Boron Nitride Nanosheets in Magnetic Resonance Imaging Equipment
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BN Nanosheets in MRI: Background and Objectives
Boron nitride nanosheets (BNNSs) have emerged as a revolutionary material in the field of magnetic resonance imaging (MRI) technology over the past decade. The evolution of this technology can be traced back to the early 2000s when two-dimensional nanomaterials began gaining significant attention following the discovery of graphene. BNNSs, often referred to as "white graphene," share a similar hexagonal structure with graphene but consist of alternating boron and nitrogen atoms instead of carbon.
The development trajectory of BNNSs has been marked by significant breakthroughs in synthesis methods, from mechanical exfoliation to chemical vapor deposition and liquid-phase exfoliation techniques. These advancements have progressively enabled the production of higher quality nanosheets with controlled thickness and lateral dimensions, which are crucial parameters for MRI applications.
Recent years have witnessed an accelerating trend toward the functionalization of BNNSs for biomedical applications, particularly in imaging contrast enhancement. The unique properties of BNNSs, including their excellent thermal stability, chemical inertness, and low cytotoxicity, make them particularly attractive for in vivo applications compared to other nanomaterials.
The integration of BNNSs into MRI technology represents a convergence of materials science and medical imaging, addressing several limitations of conventional contrast agents. Traditional gadolinium-based contrast agents, while effective, have raised concerns regarding nephrotoxicity and retention in biological tissues. BNNSs offer potential alternatives that can enhance imaging contrast while potentially reducing these risks.
The primary technical objectives for BNNSs in MRI applications include enhancing contrast efficiency, improving targeting capabilities for specific tissues or pathologies, reducing potential toxicity, and developing stimuli-responsive imaging agents. Researchers aim to leverage the unique physical and chemical properties of BNNSs to create next-generation contrast agents with superior performance characteristics.
Another critical objective is the development of multifunctional platforms where BNNSs can simultaneously serve as contrast agents for MRI and carriers for therapeutic agents, enabling theranostic applications. This dual functionality could revolutionize personalized medicine by allowing real-time monitoring of drug delivery and therapeutic response.
The field is also moving toward creating hybrid systems that combine BNNSs with magnetic nanoparticles or other functional materials to achieve synergistic effects and enhanced performance in MRI applications. These hybrid materials aim to overcome the inherent limitations of single-component systems and offer unprecedented capabilities in medical imaging.
As research progresses, the ultimate goal remains the clinical translation of BNNS-based MRI contrast agents, which requires addressing challenges related to large-scale production, standardization, biocompatibility, and regulatory approval. The potential impact on diagnostic accuracy and patient outcomes drives continued investment and innovation in this promising technological frontier.
The development trajectory of BNNSs has been marked by significant breakthroughs in synthesis methods, from mechanical exfoliation to chemical vapor deposition and liquid-phase exfoliation techniques. These advancements have progressively enabled the production of higher quality nanosheets with controlled thickness and lateral dimensions, which are crucial parameters for MRI applications.
Recent years have witnessed an accelerating trend toward the functionalization of BNNSs for biomedical applications, particularly in imaging contrast enhancement. The unique properties of BNNSs, including their excellent thermal stability, chemical inertness, and low cytotoxicity, make them particularly attractive for in vivo applications compared to other nanomaterials.
The integration of BNNSs into MRI technology represents a convergence of materials science and medical imaging, addressing several limitations of conventional contrast agents. Traditional gadolinium-based contrast agents, while effective, have raised concerns regarding nephrotoxicity and retention in biological tissues. BNNSs offer potential alternatives that can enhance imaging contrast while potentially reducing these risks.
The primary technical objectives for BNNSs in MRI applications include enhancing contrast efficiency, improving targeting capabilities for specific tissues or pathologies, reducing potential toxicity, and developing stimuli-responsive imaging agents. Researchers aim to leverage the unique physical and chemical properties of BNNSs to create next-generation contrast agents with superior performance characteristics.
Another critical objective is the development of multifunctional platforms where BNNSs can simultaneously serve as contrast agents for MRI and carriers for therapeutic agents, enabling theranostic applications. This dual functionality could revolutionize personalized medicine by allowing real-time monitoring of drug delivery and therapeutic response.
The field is also moving toward creating hybrid systems that combine BNNSs with magnetic nanoparticles or other functional materials to achieve synergistic effects and enhanced performance in MRI applications. These hybrid materials aim to overcome the inherent limitations of single-component systems and offer unprecedented capabilities in medical imaging.
As research progresses, the ultimate goal remains the clinical translation of BNNS-based MRI contrast agents, which requires addressing challenges related to large-scale production, standardization, biocompatibility, and regulatory approval. The potential impact on diagnostic accuracy and patient outcomes drives continued investment and innovation in this promising technological frontier.
