Customized Nitinol Alloys for Medical Imaging Devices
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitinol Alloy Evolution and Objectives
Nitinol, a remarkable shape memory alloy composed of nickel and titanium, has undergone significant evolution since its discovery in 1959 at the Naval Ordnance Laboratory. Initially developed for aerospace applications, its unique properties quickly garnered attention in the medical field, particularly for imaging devices. The alloy's superelastic behavior and biocompatibility have made it an ideal material for various medical applications, including guidewires, stents, and catheter systems used in medical imaging procedures.
The evolution of Nitinol alloys for medical imaging devices has been driven by the need for increasingly sophisticated and minimally invasive diagnostic and therapeutic procedures. Early applications focused on simple guidewires, but as imaging technologies advanced, so did the demands on Nitinol alloys. The development of customized Nitinol alloys has become crucial to meet the specific requirements of different medical imaging modalities, such as X-ray, MRI, and ultrasound.
One of the key objectives in researching customized Nitinol alloys for medical imaging devices is to enhance their radiopacity. This property is essential for improved visibility under X-ray imaging, allowing for more precise navigation and placement of devices within the body. Researchers have been exploring various techniques to increase radiopacity, including the addition of platinum or palladium to the alloy composition.
Another important goal is to optimize the mechanical properties of Nitinol alloys to suit specific imaging applications. This includes tailoring the transformation temperatures, improving fatigue resistance, and enhancing shape memory characteristics. For instance, in MRI-compatible devices, the focus is on developing Nitinol alloys with reduced magnetic susceptibility to minimize image artifacts.
The customization of Nitinol alloys also aims to improve their biocompatibility and reduce the risk of adverse reactions in patients. This involves developing surface modification techniques and exploring new alloy compositions that minimize nickel release while maintaining the desired mechanical properties.
As medical imaging technologies continue to advance, the objectives for Nitinol alloy research expand to include compatibility with emerging imaging modalities and integration with smart materials. This includes developing Nitinol-based sensors for real-time imaging feedback and exploring hybrid materials that combine Nitinol with other advanced materials to create multifunctional imaging devices.
The future trajectory of Nitinol alloy evolution in medical imaging devices is focused on achieving greater precision, enhanced functionality, and improved patient outcomes. Researchers are working towards developing alloys that can respond to multiple stimuli, allowing for more complex and controlled device behavior during imaging procedures. Additionally, there is a growing interest in creating Nitinol alloys that can be easily 3D printed, opening up new possibilities for customized, patient-specific imaging devices.
The evolution of Nitinol alloys for medical imaging devices has been driven by the need for increasingly sophisticated and minimally invasive diagnostic and therapeutic procedures. Early applications focused on simple guidewires, but as imaging technologies advanced, so did the demands on Nitinol alloys. The development of customized Nitinol alloys has become crucial to meet the specific requirements of different medical imaging modalities, such as X-ray, MRI, and ultrasound.
One of the key objectives in researching customized Nitinol alloys for medical imaging devices is to enhance their radiopacity. This property is essential for improved visibility under X-ray imaging, allowing for more precise navigation and placement of devices within the body. Researchers have been exploring various techniques to increase radiopacity, including the addition of platinum or palladium to the alloy composition.
Another important goal is to optimize the mechanical properties of Nitinol alloys to suit specific imaging applications. This includes tailoring the transformation temperatures, improving fatigue resistance, and enhancing shape memory characteristics. For instance, in MRI-compatible devices, the focus is on developing Nitinol alloys with reduced magnetic susceptibility to minimize image artifacts.
The customization of Nitinol alloys also aims to improve their biocompatibility and reduce the risk of adverse reactions in patients. This involves developing surface modification techniques and exploring new alloy compositions that minimize nickel release while maintaining the desired mechanical properties.
As medical imaging technologies continue to advance, the objectives for Nitinol alloy research expand to include compatibility with emerging imaging modalities and integration with smart materials. This includes developing Nitinol-based sensors for real-time imaging feedback and exploring hybrid materials that combine Nitinol with other advanced materials to create multifunctional imaging devices.
