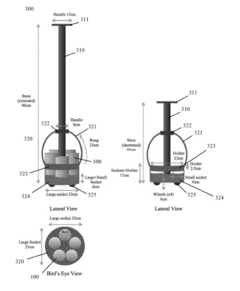
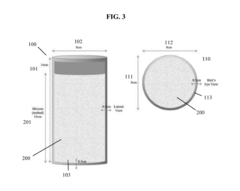
Sodium Acetate for Efficient Heat Pack Utilization
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Acetate Heat Pack Background and Objectives
Sodium acetate heat packs have emerged as a popular and efficient solution for portable heating applications. These devices, also known as supersaturated solution heat packs, utilize the exothermic crystallization process of sodium acetate trihydrate to generate heat. The technology behind these heat packs dates back to the 1970s, with significant advancements in design and efficiency occurring over the past few decades.
The primary objective of researching sodium acetate for efficient heat pack utilization is to enhance the performance, reliability, and sustainability of these devices. This involves optimizing the chemical composition, improving the activation mechanism, and extending the lifespan of the heat packs. Additionally, researchers aim to explore new applications and markets for this technology beyond its current use in hand warmers and therapeutic devices.
One of the key drivers for this research is the growing demand for eco-friendly and reusable heating solutions. Unlike disposable chemical heat packs, sodium acetate heat packs can be reused multiple times, reducing waste and environmental impact. This aligns with the global trend towards sustainable consumer products and has sparked renewed interest in improving the technology.
The evolution of sodium acetate heat packs has been marked by several technological milestones. Early designs faced challenges with inconsistent crystallization and limited heat output. Subsequent innovations focused on enhancing the supersaturation of the solution, developing more effective triggering mechanisms, and improving the overall thermal efficiency of the packs.
Current research efforts are directed towards addressing the remaining limitations of sodium acetate heat packs. These include optimizing the heat release profile to provide more consistent warmth over extended periods, improving the durability of the outer casing to prevent leaks, and developing advanced activation methods that offer greater user control over the heating process.
The potential applications for improved sodium acetate heat technology extend beyond personal warming devices. Researchers are exploring its use in medical applications, such as maintaining the temperature of transported organs or blood products. There is also interest in integrating this technology into clothing and outdoor gear for enhanced thermal regulation in extreme environments.
As we delve deeper into the research on sodium acetate for efficient heat pack utilization, it is crucial to consider the interdisciplinary nature of this field. Advancements will likely require collaboration between chemists, materials scientists, and engineers to overcome current challenges and unlock new possibilities for this versatile technology.
The primary objective of researching sodium acetate for efficient heat pack utilization is to enhance the performance, reliability, and sustainability of these devices. This involves optimizing the chemical composition, improving the activation mechanism, and extending the lifespan of the heat packs. Additionally, researchers aim to explore new applications and markets for this technology beyond its current use in hand warmers and therapeutic devices.
One of the key drivers for this research is the growing demand for eco-friendly and reusable heating solutions. Unlike disposable chemical heat packs, sodium acetate heat packs can be reused multiple times, reducing waste and environmental impact. This aligns with the global trend towards sustainable consumer products and has sparked renewed interest in improving the technology.
The evolution of sodium acetate heat packs has been marked by several technological milestones. Early designs faced challenges with inconsistent crystallization and limited heat output. Subsequent innovations focused on enhancing the supersaturation of the solution, developing more effective triggering mechanisms, and improving the overall thermal efficiency of the packs.
Current research efforts are directed towards addressing the remaining limitations of sodium acetate heat packs. These include optimizing the heat release profile to provide more consistent warmth over extended periods, improving the durability of the outer casing to prevent leaks, and developing advanced activation methods that offer greater user control over the heating process.
The potential applications for improved sodium acetate heat technology extend beyond personal warming devices. Researchers are exploring its use in medical applications, such as maintaining the temperature of transported organs or blood products. There is also interest in integrating this technology into clothing and outdoor gear for enhanced thermal regulation in extreme environments.