Market Analysis for Advanced MRI Contrast Agents
The global market for advanced MRI contrast agents is experiencing significant growth, driven by increasing prevalence of chronic diseases requiring accurate diagnosis and the rising demand for enhanced imaging technologies. The current market value for MRI contrast agents stands at approximately $2.1 billion, with projections indicating a compound annual growth rate of 3.8% through 2028. Traditional gadolinium-based contrast agents currently dominate with over 80% market share, despite growing concerns about gadolinium retention in tissues.
Boron Nitride Nanosheets (BNNS) represent an emerging segment within the advanced contrast agent market, positioned to potentially disrupt the established paradigm. Market analysis indicates that novel nanomaterial-based contrast agents, including BNNS, are expected to capture 15% of the market by 2027, representing a substantial shift from their current 3% share. This growth is primarily attributed to their superior biocompatibility profiles and enhanced imaging capabilities.
Healthcare facilities and diagnostic centers constitute the largest end-user segment for advanced MRI contrast agents, accounting for 68% of total consumption. Research institutions represent a smaller but rapidly growing segment with 18% market share, particularly for novel agents like BNNS that are still transitioning from research to clinical applications.
Geographically, North America leads the market with 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 5.7% annually, driven by expanding healthcare infrastructure and increasing adoption of advanced diagnostic technologies in countries like China, Japan, and India.
The competitive landscape for advanced MRI contrast agents features established pharmaceutical giants like Bayer, Bracco, and GE Healthcare controlling approximately 75% of the market. However, specialized materials science companies and biotech startups focused on nanomaterial-based solutions are gaining traction, with venture capital investments in this sector exceeding $450 million in the past three years.
Key market drivers for BNNS-based contrast agents include growing demand for non-gadolinium alternatives due to safety concerns, increasing prevalence of neurological and cardiovascular disorders requiring precise imaging, and technological advancements in nanosheet synthesis and functionalization. Regulatory pathways remain a significant market barrier, with FDA and EMA approval processes typically requiring 5-7 years for novel contrast agents, substantially longer than for modifications to existing agents.
Consumer willingness to pay premium prices for safer, more effective contrast agents presents a significant market opportunity, with surveys indicating that 62% of healthcare providers would adopt alternatives to gadolinium-based agents despite higher costs if safety and efficacy advantages were clearly demonstrated.
Boron Nitride Nanosheets (BNNS) represent an emerging segment within the advanced contrast agent market, positioned to potentially disrupt the established paradigm. Market analysis indicates that novel nanomaterial-based contrast agents, including BNNS, are expected to capture 15% of the market by 2027, representing a substantial shift from their current 3% share. This growth is primarily attributed to their superior biocompatibility profiles and enhanced imaging capabilities.
Healthcare facilities and diagnostic centers constitute the largest end-user segment for advanced MRI contrast agents, accounting for 68% of total consumption. Research institutions represent a smaller but rapidly growing segment with 18% market share, particularly for novel agents like BNNS that are still transitioning from research to clinical applications.
Geographically, North America leads the market with 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 5.7% annually, driven by expanding healthcare infrastructure and increasing adoption of advanced diagnostic technologies in countries like China, Japan, and India.
The competitive landscape for advanced MRI contrast agents features established pharmaceutical giants like Bayer, Bracco, and GE Healthcare controlling approximately 75% of the market. However, specialized materials science companies and biotech startups focused on nanomaterial-based solutions are gaining traction, with venture capital investments in this sector exceeding $450 million in the past three years.
Key market drivers for BNNS-based contrast agents include growing demand for non-gadolinium alternatives due to safety concerns, increasing prevalence of neurological and cardiovascular disorders requiring precise imaging, and technological advancements in nanosheet synthesis and functionalization. Regulatory pathways remain a significant market barrier, with FDA and EMA approval processes typically requiring 5-7 years for novel contrast agents, substantially longer than for modifications to existing agents.
Consumer willingness to pay premium prices for safer, more effective contrast agents presents a significant market opportunity, with surveys indicating that 62% of healthcare providers would adopt alternatives to gadolinium-based agents despite higher costs if safety and efficacy advantages were clearly demonstrated.
Current Challenges in BN Nanosheets for MRI Applications
Despite the promising potential of boron nitride nanosheets (BNNS) in magnetic resonance imaging applications, several significant challenges currently impede their widespread implementation. The primary obstacle lies in the synthesis of high-quality BNNS with consistent properties at scale. Current production methods, including chemical vapor deposition, liquid-phase exfoliation, and mechanical cleavage, often yield materials with varying thickness, lateral dimensions, and defect densities. This inconsistency directly impacts their performance in MRI applications, where uniformity is crucial for reliable imaging results.