The future trajectory of Nitinol alloy evolution in medical imaging devices is focused on achieving greater precision, enhanced functionality, and improved patient outcomes. Researchers are working towards developing alloys that can respond to multiple stimuli, allowing for more complex and controlled device behavior during imaging procedures. Additionally, there is a growing interest in creating Nitinol alloys that can be easily 3D printed, opening up new possibilities for customized, patient-specific imaging devices.
Medical Imaging Market Analysis
The medical imaging market has experienced significant growth in recent years, driven by technological advancements, increasing prevalence of chronic diseases, and growing demand for early and accurate diagnosis. The global medical imaging market was valued at approximately $39 billion in 2020 and is projected to reach $55 billion by 2025, with a compound annual growth rate (CAGR) of 7.1% during this period.
Within this market, the demand for customized Nitinol alloys in medical imaging devices has been steadily increasing. Nitinol, a nickel-titanium alloy known for its unique properties such as shape memory and superelasticity, has found extensive applications in various medical imaging modalities, including MRI, CT, and ultrasound.
The adoption of Nitinol in medical imaging devices is primarily driven by its ability to enhance image quality, improve patient comfort, and increase the overall efficiency of imaging procedures. For instance, Nitinol-based guidewires and catheters used in interventional radiology procedures offer superior flexibility and kink resistance, allowing for better navigation through complex anatomical structures.
The market for Nitinol-based medical imaging devices is segmented based on application areas, including cardiovascular imaging, neurovascular imaging, and musculoskeletal imaging. Among these, cardiovascular imaging represents the largest market share due to the rising incidence of cardiovascular diseases and the increasing adoption of minimally invasive procedures.
Geographically, North America dominates the market for Nitinol-based medical imaging devices, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare infrastructure and increasing awareness about advanced medical imaging technologies.
Key market players in the Nitinol-based medical imaging devices segment include Medtronic, Abbott Laboratories, Boston Scientific Corporation, and Cook Medical. These companies are actively investing in research and development to introduce innovative Nitinol-based products and expand their market presence.
The future outlook for customized Nitinol alloys in medical imaging devices remains promising, with ongoing research focused on developing new alloy compositions and manufacturing techniques to further enhance the material's properties and performance in medical imaging applications. As the demand for more precise and less invasive diagnostic procedures continues to grow, the market for Nitinol-based medical imaging devices is expected to expand, offering significant opportunities for both established players and new entrants in the field.
Within this market, the demand for customized Nitinol alloys in medical imaging devices has been steadily increasing. Nitinol, a nickel-titanium alloy known for its unique properties such as shape memory and superelasticity, has found extensive applications in various medical imaging modalities, including MRI, CT, and ultrasound.
The adoption of Nitinol in medical imaging devices is primarily driven by its ability to enhance image quality, improve patient comfort, and increase the overall efficiency of imaging procedures. For instance, Nitinol-based guidewires and catheters used in interventional radiology procedures offer superior flexibility and kink resistance, allowing for better navigation through complex anatomical structures.
The market for Nitinol-based medical imaging devices is segmented based on application areas, including cardiovascular imaging, neurovascular imaging, and musculoskeletal imaging. Among these, cardiovascular imaging represents the largest market share due to the rising incidence of cardiovascular diseases and the increasing adoption of minimally invasive procedures.
Geographically, North America dominates the market for Nitinol-based medical imaging devices, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare infrastructure and increasing awareness about advanced medical imaging technologies.
Key market players in the Nitinol-based medical imaging devices segment include Medtronic, Abbott Laboratories, Boston Scientific Corporation, and Cook Medical. These companies are actively investing in research and development to introduce innovative Nitinol-based products and expand their market presence.
The future outlook for customized Nitinol alloys in medical imaging devices remains promising, with ongoing research focused on developing new alloy compositions and manufacturing techniques to further enhance the material's properties and performance in medical imaging applications. As the demand for more precise and less invasive diagnostic procedures continues to grow, the market for Nitinol-based medical imaging devices is expected to expand, offering significant opportunities for both established players and new entrants in the field.
