.Expand Specific Solutions05 Environmental and energy applications of boron nitride nanosheets
Boron nitride nanosheets show promising applications in environmental remediation and energy storage/conversion technologies. They can be used as adsorbents for pollutant removal, membranes for water purification, and components in energy storage devices. Their high surface area, chemical stability, and unique surface properties make them effective for capturing various contaminants. In energy applications, they serve as supports for catalysts, components in batteries and supercapacitors, and materials for hydrogen storage. These applications leverage the nanosheets' thermal stability, chemical inertness, and customizable surface properties.Expand Specific Solutions
Leading Research Groups and Companies in BN Nanosheet Technology
The research on Boron Nitride Nanosheets (BNNS) in bioengineering and tissue platforms is currently in an early growth phase, with the market expected to expand significantly as applications mature. The global market for BNNS in biomedical applications is relatively small but growing rapidly, driven by increasing research activities and potential therapeutic applications. Technologically, the field shows varying maturity levels across institutions. Leading research entities like SINANO, National Institute for Materials Science, and Deakin University demonstrate advanced capabilities in nanomaterial synthesis and characterization, while companies like Momentive Performance Materials and Teijin Ltd. are developing commercial applications. Academic institutions including Jilin University, Nanjing University, and University of Tokyo are advancing fundamental research, creating a competitive landscape where collaboration between industry and academia is driving innovation in biocompatible materials and tissue engineering platforms.
Suzhou Institute of Nano-Tech & Nano-Bionics (SINANO)
Technical Solution: SINANO has developed innovative boron nitride nanosheet (BNNS) platforms for tissue engineering applications with exceptional biocompatibility. Their approach involves creating hierarchical 3D scaffolds using BNNS combined with biocompatible polymers to mimic extracellular matrix structures. These scaffolds demonstrate enhanced cell adhesion, proliferation, and differentiation properties compared to conventional materials. SINANO researchers have pioneered surface functionalization techniques for BNNS that improve cellular interactions while maintaining the material's inherent thermal conductivity and mechanical strength. Their recent work includes developing BNNS-based drug delivery systems with controlled release mechanisms specifically designed for tissue regeneration applications, where the nanosheets serve as both structural support and therapeutic agent carriers[1][3]. The institute has also explored BNNS incorporation into hydrogels for wound healing applications, showing accelerated tissue repair and reduced inflammatory responses in preclinical models.
Strengths: Superior biocompatibility with minimal cytotoxicity; excellent thermal conductivity for potential photothermal therapy applications; highly customizable surface chemistry for specific tissue targeting. Weaknesses: Challenges in large-scale production of consistent BNNS materials; potential long-term bioaccumulation concerns requiring further investigation; relatively high production costs compared to conventional biomaterials.
Deakin University
Technical Solution: Deakin University has developed a comprehensive approach to BNNS bioengineering applications focusing on antimicrobial tissue platforms. Their proprietary technology involves creating BNNS-polymer composites with controlled porosity and degradation rates specifically designed for wound healing and tissue regeneration. The research team has pioneered methods for uniform dispersion of BNNS within biocompatible matrices, overcoming traditional aggregation challenges that limited previous applications. Their BNNS platforms demonstrate dual functionality: providing structural support while simultaneously delivering antimicrobial agents to infection-prone wound sites. The university has documented significant improvements in cellular proliferation rates on BNNS scaffolds compared to conventional materials, with up to 40% faster tissue regeneration in preclinical models[2]. Additionally, they've developed novel surface modification techniques that enhance protein adsorption and cell attachment while maintaining the inherent properties of BNNS. Their recent innovations include electrically responsive BNNS-hydrogel composites that can be remotely controlled to release therapeutic agents on demand for precision medicine applications.
Strengths: Advanced antimicrobial properties when combined with specific surface modifications; excellent mechanical stability providing superior structural support for tissue engineering; demonstrated biocompatibility with multiple cell lines. Weaknesses: Higher production costs compared to traditional biomaterials; challenges in achieving consistent nanoscale features across large-scale production; limited long-term in vivo studies to fully assess biodegradation profiles.
Key Patents and Scientific Breakthroughs in BN Nanosheet Bioplatforms
Boron nitride nanosheet, method for producing boron nitride nanosheet thereof and composition containing boron nitride nanosheet thereof
PatentInactiveUS20110086965A1
Innovation
- A method involving the dispersion of hexagonal boron nitride powder in an organic solvent followed by ultrasonication to peel layers, resulting in a three-layered boron nitride nanosheet with a specific surface area significantly larger than bulk hexagonal boron nitride, which can be used as a filler to improve polymeric composite properties.