As we delve deeper into the research on sodium acetate for efficient heat pack utilization, it is crucial to consider the interdisciplinary nature of this field. Advancements will likely require collaboration between chemists, materials scientists, and engineers to overcome current challenges and unlock new possibilities for this versatile technology.
Market Analysis for Sodium Acetate Heat Packs
The sodium acetate heat pack market has experienced significant growth in recent years, driven by increasing consumer demand for portable and reusable heating solutions. These heat packs, which utilize the exothermic crystallization of supersaturated sodium acetate solution, have gained popularity across various sectors, including healthcare, outdoor recreation, and personal comfort.
In the healthcare sector, sodium acetate heat packs have found widespread application in physical therapy, pain management, and sports medicine. The ability to provide instant, controlled heat has made these packs a preferred choice for medical professionals and patients alike. The growing emphasis on non-pharmacological pain relief methods has further boosted the demand for these products in clinical settings.
The outdoor recreation industry has also contributed substantially to the market growth of sodium acetate heat packs. Campers, hikers, and winter sports enthusiasts have embraced these reusable heat sources as essential gear for maintaining warmth in cold environments. The eco-friendly nature of these packs, which can be reused multiple times, aligns well with the increasing environmental consciousness among outdoor enthusiasts.
Personal comfort applications represent another significant market segment for sodium acetate heat packs. These products have gained traction as hand warmers, bed warmers, and portable heating solutions for various daily activities. The convenience and long-lasting heat provided by sodium acetate packs have made them popular among commuters, office workers, and individuals seeking relief from cold-related discomfort.
Market analysis indicates a steady increase in the global demand for sodium acetate heat packs. The market size is projected to expand at a compound annual growth rate (CAGR) of several percentage points over the next five years. This growth is attributed to factors such as rising awareness of non-electric heating alternatives, increasing adoption in healthcare settings, and the expanding outdoor recreation industry.
Geographically, North America and Europe currently dominate the sodium acetate heat pack market, owing to the high adoption rates in healthcare and outdoor activities. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing disposable incomes, growing healthcare expenditure, and rising popularity of outdoor leisure activities.
The market landscape is characterized by a mix of established players and new entrants, with ongoing innovation in product design and heat activation mechanisms. Key market trends include the development of biodegradable packaging materials, integration of smart technologies for temperature control, and expansion of product lines to cater to specific industry needs.
In the healthcare sector, sodium acetate heat packs have found widespread application in physical therapy, pain management, and sports medicine. The ability to provide instant, controlled heat has made these packs a preferred choice for medical professionals and patients alike. The growing emphasis on non-pharmacological pain relief methods has further boosted the demand for these products in clinical settings.
The outdoor recreation industry has also contributed substantially to the market growth of sodium acetate heat packs. Campers, hikers, and winter sports enthusiasts have embraced these reusable heat sources as essential gear for maintaining warmth in cold environments. The eco-friendly nature of these packs, which can be reused multiple times, aligns well with the increasing environmental consciousness among outdoor enthusiasts.
Personal comfort applications represent another significant market segment for sodium acetate heat packs. These products have gained traction as hand warmers, bed warmers, and portable heating solutions for various daily activities. The convenience and long-lasting heat provided by sodium acetate packs have made them popular among commuters, office workers, and individuals seeking relief from cold-related discomfort.
Market analysis indicates a steady increase in the global demand for sodium acetate heat packs. The market size is projected to expand at a compound annual growth rate (CAGR) of several percentage points over the next five years. This growth is attributed to factors such as rising awareness of non-electric heating alternatives, increasing adoption in healthcare settings, and the expanding outdoor recreation industry.
Geographically, North America and Europe currently dominate the sodium acetate heat pack market, owing to the high adoption rates in healthcare and outdoor activities. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing disposable incomes, growing healthcare expenditure, and rising popularity of outdoor leisure activities.