Nitinol Challenges in Medical Imaging
Nitinol, a unique alloy of nickel and titanium, has revolutionized the field of medical imaging devices due to its exceptional shape memory and superelastic properties. However, its application in this domain is not without challenges. The primary obstacle lies in achieving precise control over the alloy's transformation temperatures, which are crucial for its functionality in medical imaging equipment.
One of the most significant challenges is the customization of Nitinol's composition to meet the specific requirements of different medical imaging devices. Each application may demand a unique set of mechanical properties and transformation temperatures, necessitating careful adjustment of the nickel-titanium ratio and the addition of tertiary elements. This fine-tuning process is complex and requires extensive research and development efforts.
Another major hurdle is the manufacturing of Nitinol components with consistent and reproducible properties. The alloy's characteristics are highly sensitive to processing conditions, including heat treatment, cold working, and surface finishing. Achieving uniformity across batches while maintaining the desired properties is a persistent challenge for manufacturers of medical imaging devices.
The biocompatibility of Nitinol is also a critical concern in medical applications. While generally considered biocompatible, the potential release of nickel ions from the alloy surface remains a subject of ongoing research and debate. Ensuring long-term stability and minimizing nickel leaching in the biological environment is crucial for the safety and efficacy of Nitinol-based medical imaging devices.
Furthermore, the integration of Nitinol components into complex medical imaging systems presents its own set of challenges. The alloy's unique properties must be carefully considered in the design and assembly processes to ensure optimal performance and reliability. This often requires specialized knowledge and expertise in both materials science and medical device engineering.
The fatigue behavior of Nitinol under cyclic loading conditions is another area of concern, particularly in applications involving repeated shape changes or stress cycles. Understanding and predicting the long-term performance of Nitinol components in medical imaging devices is essential for ensuring device longevity and patient safety.
Lastly, the regulatory landscape surrounding the use of Nitinol in medical devices adds another layer of complexity. Manufacturers must navigate stringent approval processes and demonstrate the safety and efficacy of their Nitinol-based products, which can be particularly challenging given the material's unique properties and behavior.
One of the most significant challenges is the customization of Nitinol's composition to meet the specific requirements of different medical imaging devices. Each application may demand a unique set of mechanical properties and transformation temperatures, necessitating careful adjustment of the nickel-titanium ratio and the addition of tertiary elements. This fine-tuning process is complex and requires extensive research and development efforts.
Another major hurdle is the manufacturing of Nitinol components with consistent and reproducible properties. The alloy's characteristics are highly sensitive to processing conditions, including heat treatment, cold working, and surface finishing. Achieving uniformity across batches while maintaining the desired properties is a persistent challenge for manufacturers of medical imaging devices.
The biocompatibility of Nitinol is also a critical concern in medical applications. While generally considered biocompatible, the potential release of nickel ions from the alloy surface remains a subject of ongoing research and debate. Ensuring long-term stability and minimizing nickel leaching in the biological environment is crucial for the safety and efficacy of Nitinol-based medical imaging devices.
Furthermore, the integration of Nitinol components into complex medical imaging systems presents its own set of challenges. The alloy's unique properties must be carefully considered in the design and assembly processes to ensure optimal performance and reliability. This often requires specialized knowledge and expertise in both materials science and medical device engineering.
The fatigue behavior of Nitinol under cyclic loading conditions is another area of concern, particularly in applications involving repeated shape changes or stress cycles. Understanding and predicting the long-term performance of Nitinol components in medical imaging devices is essential for ensuring device longevity and patient safety.
Lastly, the regulatory landscape surrounding the use of Nitinol in medical devices adds another layer of complexity. Manufacturers must navigate stringent approval processes and demonstrate the safety and efficacy of their Nitinol-based products, which can be particularly challenging given the material's unique properties and behavior.