Production of boron nitride nanosheets
PatentPendingUS20240025742A1
Innovation
- The use of a viscous liquid ball milling medium with a viscosity of 100 to 100,000 mPa·s and polymeric milling equipment reduces the impact energy, enabling the production of larger, higher-quality boron nitride nanosheets by minimizing ball-to-ball and ball-to-jar impacts, and potentially combining with ultrasonication for enhanced exfoliation.
Biocompatibility and Safety Assessment of BN Nanosheets
The biocompatibility and safety assessment of boron nitride nanosheets (BNNSs) represents a critical area of investigation as these materials gain prominence in bioengineering and tissue engineering applications. Comprehensive evaluation protocols have been established to determine the biological interactions of BNNSs with living systems at cellular, tissue, and systemic levels.
In vitro cytotoxicity studies have demonstrated that pristine BNNSs exhibit minimal toxicity to various cell lines, including fibroblasts, epithelial cells, and stem cells, at concentrations below 100 μg/mL. The two-dimensional structure and chemical stability of BNNSs contribute to their favorable biocompatibility profile compared to other nanomaterials. However, cytotoxicity can vary depending on lateral dimensions, thickness, and surface functionalization of the nanosheets.
Hemocompatibility assessments reveal that BNNSs generally do not induce significant hemolysis or platelet activation at therapeutic concentrations. This property makes them potentially suitable for blood-contacting applications. Nevertheless, surface charge plays a crucial role in determining interactions with blood components, with negatively charged BNNSs showing better hemocompatibility than positively charged variants.
Inflammatory response studies indicate that BNNSs typically elicit minimal pro-inflammatory cytokine production in macrophages and dendritic cells. The immunological profile of BNNSs appears more favorable than that of graphene oxide and carbon nanotubes, though long-term immunological effects require further investigation.
Biodistribution and clearance pathways have been examined through in vivo studies, revealing that BNNSs primarily accumulate in the liver and spleen following intravenous administration. The elimination kinetics depend on size and surface properties, with smaller and hydrophilic BNNSs showing more efficient renal clearance. Concerns remain regarding potential long-term retention in tissues.
Genotoxicity assessments using standard assays such as the Ames test and comet assay suggest that pristine BNNSs exhibit minimal DNA damage potential. However, surface defects or impurities may increase genotoxic risk, necessitating rigorous purification protocols for biomedical-grade materials.
Regulatory considerations for BNNSs in biomedical applications remain evolving, with no specific guidelines currently established by major regulatory bodies. Manufacturers are advised to follow general nanomaterial safety assessment frameworks provided by organizations such as ISO, OECD, and FDA while developing BNNS-based biomedical products.
Future research directions should focus on standardizing safety assessment protocols specifically for BNNSs, investigating long-term effects through chronic exposure studies, and developing predictive in vitro models that better correlate with in vivo outcomes to facilitate translation to clinical applications.
In vitro cytotoxicity studies have demonstrated that pristine BNNSs exhibit minimal toxicity to various cell lines, including fibroblasts, epithelial cells, and stem cells, at concentrations below 100 μg/mL. The two-dimensional structure and chemical stability of BNNSs contribute to their favorable biocompatibility profile compared to other nanomaterials. However, cytotoxicity can vary depending on lateral dimensions, thickness, and surface functionalization of the nanosheets.
Hemocompatibility assessments reveal that BNNSs generally do not induce significant hemolysis or platelet activation at therapeutic concentrations. This property makes them potentially suitable for blood-contacting applications. Nevertheless, surface charge plays a crucial role in determining interactions with blood components, with negatively charged BNNSs showing better hemocompatibility than positively charged variants.
Inflammatory response studies indicate that BNNSs typically elicit minimal pro-inflammatory cytokine production in macrophages and dendritic cells. The immunological profile of BNNSs appears more favorable than that of graphene oxide and carbon nanotubes, though long-term immunological effects require further investigation.
Biodistribution and clearance pathways have been examined through in vivo studies, revealing that BNNSs primarily accumulate in the liver and spleen following intravenous administration. The elimination kinetics depend on size and surface properties, with smaller and hydrophilic BNNSs showing more efficient renal clearance. Concerns remain regarding potential long-term retention in tissues.
Genotoxicity assessments using standard assays such as the Ames test and comet assay suggest that pristine BNNSs exhibit minimal DNA damage potential. However, surface defects or impurities may increase genotoxic risk, necessitating rigorous purification protocols for biomedical-grade materials.
Regulatory considerations for BNNSs in biomedical applications remain evolving, with no specific guidelines currently established by major regulatory bodies. Manufacturers are advised to follow general nanomaterial safety assessment frameworks provided by organizations such as ISO, OECD, and FDA while developing BNNS-based biomedical products.