The market landscape is characterized by a mix of established players and new entrants, with ongoing innovation in product design and heat activation mechanisms. Key market trends include the development of biodegradable packaging materials, integration of smart technologies for temperature control, and expansion of product lines to cater to specific industry needs.
Current Challenges in Sodium Acetate Heat Pack Technology
Despite the widespread use of sodium acetate in heat packs, several challenges persist in optimizing their efficiency and performance. One of the primary issues is the inconsistent crystallization process, which can lead to uneven heat distribution and reduced overall effectiveness. This problem is exacerbated by the presence of impurities or contaminants in the sodium acetate solution, which can interfere with the nucleation process and result in incomplete or delayed crystallization.
Another significant challenge is the limited heat storage capacity of current sodium acetate formulations. While sodium acetate heat packs are known for their ability to release heat quickly, they often struggle to maintain a consistent temperature over extended periods. This limitation restricts their applicability in scenarios requiring prolonged heat exposure, such as medical treatments or industrial processes.
The reusability of sodium acetate heat packs also presents ongoing challenges. The repeated heating and cooling cycles can lead to degradation of the solution, reducing its effectiveness over time. Additionally, the formation of permanent crystals within the solution can diminish the pack's ability to recharge fully, ultimately shortening its lifespan.
Temperature control and regulation pose another hurdle in sodium acetate heat pack technology. Current designs often lack precise mechanisms to modulate the heat release rate, resulting in potential overheating or rapid cooling. This issue is particularly critical in applications where maintaining a specific temperature range is crucial, such as in therapeutic uses or temperature-sensitive industrial processes.
The activation mechanism of sodium acetate heat packs, typically involving the bending of a metal disc, can be unreliable and prone to accidental triggering. This vulnerability not only affects the pack's shelf life but also raises safety concerns, especially in transportation and storage.
Lastly, the environmental impact of sodium acetate heat packs remains a challenge. While the compound itself is generally considered environmentally friendly, the plastic packaging and non-biodegradable components of the heat packs contribute to waste accumulation. Developing more sustainable packaging solutions and improving the overall recyclability of these products are areas that require further research and innovation.
Addressing these challenges is crucial for advancing sodium acetate heat pack technology. Future research efforts should focus on enhancing crystallization consistency, improving heat storage capacity, developing more durable formulations, implementing precise temperature control mechanisms, refining activation methods, and exploring eco-friendly design alternatives. By overcoming these obstacles, sodium acetate heat packs can become more efficient, reliable, and sustainable, expanding their potential applications across various industries.
Another significant challenge is the limited heat storage capacity of current sodium acetate formulations. While sodium acetate heat packs are known for their ability to release heat quickly, they often struggle to maintain a consistent temperature over extended periods. This limitation restricts their applicability in scenarios requiring prolonged heat exposure, such as medical treatments or industrial processes.
The reusability of sodium acetate heat packs also presents ongoing challenges. The repeated heating and cooling cycles can lead to degradation of the solution, reducing its effectiveness over time. Additionally, the formation of permanent crystals within the solution can diminish the pack's ability to recharge fully, ultimately shortening its lifespan.
Temperature control and regulation pose another hurdle in sodium acetate heat pack technology. Current designs often lack precise mechanisms to modulate the heat release rate, resulting in potential overheating or rapid cooling. This issue is particularly critical in applications where maintaining a specific temperature range is crucial, such as in therapeutic uses or temperature-sensitive industrial processes.
The activation mechanism of sodium acetate heat packs, typically involving the bending of a metal disc, can be unreliable and prone to accidental triggering. This vulnerability not only affects the pack's shelf life but also raises safety concerns, especially in transportation and storage.