Current Nitinol Customization Techniques
01 Composition and properties of Nitinol alloys
Nitinol alloys are shape memory alloys composed of nickel and titanium. They exhibit unique properties such as superelasticity and shape memory effect, making them suitable for various applications in medical devices, aerospace, and automotive industries. The specific composition and heat treatment processes can be tailored to achieve desired mechanical and thermal properties.- Composition and properties of Nitinol alloys: Nitinol alloys are shape memory alloys composed of nickel and titanium. They exhibit unique properties such as superelasticity and shape memory effect, making them suitable for various applications in medical devices, aerospace, and automotive industries. The specific composition and heat treatment processes can be tailored to achieve desired mechanical and thermal properties.
- Medical applications of Nitinol alloys: Nitinol alloys are widely used in medical devices due to their biocompatibility and unique properties. Applications include stents, guidewires, orthodontic archwires, and surgical instruments. The superelastic behavior of Nitinol allows for minimally invasive procedures and improved patient outcomes.
- Actuators and sensors using Nitinol alloys: The shape memory effect of Nitinol alloys is utilized in the development of actuators and sensors. These devices can respond to temperature changes or applied stress, making them suitable for various applications in robotics, automotive systems, and aerospace engineering. The ability to recover large strains and generate significant forces makes Nitinol-based actuators particularly attractive.
- Manufacturing processes for Nitinol alloys: Various manufacturing processes are employed to produce Nitinol alloys with desired properties. These include vacuum induction melting, vacuum arc remelting, and powder metallurgy techniques. Post-processing methods such as heat treatment, cold working, and shape setting are crucial for achieving the required shape memory and superelastic characteristics.
- Joining and fastening applications of Nitinol alloys: Nitinol alloys are used in joining and fastening applications due to their unique properties. Shape memory couplings, fasteners, and connectors can be designed to provide secure connections that can be easily released when needed. These applications are particularly useful in industries where traditional joining methods may be challenging or impractical.
02 Medical applications of Nitinol alloys
Nitinol alloys are widely used in medical devices due to their biocompatibility and unique properties. They are employed in stents, guidewires, orthodontic archwires, and surgical instruments. The superelastic nature of Nitinol allows for minimally invasive procedures and improved patient outcomes in various medical treatments.Expand Specific Solutions03 Actuators and sensors using Nitinol alloys
Nitinol alloys are utilized in the development of actuators and sensors for various applications. Their shape memory effect allows for the creation of compact and efficient actuators that can be activated by temperature changes. These actuators find use in robotics, automotive systems, and aerospace applications. Nitinol-based sensors can detect changes in temperature or stress, making them valuable in monitoring systems.Expand Specific Solutions04 Manufacturing processes for Nitinol alloys
The production of Nitinol alloys involves specialized manufacturing processes to achieve the desired properties. These processes may include vacuum induction melting, vacuum arc remelting, and precise heat treatment procedures. Advanced techniques such as powder metallurgy and additive manufacturing are also being explored to create complex Nitinol structures with tailored properties.Expand Specific Solutions05 Nitinol alloys in industrial applications
Nitinol alloys find applications in various industrial sectors due to their unique properties. They are used in couplings, fasteners, and seals in the oil and gas industry, where their superelasticity and corrosion resistance are advantageous. In the automotive industry, Nitinol alloys are employed in valves, actuators, and safety systems. The aerospace sector utilizes these alloys in vibration damping systems and deployable structures.Expand Specific Solutions
Key Nitinol Manufacturers
The research on customized Nitinol alloys for medical imaging devices is in a growth phase, with increasing market demand driven by advancements in minimally invasive procedures. The global market for Nitinol-based medical devices is expanding, estimated to reach several billion dollars by 2025. Technologically, the field is progressing rapidly, with companies like Abbott Laboratories, Cook Medical Technologies LLC, and W. L. Gore & Associates leading innovation. These firms are investing heavily in R&D to develop more sophisticated Nitinol alloys with enhanced properties for specific medical applications. Academic institutions such as South China University of Technology and Jilin University are also contributing significantly to the advancement of Nitinol technology, bridging the gap between fundamental research and practical applications in the medical device industry.