Surface functionalization represents another major challenge. While pristine BNNS exhibits excellent thermal and chemical stability, its surface is relatively inert, making it difficult to attach functional groups necessary for biocompatibility, targeting capabilities, and contrast enhancement. Researchers are actively exploring various chemical modification strategies, but achieving selective functionalization without compromising the intrinsic properties of BNNS remains problematic.
Biocompatibility and toxicity concerns constitute significant barriers to clinical translation. Although preliminary studies suggest that BNNS may have lower cytotoxicity compared to other nanomaterials, comprehensive long-term toxicity studies are lacking. The biological fate, including biodistribution, metabolism, and clearance pathways of BNNS in living organisms, requires thorough investigation before clinical applications can be considered.
The colloidal stability of BNNS in physiological environments presents another challenge. BNNS tends to aggregate in biological media due to their hydrophobic nature and strong van der Waals interactions. This aggregation not only reduces their effectiveness as MRI contrast agents but also potentially increases toxicity risks. Developing stable dispersions without using toxic surfactants remains an ongoing research focus.
From an imaging perspective, optimizing the magnetic properties of BNNS for enhanced MRI contrast is technically demanding. While BNNS can be doped or functionalized with paramagnetic ions to improve contrast, achieving the right balance between loading capacity and maintaining the structural integrity of the nanosheets is challenging. Additionally, controlling relaxivity properties to match specific imaging requirements requires precise engineering at the nanoscale.
Regulatory hurdles and standardization issues further complicate the path to clinical implementation. The lack of standardized characterization methods for BNNS makes it difficult to establish reproducible quality control protocols, which are essential for regulatory approval. The novel nature of these nanomaterials also means that existing regulatory frameworks may not adequately address their unique properties and potential risks.
Surface functionalization represents another major challenge. While pristine BNNS exhibits excellent thermal and chemical stability, its surface is relatively inert, making it difficult to attach functional groups necessary for biocompatibility, targeting capabilities, and contrast enhancement. Researchers are actively exploring various chemical modification strategies, but achieving selective functionalization without compromising the intrinsic properties of BNNS remains problematic.