Lastly, the environmental impact of sodium acetate heat packs remains a challenge. While the compound itself is generally considered environmentally friendly, the plastic packaging and non-biodegradable components of the heat packs contribute to waste accumulation. Developing more sustainable packaging solutions and improving the overall recyclability of these products are areas that require further research and innovation.
Addressing these challenges is crucial for advancing sodium acetate heat pack technology. Future research efforts should focus on enhancing crystallization consistency, improving heat storage capacity, developing more durable formulations, implementing precise temperature control mechanisms, refining activation methods, and exploring eco-friendly design alternatives. By overcoming these obstacles, sodium acetate heat packs can become more efficient, reliable, and sustainable, expanding their potential applications across various industries.
Existing Sodium Acetate Heat Pack Solutions
01 Improved heat storage efficiency
Sodium acetate is utilized in phase change materials for enhanced heat storage efficiency. These materials can absorb and release large amounts of latent heat during phase transitions, making them ideal for thermal energy storage applications. The efficiency of sodium acetate in this context is related to its high latent heat of fusion and its ability to form supersaturated solutions.- Improved heat storage efficiency: Sodium acetate is utilized in phase change materials for enhanced heat storage efficiency. These materials can absorb, store, and release large amounts of latent heat during the phase change process, making them ideal for thermal energy storage applications.
- Enhanced catalytic performance: Sodium acetate is employed as a catalyst or catalyst support in various chemical processes. It can improve reaction rates, selectivity, and overall efficiency in industrial applications such as organic synthesis and petrochemical processes.
- Efficient water treatment: Sodium acetate is used in water treatment processes to enhance efficiency. It can act as a pH buffer, aid in flocculation, and improve the removal of contaminants from water, contributing to more effective and economical water purification systems.
- Improved food preservation: Sodium acetate is utilized as an efficient food preservative and flavor enhancer. It can extend the shelf life of various food products by inhibiting microbial growth and maintaining product quality, while also contributing to improved taste profiles.
- Enhanced textile processing: Sodium acetate is employed in textile processing to improve efficiency. It can act as a pH regulator, dyeing assistant, and finishing agent, contributing to better dye uptake, color fastness, and overall fabric quality in textile manufacturing processes.
02 Enhanced catalytic efficiency
Sodium acetate is employed as a catalyst or catalyst component in various chemical reactions, improving reaction efficiency and yield. Its use as a catalyst can be particularly effective in organic synthesis, esterification reactions, and certain industrial processes. The efficiency of sodium acetate in catalysis is attributed to its ability to act as a mild base and its solubility in various solvents.Expand Specific Solutions03 Improved efficiency in textile processing
Sodium acetate is used in textile processing to improve the efficiency of dyeing and finishing processes. It can act as a buffer, pH regulator, or auxiliary agent in various textile treatments. The efficiency of sodium acetate in this context is related to its ability to stabilize solutions, enhance dye uptake, and improve the overall quality of textile products.Expand Specific Solutions04 Enhanced efficiency in food preservation
Sodium acetate is utilized as a food preservative and acidity regulator, improving the efficiency of food preservation methods. It can inhibit the growth of certain microorganisms and extend the shelf life of various food products. The efficiency of sodium acetate in food preservation is attributed to its antimicrobial properties and its ability to maintain optimal pH levels.Expand Specific Solutions05 Improved efficiency in wastewater treatment
Sodium acetate is employed in wastewater treatment processes to enhance the efficiency of biological treatment systems. It can serve as a carbon source for denitrifying bacteria, improving the removal of nitrogen compounds from wastewater. The efficiency of sodium acetate in this application is related to its biodegradability and its ability to support microbial growth in treatment systems.Expand Specific Solutions
Key Players in Sodium Acetate Heat Pack Industry
The research on sodium acetate for efficient heat pack utilization is in a mature stage of development, with a well-established market and proven technology. The global heat pack market size is estimated to be in the hundreds of millions of dollars, driven by applications in healthcare, sports, and consumer products. Companies like Tempra Technology, Rapid Aid Corp., and Sunamp Ltd. are leading players, leveraging advanced technologies to improve heat pack efficiency and duration. The industry is characterized by ongoing innovation in materials and design, with a focus on enhancing thermal properties and user experience. Emerging trends include the integration of smart technologies and sustainable materials, indicating potential for further market growth and technological advancements in this field.