Cook Medical Technologies LLC
Technical Solution: Cook Medical Technologies LLC has developed customized Nitinol alloys for medical imaging devices, focusing on improving the radiopacity and mechanical properties. Their approach involves fine-tuning the composition of Nitinol by adding small amounts of platinum or palladium to enhance visibility under X-ray imaging[1]. The company has also implemented advanced heat treatment processes to optimize the superelastic behavior of Nitinol, allowing for the creation of self-expanding stents and guidewires with improved flexibility and kink resistance[2]. Cook's research has led to the development of Nitinol-based devices with enhanced fatigue resistance, crucial for long-term implantable devices used in cardiovascular and peripheral interventions[3].
Strengths: Improved radiopacity for better visibility during procedures, enhanced mechanical properties for durability. Weaknesses: Potential increase in production costs due to the addition of precious metals, complexity in manufacturing process.
W. L. Gore & Associates, Inc.
Technical Solution: W. L. Gore & Associates has focused on developing Nitinol-based composite materials for medical imaging devices. Their research involves creating hybrid structures that combine Nitinol with other biocompatible materials such as ePTFE (expanded polytetrafluoroethylene)[1]. This approach aims to leverage Nitinol's shape memory and superelastic properties while addressing its limitations in certain applications. Gore has developed a proprietary surface modification technique for Nitinol that enhances its biocompatibility and reduces the risk of nickel ion release, a common concern with Nitinol implants[2]. The company has also explored the use of thin-film Nitinol in medical imaging devices, allowing for the creation of ultra-thin, flexible structures with excellent radiopacity[3].
Strengths: Enhanced biocompatibility, reduced risk of adverse reactions, ability to create complex composite structures. Weaknesses: Potentially higher manufacturing costs, challenges in scaling up production of composite materials.
Innovative Nitinol Alloy Patents
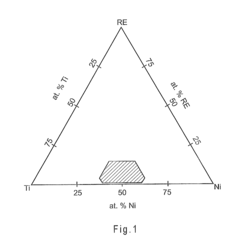
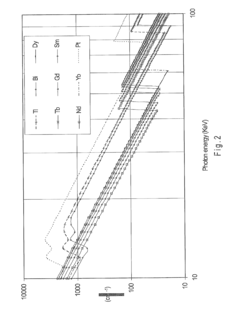
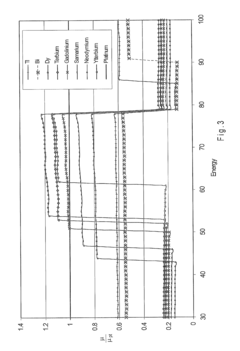
Nickel-titanium-rare earth alloy and method of processing the alloy
PatentActiveEP2501833A1
Innovation
- A nickel-titanium-rare earth alloy with specific composition ranges, including boron, that exhibits improved radiopacity and workability through a method of processing involving homogenization heat treatment below a critical temperature to form spheroids of rare earth-rich second phases, enhancing ductility and mechanical properties.
Nickel-Titanium-Rare Earth Alloy and Method of Processing the Alloy
PatentActiveUS20110114230A1
Innovation
- Development of nickel-titanium-rare earth (Ni—Ti-RE) alloys with specific compositions and processing methods to enhance radiopacity and workability, including the addition of boron to improve ductility and the formation of rare earth-rich second phases, which provide improved visibility under x-ray imaging and ease of shaping.
Regulatory Framework for Medical Alloys
The regulatory framework for medical alloys, particularly Nitinol alloys used in medical imaging devices, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the safety and efficacy of medical devices, including those incorporating Nitinol alloys. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating medical devices and radiation-emitting products.