Biocompatibility and toxicity concerns constitute significant barriers to clinical translation. Although preliminary studies suggest that BNNS may have lower cytotoxicity compared to other nanomaterials, comprehensive long-term toxicity studies are lacking. The biological fate, including biodistribution, metabolism, and clearance pathways of BNNS in living organisms, requires thorough investigation before clinical applications can be considered.
The colloidal stability of BNNS in physiological environments presents another challenge. BNNS tends to aggregate in biological media due to their hydrophobic nature and strong van der Waals interactions. This aggregation not only reduces their effectiveness as MRI contrast agents but also potentially increases toxicity risks. Developing stable dispersions without using toxic surfactants remains an ongoing research focus.
From an imaging perspective, optimizing the magnetic properties of BNNS for enhanced MRI contrast is technically demanding. While BNNS can be doped or functionalized with paramagnetic ions to improve contrast, achieving the right balance between loading capacity and maintaining the structural integrity of the nanosheets is challenging. Additionally, controlling relaxivity properties to match specific imaging requirements requires precise engineering at the nanoscale.
Regulatory hurdles and standardization issues further complicate the path to clinical implementation. The lack of standardized characterization methods for BNNS makes it difficult to establish reproducible quality control protocols, which are essential for regulatory approval. The novel nature of these nanomaterials also means that existing regulatory frameworks may not adequately address their unique properties and potential risks.
Current BN Nanosheet Synthesis and Functionalization Methods
01 Synthesis methods for boron nitride nanosheets
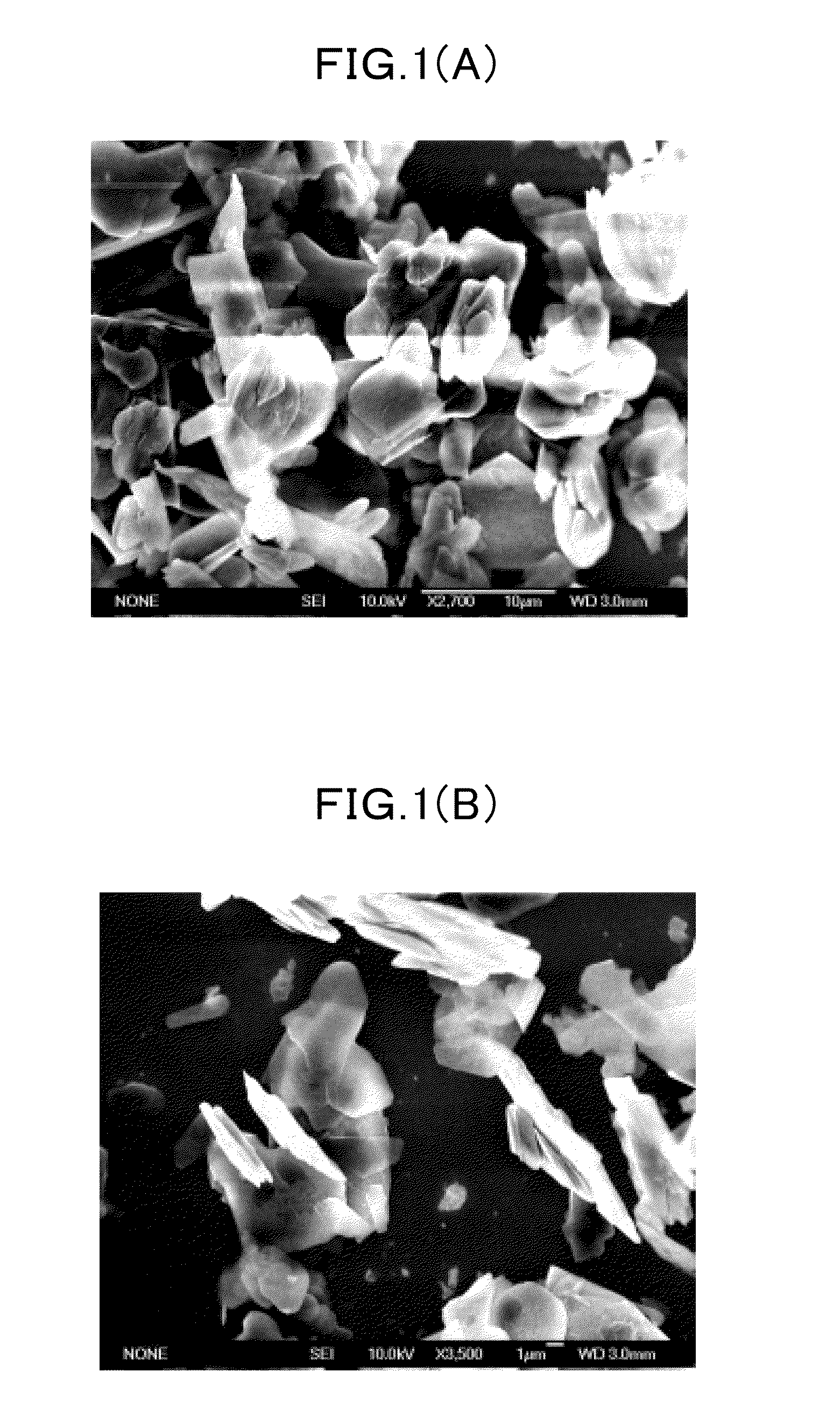
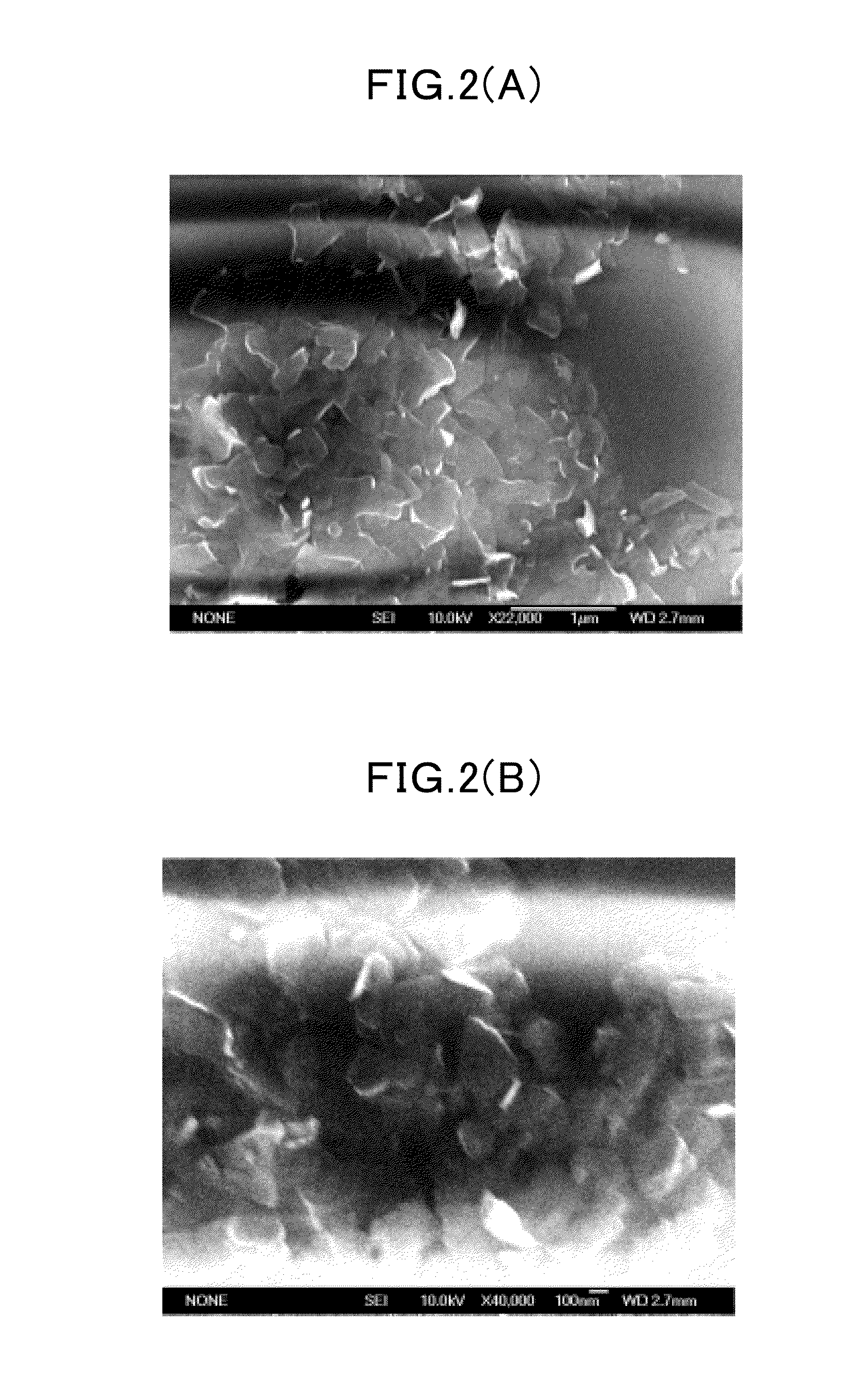
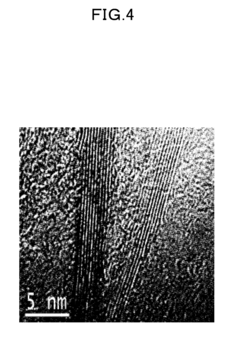
Various methods can be employed to synthesize boron nitride nanosheets, including chemical vapor deposition, exfoliation techniques, and chemical synthesis routes. These methods allow for the production of high-quality nanosheets with controlled thickness, size, and crystallinity. The synthesis parameters can be optimized to achieve desired properties such as high surface area, few-layer structure, and minimal defects. Different precursors and catalysts can be used to enhance the growth process and improve the quality of the resulting nanosheets.- Synthesis methods for boron nitride nanosheets: Various methods have been developed for synthesizing boron nitride nanosheets with controlled properties. These include chemical vapor deposition, liquid exfoliation, and thermal treatments. The synthesis parameters significantly influence the thickness, lateral size, and crystallinity of the resulting nanosheets. Advanced techniques allow for the production of few-layer or even single-layer boron nitride nanosheets with high purity and well-defined structures.