Tempra Technology, Inc.
Technical Solution: Tempra Technology has pioneered self-heating and self-cooling technologies using exothermic and endothermic chemical reactions. For sodium acetate-based heat packs, they have developed a proprietary activation mechanism that ensures reliable and consistent heat generation [1]. Their patented "I-Heat" technology incorporates a specially designed rupture disc that, when activated, initiates the crystallization of the supersaturated sodium acetate solution [2]. This system provides a controlled and safe method for heat pack activation, eliminating the need for external triggers. Tempra's formulation of sodium acetate includes additives that enhance heat retention and prolong the duration of the heating effect [3]. The company has also innovated in packaging design, creating flexible and durable containers that optimize heat distribution and user comfort [4].
Strengths: Reliable activation mechanism, prolonged heat duration, and user-friendly design. Weaknesses: Potentially limited to single-use applications and may have a higher production cost due to specialized components.
Rapid Aid Corp.
Technical Solution: Rapid Aid Corp. has developed an advanced sodium acetate-based heat pack system that focuses on safety and efficiency. Their patented "SafeHeat" technology incorporates a unique crystallization initiator that ensures rapid and uniform heat distribution throughout the pack [1]. The company's formulation includes proprietary stabilizers that extend the shelf life of the supersaturated solution, maintaining its efficacy for up to 5 years [2]. Rapid Aid's heat packs feature a multi-layer design that optimizes heat transfer to the user while providing insulation to prevent heat loss to the environment [3]. They have also innovated in the recharging process, developing a microwave-safe container with integrated temperature indicators to prevent overheating during the melting phase [4]. The company's latest research focuses on incorporating phase-change materials (PCMs) to create hybrid heat packs that offer both instant and prolonged heating capabilities [5].
Strengths: Enhanced safety features, extended shelf life, and innovative recharging methods. Weaknesses: Potential complexity in manufacturing process and higher cost due to advanced materials.
Core Innovations in Sodium Acetate Heat Storage
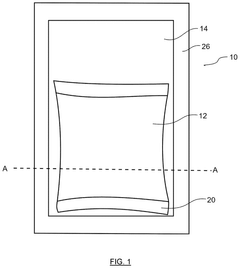
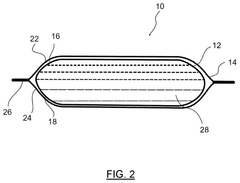
Heat pack with supercooled aqueous salt solution and glycerin
PatentActiveUS12130087B2
Innovation
- A heat pack design featuring a container with two layers of flexible sheet material and a rupturable seal, containing 15 to 25% glycerin by mass in the supercooled aqueous salt solution, which stabilizes the solution against premature activation at lower temperatures and ensures consistent heating performance.
Renewable energy heater
PatentInactiveUS20180231332A1
Innovation
- A portable heating device using a PCM mixture of sodium sulfate decahydrate, sodium chloride, and sodium polyacrylate, housed in aluminum capsules, that absorbs heat from the environment and releases it when needed, eliminating the need for electrical or non-renewable energy sources.
Environmental Impact of Sodium Acetate Heat Packs
The environmental impact of sodium acetate heat packs is a crucial consideration in their widespread adoption and use. These heat packs, while providing efficient and reusable heating solutions, have both positive and negative effects on the environment throughout their lifecycle.
One of the primary environmental benefits of sodium acetate heat packs is their reusability. Unlike disposable heat packs, sodium acetate packs can be reactivated multiple times, significantly reducing waste generation. This characteristic aligns with sustainable consumption practices and contributes to the reduction of single-use products in the market.