For Nitinol alloys in medical imaging devices, manufacturers must comply with the FDA's premarket approval (PMA) process or the 510(k) clearance pathway, depending on the device classification. Class III devices, which are typically high-risk and novel, require a PMA, while Class II devices may be eligible for the less stringent 510(k) process. The FDA's guidance document "Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol" provides specific recommendations for evaluating Nitinol-based devices.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval and marketing of medical devices. These regulations, which came into full effect in May 2021, have significantly increased the requirements for clinical evidence and post-market surveillance. Manufacturers of Nitinol-based medical imaging devices must obtain CE marking to demonstrate compliance with these regulations.
International standards also play a crucial role in the regulatory framework. ISO 13485, which specifies requirements for quality management systems in the medical device industry, is widely recognized and often required by regulatory bodies. ASTM F2063 specifically addresses the standard specification for wrought Nickel-Titanium shape memory alloys for medical devices and surgical implants.
The regulatory landscape also emphasizes biocompatibility testing, as outlined in ISO 10993 series. For Nitinol alloys, particular attention is given to nickel ion release and potential allergic reactions. Manufacturers must conduct thorough biocompatibility assessments and provide comprehensive data on the material's safety profile.
Post-market surveillance is an increasingly important aspect of the regulatory framework. Both the FDA and EU regulations require manufacturers to actively monitor the performance and safety of their devices after market introduction. This includes implementing systems for collecting and analyzing real-world data, as well as reporting adverse events to regulatory authorities.
As the field of medical imaging continues to advance, regulatory bodies are adapting their frameworks to address emerging technologies and materials. This includes considerations for 3D-printed Nitinol components and the integration of smart materials in medical devices. Manufacturers and researchers must stay abreast of these evolving regulations to ensure compliance and facilitate innovation in customized Nitinol alloys for medical imaging devices.
For Nitinol alloys in medical imaging devices, manufacturers must comply with the FDA's premarket approval (PMA) process or the 510(k) clearance pathway, depending on the device classification. Class III devices, which are typically high-risk and novel, require a PMA, while Class II devices may be eligible for the less stringent 510(k) process. The FDA's guidance document "Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol" provides specific recommendations for evaluating Nitinol-based devices.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval and marketing of medical devices. These regulations, which came into full effect in May 2021, have significantly increased the requirements for clinical evidence and post-market surveillance. Manufacturers of Nitinol-based medical imaging devices must obtain CE marking to demonstrate compliance with these regulations.
International standards also play a crucial role in the regulatory framework. ISO 13485, which specifies requirements for quality management systems in the medical device industry, is widely recognized and often required by regulatory bodies. ASTM F2063 specifically addresses the standard specification for wrought Nickel-Titanium shape memory alloys for medical devices and surgical implants.
The regulatory landscape also emphasizes biocompatibility testing, as outlined in ISO 10993 series. For Nitinol alloys, particular attention is given to nickel ion release and potential allergic reactions. Manufacturers must conduct thorough biocompatibility assessments and provide comprehensive data on the material's safety profile.
Post-market surveillance is an increasingly important aspect of the regulatory framework. Both the FDA and EU regulations require manufacturers to actively monitor the performance and safety of their devices after market introduction. This includes implementing systems for collecting and analyzing real-world data, as well as reporting adverse events to regulatory authorities.
As the field of medical imaging continues to advance, regulatory bodies are adapting their frameworks to address emerging technologies and materials. This includes considerations for 3D-printed Nitinol components and the integration of smart materials in medical devices. Manufacturers and researchers must stay abreast of these evolving regulations to ensure compliance and facilitate innovation in customized Nitinol alloys for medical imaging devices.
Biocompatibility Considerations
Biocompatibility is a critical consideration in the development of customized Nitinol alloys for medical imaging devices. The unique properties of Nitinol, including its shape memory and superelasticity, make it an attractive material for various medical applications. However, ensuring the biocompatibility of these alloys is paramount to their successful implementation in medical imaging devices.
One of the primary concerns in biocompatibility is the potential release of nickel ions from Nitinol alloys. Nickel is a known allergen and can cause adverse reactions in some patients. To address this issue, researchers have focused on developing surface modification techniques to create a protective layer that minimizes nickel release. These techniques include oxidation treatments, coating with biocompatible materials, and ion implantation.