- Functionalization and modification of boron nitride nanosheets: Boron nitride nanosheets can be functionalized or modified to enhance their properties and compatibility with various matrices. Surface modifications include chemical functionalization with organic groups, doping with heteroatoms, and creation of defects. These modifications can improve dispersibility in solvents, enhance interaction with polymer matrices, and introduce new properties such as catalytic activity or enhanced thermal conductivity while maintaining the inherent thermal stability of the nanosheets.
- Thermal management applications of boron nitride nanosheets: Boron nitride nanosheets exhibit exceptional thermal conductivity and electrical insulation properties, making them ideal for thermal management applications. They can be incorporated into thermal interface materials, heat spreaders, and thermally conductive composites. When dispersed in polymers or other matrices, they create efficient pathways for heat dissipation while maintaining electrical insulation. These properties are particularly valuable in electronics cooling, where heat management is critical for device performance and reliability.
- Mechanical reinforcement in composite materials: Boron nitride nanosheets serve as effective reinforcing fillers in various composite materials due to their high mechanical strength and thermal stability. When incorporated into polymers, ceramics, or metals, they can significantly enhance mechanical properties such as tensile strength, modulus, and hardness. The two-dimensional structure of these nanosheets provides large interfacial areas for stress transfer, while their chemical stability ensures durability under harsh conditions. The resulting composites find applications in aerospace, automotive, and structural components.
- Environmental and biomedical applications: Boron nitride nanosheets have emerging applications in environmental remediation and biomedical fields. Their high surface area and adsorption capabilities make them effective for water purification and removal of pollutants. In biomedical applications, they show promise as drug delivery vehicles, bioimaging agents, and components in tissue engineering scaffolds. Their biocompatibility, combined with their unique physical and chemical properties, enables novel approaches in sensing, therapy, and diagnostic applications. Research continues to explore their potential in addressing environmental challenges and advancing healthcare technologies.