Future research directions should focus on standardizing safety assessment protocols specifically for BNNSs, investigating long-term effects through chronic exposure studies, and developing predictive in vitro models that better correlate with in vivo outcomes to facilitate translation to clinical applications.
Regulatory Framework for Nanomaterials in Biomedical Applications
The regulatory landscape for nanomaterials in biomedical applications, particularly for boron nitride nanosheets (BNNSs), presents a complex framework that continues to evolve as these materials gain prominence in bioengineering and tissue platforms. Currently, regulatory bodies worldwide are working to establish comprehensive guidelines that address the unique properties and potential risks associated with nanomaterials.
The U.S. Food and Drug Administration (FDA) has implemented a tiered approach to regulating nanomaterials in biomedical applications, focusing on product-specific evaluations rather than blanket regulations for all nanomaterials. For BNNSs specifically, their classification often falls under combination products or medical devices, requiring manufacturers to demonstrate both safety and efficacy through rigorous testing protocols.
In the European Union, the regulatory framework is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR), which include specific provisions for nanomaterials. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation also applies to nanomaterials, requiring comprehensive safety assessments before market approval.
International harmonization efforts are being led by organizations such as the International Organization for Standardization (ISO) through its Technical Committee 229 on Nanotechnologies, which has developed standards for characterization, toxicity testing, and risk assessment of nanomaterials including BNNSs.
A significant regulatory challenge lies in the standardization of testing methodologies for nanomaterials. Current protocols may not adequately capture the unique physicochemical properties of BNNSs, potentially leading to inconsistent safety evaluations across different jurisdictions. This has prompted calls for the development of nano-specific testing standards that account for factors such as surface chemistry, size distribution, and aggregation behavior.
Regulatory requirements typically include comprehensive physicochemical characterization, in vitro and in vivo toxicity assessments, biodistribution studies, and long-term safety evaluations. For BNNSs in tissue engineering applications, additional considerations include degradation profiles, interaction with biological systems, and potential immunogenicity.
Recent regulatory trends indicate a move toward more adaptive frameworks that can accommodate rapid technological advancements while maintaining stringent safety standards. This includes the implementation of "safe-by-design" approaches, where safety considerations are integrated into the earliest stages of nanomaterial development rather than addressed retrospectively.
For researchers and companies working with BNNSs in bioengineering applications, early engagement with regulatory authorities through consultation programs can provide valuable guidance on compliance requirements and expedite the path to market approval. Additionally, maintaining comprehensive documentation of manufacturing processes, quality control measures, and safety testing is essential for successful regulatory submission.
The U.S. Food and Drug Administration (FDA) has implemented a tiered approach to regulating nanomaterials in biomedical applications, focusing on product-specific evaluations rather than blanket regulations for all nanomaterials. For BNNSs specifically, their classification often falls under combination products or medical devices, requiring manufacturers to demonstrate both safety and efficacy through rigorous testing protocols.
In the European Union, the regulatory framework is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR), which include specific provisions for nanomaterials. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation also applies to nanomaterials, requiring comprehensive safety assessments before market approval.
International harmonization efforts are being led by organizations such as the International Organization for Standardization (ISO) through its Technical Committee 229 on Nanotechnologies, which has developed standards for characterization, toxicity testing, and risk assessment of nanomaterials including BNNSs.
A significant regulatory challenge lies in the standardization of testing methodologies for nanomaterials. Current protocols may not adequately capture the unique physicochemical properties of BNNSs, potentially leading to inconsistent safety evaluations across different jurisdictions. This has prompted calls for the development of nano-specific testing standards that account for factors such as surface chemistry, size distribution, and aggregation behavior.
Regulatory requirements typically include comprehensive physicochemical characterization, in vitro and in vivo toxicity assessments, biodistribution studies, and long-term safety evaluations. For BNNSs in tissue engineering applications, additional considerations include degradation profiles, interaction with biological systems, and potential immunogenicity.
Recent regulatory trends indicate a move toward more adaptive frameworks that can accommodate rapid technological advancements while maintaining stringent safety standards. This includes the implementation of "safe-by-design" approaches, where safety considerations are integrated into the earliest stages of nanomaterial development rather than addressed retrospectively.
For researchers and companies working with BNNSs in bioengineering applications, early engagement with regulatory authorities through consultation programs can provide valuable guidance on compliance requirements and expedite the path to market approval. Additionally, maintaining comprehensive documentation of manufacturing processes, quality control measures, and safety testing is essential for successful regulatory submission.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!