The interaction between Nitinol alloys and surrounding tissues is another crucial aspect of biocompatibility. Studies have shown that Nitinol generally exhibits good tissue compatibility, with minimal inflammatory responses observed in in vivo experiments. However, the specific composition and surface properties of customized Nitinol alloys can influence their biological performance. Researchers are investigating ways to optimize these properties to enhance tissue integration and reduce the risk of complications.
Corrosion resistance is a key factor in ensuring the long-term biocompatibility of Nitinol alloys in medical imaging devices. The corrosive environment within the human body can potentially lead to material degradation and the release of harmful byproducts. Efforts are being made to improve the corrosion resistance of Nitinol alloys through alloying with other elements, such as tantalum or platinum, and developing advanced surface treatments.
The sterilization process for medical devices made from customized Nitinol alloys must also be carefully considered. Common sterilization methods, such as steam autoclaving or ethylene oxide treatment, can potentially affect the properties of Nitinol. Research is ongoing to determine the most suitable sterilization techniques that maintain the alloy's performance while ensuring complete sterility.
Regulatory compliance is an essential aspect of biocompatibility considerations for Nitinol alloys in medical imaging devices. Manufacturers must adhere to stringent guidelines set by regulatory bodies such as the FDA and European Medicines Agency. This includes conducting comprehensive biocompatibility testing according to ISO 10993 standards, which evaluate various aspects of material-tissue interactions, including cytotoxicity, sensitization, and genotoxicity.
As research in customized Nitinol alloys for medical imaging devices progresses, there is a growing focus on developing alloys with enhanced biocompatibility profiles. This includes exploring new alloying elements and optimizing processing techniques to create materials that not only meet the functional requirements of medical imaging devices but also exhibit superior biocompatibility characteristics.
One of the primary concerns in biocompatibility is the potential release of nickel ions from Nitinol alloys. Nickel is a known allergen and can cause adverse reactions in some patients. To address this issue, researchers have focused on developing surface modification techniques to create a protective layer that minimizes nickel release. These techniques include oxidation treatments, coating with biocompatible materials, and ion implantation.
The interaction between Nitinol alloys and surrounding tissues is another crucial aspect of biocompatibility. Studies have shown that Nitinol generally exhibits good tissue compatibility, with minimal inflammatory responses observed in in vivo experiments. However, the specific composition and surface properties of customized Nitinol alloys can influence their biological performance. Researchers are investigating ways to optimize these properties to enhance tissue integration and reduce the risk of complications.
Corrosion resistance is a key factor in ensuring the long-term biocompatibility of Nitinol alloys in medical imaging devices. The corrosive environment within the human body can potentially lead to material degradation and the release of harmful byproducts. Efforts are being made to improve the corrosion resistance of Nitinol alloys through alloying with other elements, such as tantalum or platinum, and developing advanced surface treatments.
The sterilization process for medical devices made from customized Nitinol alloys must also be carefully considered. Common sterilization methods, such as steam autoclaving or ethylene oxide treatment, can potentially affect the properties of Nitinol. Research is ongoing to determine the most suitable sterilization techniques that maintain the alloy's performance while ensuring complete sterility.
Regulatory compliance is an essential aspect of biocompatibility considerations for Nitinol alloys in medical imaging devices. Manufacturers must adhere to stringent guidelines set by regulatory bodies such as the FDA and European Medicines Agency. This includes conducting comprehensive biocompatibility testing according to ISO 10993 standards, which evaluate various aspects of material-tissue interactions, including cytotoxicity, sensitization, and genotoxicity.
As research in customized Nitinol alloys for medical imaging devices progresses, there is a growing focus on developing alloys with enhanced biocompatibility profiles. This includes exploring new alloying elements and optimizing processing techniques to create materials that not only meet the functional requirements of medical imaging devices but also exhibit superior biocompatibility characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!