02 Functionalization and modification of boron nitride nanosheets
Boron nitride nanosheets can be functionalized or modified to enhance their properties and expand their applications. Surface modification techniques include chemical functionalization, doping with heteroatoms, and creating hybrid structures with other nanomaterials. These modifications can improve the dispersibility, compatibility with matrices, and introduce new properties such as enhanced thermal conductivity, electrical properties, or mechanical strength. Functionalized boron nitride nanosheets can be better integrated into various composite materials for specific applications.Expand Specific Solutions03 Composite materials incorporating boron nitride nanosheets
Boron nitride nanosheets can be incorporated into various matrices to form composite materials with enhanced properties. These composites can include polymer-based, ceramic-based, or metal-based matrices. The addition of boron nitride nanosheets can significantly improve thermal conductivity, mechanical strength, and thermal stability of the resulting composites. The nanosheets can also provide electrical insulation properties while maintaining good thermal management capabilities, making these composites suitable for electronic packaging, thermal interface materials, and structural applications.Expand Specific Solutions04 Applications in thermal management and electronics
Boron nitride nanosheets exhibit excellent thermal conductivity and electrical insulation properties, making them ideal for thermal management applications in electronics. They can be used as fillers in thermal interface materials, heat spreaders, and cooling solutions for electronic devices. The nanosheets help dissipate heat efficiently while maintaining electrical isolation, which is crucial for high-power electronic components. Their high temperature stability also allows for applications in extreme environments where conventional materials might fail.Expand Specific Solutions05 Environmental and energy applications of boron nitride nanosheets
Boron nitride nanosheets have promising applications in environmental remediation and energy storage/conversion technologies. They can be used as adsorbents for pollutant removal, catalysts or catalyst supports for various reactions, and components in energy storage devices such as batteries and supercapacitors. Their high surface area, chemical stability, and unique surface properties make them effective for water purification, gas separation, and as platforms for energy-related applications. Modified boron nitride nanosheets can also exhibit enhanced performance in photocatalysis and electrochemical applications.Expand Specific Solutions
Key Industry Players in MRI Contrast Agent Development
The boron nitride nanosheets (BNNS) in magnetic resonance imaging equipment market is in an early growth phase, characterized by intensive research and development rather than widespread commercial deployment. The global market size remains relatively modest but is projected to expand significantly as applications in MRI technology mature. From a technical maturity perspective, the field is still evolving, with academic institutions leading research efforts. Key players include Deakin University, National Institute for Materials Science, and Suzhou Institute of Nano-Tech & Nano-Bionics, who are pioneering fundamental research, while companies like Li-S Energy and White Graphene Ltd. are working toward commercialization. Universities such as Korea Advanced Institute of Science & Technology and Northwestern University are advancing the theoretical understanding, creating a competitive landscape dominated by research-focused entities rather than established commercial players.
Suzhou Institute of Nano-Tech & Nano-Bionics (SINANO)
Technical Solution: SINANO has developed innovative boron nitride nanosheet (BNNS) composites specifically engineered for MRI contrast enhancement. Their approach involves synthesizing ultrathin BNNS with controlled defect structures that can be functionalized with paramagnetic ions (such as Gd3+) to create highly efficient T1 contrast agents. The institute has pioneered a chemical vapor deposition method that produces high-quality BNNS with precisely controlled thickness (2-5 nm) and lateral dimensions (0.5-2 μm), optimized for biomedical applications. Their technology incorporates surface modification strategies using biocompatible polymers like polyethylene glycol to improve colloidal stability and circulation time in biological systems. SINANO's BNNS-based contrast agents have demonstrated superior relaxivity values (r1 > 20 mM−1s−1) compared to conventional gadolinium chelates, while maintaining lower toxicity profiles in preclinical studies.
Strengths: Superior relaxivity performance compared to conventional contrast agents, excellent biocompatibility, and reduced toxicity concerns. Their precise synthesis control allows for consistent quality and reproducible imaging results. Weaknesses: Relatively complex manufacturing process may increase production costs, and the technology still requires more extensive clinical validation before widespread adoption in medical settings.
National Institute for Materials Science IAI
Technical Solution: The National Institute for Materials Science (NIMS) has developed a groundbreaking approach to BNNS-based MRI contrast agents through their exfoliation-functionalization technique. Their method produces atomically thin BNNS (1-3 layers) with high structural integrity and minimal defects, which are then decorated with superparamagnetic iron oxide nanoparticles (SPIONs) to create dual-mode T1/T2 contrast agents. NIMS researchers have optimized the BNNS-SPION interface to maximize magnetic coupling effects, resulting in enhanced relaxivity performance. Their proprietary surface chemistry enables stable aqueous dispersions with extended shelf-life and resistance to aggregation in biological media. The institute has also pioneered targeted delivery approaches by conjugating specific antibodies or peptides to their BNNS platforms, allowing for tissue-specific MRI enhancement. Recent studies have demonstrated successful application in detecting early-stage tumors with significantly improved signal-to-noise ratios compared to conventional contrast agents.