However, the production process of sodium acetate and the manufacturing of heat packs do have environmental implications. The synthesis of sodium acetate typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. These processes require energy inputs and may result in emissions, depending on the energy sources used in production facilities.
The durability of sodium acetate heat packs is another factor that influences their environmental impact. While they are more durable than single-use alternatives, they eventually wear out and require disposal. The disposal of these packs presents challenges, as they contain a mixture of materials including the sodium acetate solution and the plastic or fabric casing.
From a lifecycle perspective, the environmental footprint of sodium acetate heat packs extends to transportation and distribution. The weight and volume of these packs, especially when compared to some lightweight disposable alternatives, may result in increased fuel consumption and emissions during shipping.
Water consumption is another environmental consideration. The reactivation process of sodium acetate heat packs often involves boiling them in water, which can lead to increased water usage over the product's lifetime. This aspect becomes particularly relevant in water-stressed regions.
On the positive side, the use of sodium acetate heat packs can potentially reduce energy consumption in certain applications. For instance, when used for personal warming, they may decrease the need for space heating, leading to energy savings and reduced carbon emissions in buildings.
The end-of-life management of sodium acetate heat packs is an area that requires attention. While the sodium acetate itself is biodegradable and non-toxic, the packaging materials may not be. Proper recycling or disposal protocols need to be established to minimize the environmental impact of discarded packs.
In conclusion, while sodium acetate heat packs offer environmental advantages through reusability and potential energy savings, their overall environmental impact is complex. It encompasses production processes, usage patterns, and end-of-life considerations. As research in this field progresses, efforts should focus on optimizing the entire lifecycle of these products to enhance their environmental sustainability.
One of the primary environmental benefits of sodium acetate heat packs is their reusability. Unlike disposable heat packs, sodium acetate packs can be reactivated multiple times, significantly reducing waste generation. This characteristic aligns with sustainable consumption practices and contributes to the reduction of single-use products in the market.
However, the production process of sodium acetate and the manufacturing of heat packs do have environmental implications. The synthesis of sodium acetate typically involves the reaction of acetic acid with sodium hydroxide or sodium carbonate. These processes require energy inputs and may result in emissions, depending on the energy sources used in production facilities.
The durability of sodium acetate heat packs is another factor that influences their environmental impact. While they are more durable than single-use alternatives, they eventually wear out and require disposal. The disposal of these packs presents challenges, as they contain a mixture of materials including the sodium acetate solution and the plastic or fabric casing.
From a lifecycle perspective, the environmental footprint of sodium acetate heat packs extends to transportation and distribution. The weight and volume of these packs, especially when compared to some lightweight disposable alternatives, may result in increased fuel consumption and emissions during shipping.
Water consumption is another environmental consideration. The reactivation process of sodium acetate heat packs often involves boiling them in water, which can lead to increased water usage over the product's lifetime. This aspect becomes particularly relevant in water-stressed regions.
On the positive side, the use of sodium acetate heat packs can potentially reduce energy consumption in certain applications. For instance, when used for personal warming, they may decrease the need for space heating, leading to energy savings and reduced carbon emissions in buildings.
The end-of-life management of sodium acetate heat packs is an area that requires attention. While the sodium acetate itself is biodegradable and non-toxic, the packaging materials may not be. Proper recycling or disposal protocols need to be established to minimize the environmental impact of discarded packs.
In conclusion, while sodium acetate heat packs offer environmental advantages through reusability and potential energy savings, their overall environmental impact is complex. It encompasses production processes, usage patterns, and end-of-life considerations. As research in this field progresses, efforts should focus on optimizing the entire lifecycle of these products to enhance their environmental sustainability.