Strengths: Dual-mode imaging capability provides complementary diagnostic information, high colloidal stability in biological environments, and potential for targeted imaging applications. Weaknesses: The complex synthesis and functionalization processes may present challenges for large-scale production, and the long-term fate of these nanomaterials in the body requires further investigation.
Critical Patents and Research on BN Nanosheets for MRI
Boron nitride nanosheet, method for producing boron nitride nanosheet thereof and composition containing boron nitride nanosheet thereof
PatentInactiveUS20110086965A1
Innovation
- A method involving the dispersion of hexagonal boron nitride powder in an organic solvent followed by ultrasonication to peel layers, resulting in a three-layered boron nitride nanosheet with a specific surface area significantly larger than bulk hexagonal boron nitride, which can be used as a filler to improve polymeric composite properties.
Boron nitride nanosheet-containing fluid dispersion, boron nitride nanosheet composite and production method thereof
PatentActiveJP2015187057A
Innovation
- The use of ionic liquids to disperse and peel boron nitride nanosheets, utilizing cation-π and anion-π interactions to enhance dispersibility, with methods including sonication, stirring, and grinding treatments to achieve high dispersion stability and efficiency.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of boron nitride nanosheets (BNNSs) represents a critical consideration for their application in magnetic resonance imaging (MRI) equipment. Current research indicates that BNNSs exhibit promising biocompatibility characteristics compared to other nanomaterials, with studies demonstrating minimal cytotoxicity at concentrations relevant for imaging applications. In vitro assessments using various cell lines have shown that properly functionalized BNNSs maintain cell viability above 85% even after prolonged exposure periods.
The surface chemistry of BNNSs plays a determinant role in their biological interactions. Pristine BNNSs tend to aggregate in physiological environments, potentially leading to increased toxicity. However, surface modifications through PEGylation, protein coating, or polysaccharide functionalization significantly improve their dispersibility in biological fluids and reduce potential immunogenic responses. These modifications create a hydrophilic shell that prevents opsonization and subsequent recognition by the reticuloendothelial system.
Biodistribution and clearance pathways represent another crucial safety aspect. Studies tracking radiolabeled BNNSs have demonstrated that these nanomaterials primarily accumulate in the liver and spleen following intravenous administration, with minimal retention in other organs. The elimination half-life varies between 12-36 hours depending on size and surface functionalization, with renal clearance being the predominant excretion route for smaller (<10 nm) functionalized BNNSs.
Hemocompatibility assessments have shown that properly engineered BNNSs do not induce significant hemolysis or platelet activation at concentrations below 100 μg/mL. However, higher concentrations may trigger complement activation, necessitating careful dosage control in clinical applications. Long-term toxicity studies in animal models have revealed no significant adverse effects on major organ functions when administered at imaging-relevant doses.
Regulatory considerations for BNNSs in MRI applications remain complex due to their novel nature. The FDA currently evaluates such nanomaterials on a case-by-case basis, requiring comprehensive toxicological profiles and manufacturing consistency data. International regulatory bodies have begun developing specific guidelines for nanomaterials in medical imaging, with particular emphasis on characterization standards, sterility assurance, and stability assessments.
Future safety research directions should focus on standardizing toxicity evaluation protocols specifically for BNNSs in MRI applications, investigating potential long-term accumulation effects, and establishing clear dose-response relationships across different patient populations. Additionally, developing sensitive analytical methods for detecting trace amounts of BNNSs in biological samples will be essential for monitoring potential bioaccumulation in clinical settings.
The surface chemistry of BNNSs plays a determinant role in their biological interactions. Pristine BNNSs tend to aggregate in physiological environments, potentially leading to increased toxicity. However, surface modifications through PEGylation, protein coating, or polysaccharide functionalization significantly improve their dispersibility in biological fluids and reduce potential immunogenic responses. These modifications create a hydrophilic shell that prevents opsonization and subsequent recognition by the reticuloendothelial system.
Biodistribution and clearance pathways represent another crucial safety aspect. Studies tracking radiolabeled BNNSs have demonstrated that these nanomaterials primarily accumulate in the liver and spleen following intravenous administration, with minimal retention in other organs. The elimination half-life varies between 12-36 hours depending on size and surface functionalization, with renal clearance being the predominant excretion route for smaller (<10 nm) functionalized BNNSs.
Hemocompatibility assessments have shown that properly engineered BNNSs do not induce significant hemolysis or platelet activation at concentrations below 100 μg/mL. However, higher concentrations may trigger complement activation, necessitating careful dosage control in clinical applications. Long-term toxicity studies in animal models have revealed no significant adverse effects on major organ functions when administered at imaging-relevant doses.