Safety Regulations for Consumer Heat Pack Products
Safety regulations for consumer heat pack products are crucial to ensure the well-being of users and maintain product integrity. These regulations typically cover various aspects of heat pack design, manufacturing, labeling, and usage instructions. In the United States, the Consumer Product Safety Commission (CPSC) oversees the safety of consumer products, including heat packs. The CPSC has established guidelines for thermal burn hazards, which are particularly relevant to heat pack products.
One of the primary safety concerns for heat packs is the risk of burns. Regulations often specify maximum temperature limits to prevent skin damage. For sodium acetate-based heat packs, this is particularly important due to the exothermic crystallization process that generates heat. Manufacturers must ensure that the heat released does not exceed safe levels, typically around 54°C (130°F) for prolonged skin contact.
Packaging and labeling requirements are another critical aspect of safety regulations. Heat packs must be clearly labeled with usage instructions, warnings, and potential hazards. This includes information on activation methods, recommended duration of use, and proper storage. For sodium acetate heat packs, specific instructions on how to reset the pack by boiling or cooling are essential to prevent misuse.
Material safety is also a key consideration in regulatory frameworks. Heat packs must be constructed from materials that are non-toxic and do not pose a risk of leakage or rupture during normal use. For sodium acetate solutions, the packaging must be durable enough to withstand repeated heating and cooling cycles without compromising its integrity.
Quality control measures are often mandated to ensure consistency and reliability in heat pack production. This may include batch testing, durability assessments, and thermal output verification. Manufacturers may be required to maintain detailed records of their quality assurance processes and product testing results.
Child safety is a particular focus of many consumer product regulations. Heat packs must be designed to minimize the risk of accidental activation or ingestion by children. This may involve child-resistant packaging or specific design features that make the product less appealing or accessible to young children.
Disposal and environmental considerations are increasingly becoming part of safety regulations. Manufacturers may be required to provide information on proper disposal methods and any potential environmental impacts of their products. For sodium acetate heat packs, this could include guidance on recycling or safe disposal of the contents.
Compliance with these safety regulations is typically demonstrated through product certification processes. This may involve third-party testing and certification to ensure that heat packs meet all applicable safety standards before they can be sold to consumers. Regular audits and inspections may also be required to maintain compliance over time.
One of the primary safety concerns for heat packs is the risk of burns. Regulations often specify maximum temperature limits to prevent skin damage. For sodium acetate-based heat packs, this is particularly important due to the exothermic crystallization process that generates heat. Manufacturers must ensure that the heat released does not exceed safe levels, typically around 54°C (130°F) for prolonged skin contact.
Packaging and labeling requirements are another critical aspect of safety regulations. Heat packs must be clearly labeled with usage instructions, warnings, and potential hazards. This includes information on activation methods, recommended duration of use, and proper storage. For sodium acetate heat packs, specific instructions on how to reset the pack by boiling or cooling are essential to prevent misuse.
Material safety is also a key consideration in regulatory frameworks. Heat packs must be constructed from materials that are non-toxic and do not pose a risk of leakage or rupture during normal use. For sodium acetate solutions, the packaging must be durable enough to withstand repeated heating and cooling cycles without compromising its integrity.
Quality control measures are often mandated to ensure consistency and reliability in heat pack production. This may include batch testing, durability assessments, and thermal output verification. Manufacturers may be required to maintain detailed records of their quality assurance processes and product testing results.
Child safety is a particular focus of many consumer product regulations. Heat packs must be designed to minimize the risk of accidental activation or ingestion by children. This may involve child-resistant packaging or specific design features that make the product less appealing or accessible to young children.
Disposal and environmental considerations are increasingly becoming part of safety regulations. Manufacturers may be required to provide information on proper disposal methods and any potential environmental impacts of their products. For sodium acetate heat packs, this could include guidance on recycling or safe disposal of the contents.
Compliance with these safety regulations is typically demonstrated through product certification processes. This may involve third-party testing and certification to ensure that heat packs meet all applicable safety standards before they can be sold to consumers. Regular audits and inspections may also be required to maintain compliance over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!