Regulatory considerations for BNNSs in MRI applications remain complex due to their novel nature. The FDA currently evaluates such nanomaterials on a case-by-case basis, requiring comprehensive toxicological profiles and manufacturing consistency data. International regulatory bodies have begun developing specific guidelines for nanomaterials in medical imaging, with particular emphasis on characterization standards, sterility assurance, and stability assessments.
Future safety research directions should focus on standardizing toxicity evaluation protocols specifically for BNNSs in MRI applications, investigating potential long-term accumulation effects, and establishing clear dose-response relationships across different patient populations. Additionally, developing sensitive analytical methods for detecting trace amounts of BNNSs in biological samples will be essential for monitoring potential bioaccumulation in clinical settings.
Regulatory Pathway for Novel MRI Contrast Agents
The regulatory landscape for novel MRI contrast agents, particularly those incorporating Boron Nitride Nanosheets (BNNSs), presents a complex pathway requiring careful navigation. The FDA in the United States and the EMA in Europe have established specific protocols for the approval of new contrast agents, categorizing them as drug-device combination products that require comprehensive safety and efficacy evaluations.
For BNNSs-based contrast agents, the regulatory journey typically begins with preclinical testing, including in vitro cytotoxicity studies and in vivo biodistribution analyses. These studies must demonstrate acceptable safety profiles, particularly regarding potential accumulation in vital organs and clearance pathways. The unique properties of BNNSs, including their stability and surface chemistry, necessitate specialized toxicological assessments beyond those required for conventional gadolinium-based agents.
Clinical trials for novel MRI contrast agents follow a phased approach, beginning with Phase I safety studies in healthy volunteers to establish pharmacokinetics and initial safety parameters. Phase II trials then evaluate efficacy in small patient populations, while Phase III involves larger multicenter studies comparing the new agent with existing standards. For BNNSs, additional emphasis is placed on long-term safety monitoring due to limited historical data on nanomaterial biocompatibility.
Regulatory submissions must address specific concerns related to nanomaterials in medical applications, including manufacturing consistency, sterilization validation, and shelf-life stability. The FDA's Center for Drug Evaluation and Research (CDER) typically takes primary jurisdiction, though coordination with the Center for Devices and Radiological Health (CDRH) may be necessary due to the imaging equipment interface considerations.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines that facilitate global development strategies. However, regional differences persist, with Japan's PMDA often requiring country-specific data and China's NMPA implementing additional testing requirements for nanomaterials.
Post-market surveillance represents a critical component of the regulatory pathway, with manufacturers required to implement risk management plans and conduct phase IV studies. For novel nanomaterials like BNNSs, these monitoring requirements are typically more stringent, often including specialized registries to track potential long-term effects that may not emerge during pre-approval studies.
Accelerated approval pathways may be available for BNNSs-based contrast agents that demonstrate significant advantages over existing options, particularly regarding reduced nephrotoxicity or enhanced imaging capabilities for specific clinical applications. However, these pathways still require substantial evidence of safety and preliminary efficacy.
For BNNSs-based contrast agents, the regulatory journey typically begins with preclinical testing, including in vitro cytotoxicity studies and in vivo biodistribution analyses. These studies must demonstrate acceptable safety profiles, particularly regarding potential accumulation in vital organs and clearance pathways. The unique properties of BNNSs, including their stability and surface chemistry, necessitate specialized toxicological assessments beyond those required for conventional gadolinium-based agents.
Clinical trials for novel MRI contrast agents follow a phased approach, beginning with Phase I safety studies in healthy volunteers to establish pharmacokinetics and initial safety parameters. Phase II trials then evaluate efficacy in small patient populations, while Phase III involves larger multicenter studies comparing the new agent with existing standards. For BNNSs, additional emphasis is placed on long-term safety monitoring due to limited historical data on nanomaterial biocompatibility.
Regulatory submissions must address specific concerns related to nanomaterials in medical applications, including manufacturing consistency, sterilization validation, and shelf-life stability. The FDA's Center for Drug Evaluation and Research (CDER) typically takes primary jurisdiction, though coordination with the Center for Devices and Radiological Health (CDRH) may be necessary due to the imaging equipment interface considerations.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines that facilitate global development strategies. However, regional differences persist, with Japan's PMDA often requiring country-specific data and China's NMPA implementing additional testing requirements for nanomaterials.
Post-market surveillance represents a critical component of the regulatory pathway, with manufacturers required to implement risk management plans and conduct phase IV studies. For novel nanomaterials like BNNSs, these monitoring requirements are typically more stringent, often including specialized registries to track potential long-term effects that may not emerge during pre-approval studies.
Accelerated approval pathways may be available for BNNSs-based contrast agents that demonstrate significant advantages over existing options, particularly regarding reduced nephrotoxicity or enhanced imaging capabilities for specific clinical applications. However, these pathways still require substantial evidence of safety and preliminary efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!