Role of Electrolytic Cells in Zero Emission Industrial Goals
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolytic Cells: Background and Zero-Emission Objectives
Electrolytic cells have played a pivotal role in industrial processes for over a century, with their origins dating back to the early 1800s. These electrochemical devices have been instrumental in various sectors, including metal production, chemical manufacturing, and energy storage. As global efforts to combat climate change intensify, the significance of electrolytic cells in achieving zero-emission industrial goals has come to the forefront.
The evolution of electrolytic cell technology has been marked by continuous improvements in efficiency, scalability, and environmental impact. From the initial Downs cell used in sodium production to modern membrane cells employed in chlor-alkali processes, the industry has witnessed substantial advancements. These developments have not only enhanced productivity but also reduced energy consumption and environmental footprint.
In recent years, the focus has shifted towards leveraging electrolytic cells as a cornerstone of sustainable industrial practices. The primary objective is to harness these devices to facilitate the transition from fossil fuel-dependent processes to clean, electrically-driven alternatives. This aligns with the broader goal of decarbonizing industries and achieving net-zero emissions targets set by governments and corporations worldwide.
One of the most promising applications of electrolytic cells in the context of zero-emission goals is in the production of green hydrogen. By using renewable electricity to power water electrolysis, industries can generate hydrogen without associated carbon emissions. This green hydrogen can then serve as a clean energy carrier or feedstock for various industrial processes, effectively replacing fossil fuels.
Moreover, electrolytic cells are being explored for their potential in carbon capture and utilization technologies. By employing these cells in electrochemical reduction processes, captured CO2 can be converted into valuable chemicals or fuels, creating a circular economy approach to emissions management.
The integration of electrolytic cells into industrial systems also opens up possibilities for energy storage and grid balancing. As renewable energy sources become more prevalent, electrolytic cells can help manage intermittency issues by converting excess electricity into storable forms like hydrogen or other chemical compounds.
As we look towards the future, the role of electrolytic cells in achieving zero-emission industrial goals is set to expand further. Research and development efforts are focused on improving cell efficiency, reducing costs, and scaling up technologies to meet industrial demands. The ultimate aim is to establish electrolytic processes as the backbone of a sustainable, carbon-neutral industrial landscape.
The evolution of electrolytic cell technology has been marked by continuous improvements in efficiency, scalability, and environmental impact. From the initial Downs cell used in sodium production to modern membrane cells employed in chlor-alkali processes, the industry has witnessed substantial advancements. These developments have not only enhanced productivity but also reduced energy consumption and environmental footprint.
In recent years, the focus has shifted towards leveraging electrolytic cells as a cornerstone of sustainable industrial practices. The primary objective is to harness these devices to facilitate the transition from fossil fuel-dependent processes to clean, electrically-driven alternatives. This aligns with the broader goal of decarbonizing industries and achieving net-zero emissions targets set by governments and corporations worldwide.
One of the most promising applications of electrolytic cells in the context of zero-emission goals is in the production of green hydrogen. By using renewable electricity to power water electrolysis, industries can generate hydrogen without associated carbon emissions. This green hydrogen can then serve as a clean energy carrier or feedstock for various industrial processes, effectively replacing fossil fuels.
Moreover, electrolytic cells are being explored for their potential in carbon capture and utilization technologies. By employing these cells in electrochemical reduction processes, captured CO2 can be converted into valuable chemicals or fuels, creating a circular economy approach to emissions management.
The integration of electrolytic cells into industrial systems also opens up possibilities for energy storage and grid balancing. As renewable energy sources become more prevalent, electrolytic cells can help manage intermittency issues by converting excess electricity into storable forms like hydrogen or other chemical compounds.
As we look towards the future, the role of electrolytic cells in achieving zero-emission industrial goals is set to expand further. Research and development efforts are focused on improving cell efficiency, reducing costs, and scaling up technologies to meet industrial demands. The ultimate aim is to establish electrolytic processes as the backbone of a sustainable, carbon-neutral industrial landscape.
Market Demand for Green Industrial Processes
The global market for green industrial processes has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations aimed at reducing carbon emissions. Electrolytic cells play a crucial role in this shift towards zero-emission industrial goals, particularly in sectors such as chemical manufacturing, metal production, and energy storage.
In the chemical industry, there is a growing demand for electrolytic processes that can replace traditional, carbon-intensive methods. For instance, the production of chlorine and caustic soda, which are essential chemicals used in various industries, is increasingly moving towards membrane cell electrolysis technology. This method not only reduces energy consumption but also eliminates mercury-related environmental concerns associated with older technologies.
The metal production sector, especially aluminum manufacturing, is another area where electrolytic cells are in high demand. As the industry seeks to reduce its carbon footprint, there is a strong push for the development and implementation of inert anode technology in aluminum smelting. This innovation has the potential to eliminate direct CO2 emissions from the smelting process, making it a highly sought-after solution in the market.
In the energy storage sector, the rising adoption of renewable energy sources has created a substantial market for electrolytic hydrogen production. Green hydrogen, produced through water electrolysis powered by renewable electricity, is seen as a key component in decarbonizing various industries, including transportation, heating, and industrial processes. The demand for electrolyzers is expected to grow exponentially as countries and companies invest in hydrogen infrastructure as part of their zero-emission strategies.
The market for electrolytic cells is also being driven by the increasing focus on circular economy principles. Industries are looking for ways to recycle and recover valuable materials from waste streams, and electrolytic processes offer efficient solutions for metal recovery and wastewater treatment. This trend is particularly evident in the electronics industry, where there is a growing need for sustainable methods to recover precious metals from e-waste.
Furthermore, the automotive industry's shift towards electric vehicles has created a new market for electrolytic cells in battery production and recycling. As the demand for lithium-ion batteries continues to rise, there is an increasing need for efficient and environmentally friendly processes for battery material production and end-of-life recycling, where electrolytic techniques play a vital role.
In the chemical industry, there is a growing demand for electrolytic processes that can replace traditional, carbon-intensive methods. For instance, the production of chlorine and caustic soda, which are essential chemicals used in various industries, is increasingly moving towards membrane cell electrolysis technology. This method not only reduces energy consumption but also eliminates mercury-related environmental concerns associated with older technologies.
The metal production sector, especially aluminum manufacturing, is another area where electrolytic cells are in high demand. As the industry seeks to reduce its carbon footprint, there is a strong push for the development and implementation of inert anode technology in aluminum smelting. This innovation has the potential to eliminate direct CO2 emissions from the smelting process, making it a highly sought-after solution in the market.
In the energy storage sector, the rising adoption of renewable energy sources has created a substantial market for electrolytic hydrogen production. Green hydrogen, produced through water electrolysis powered by renewable electricity, is seen as a key component in decarbonizing various industries, including transportation, heating, and industrial processes. The demand for electrolyzers is expected to grow exponentially as countries and companies invest in hydrogen infrastructure as part of their zero-emission strategies.
The market for electrolytic cells is also being driven by the increasing focus on circular economy principles. Industries are looking for ways to recycle and recover valuable materials from waste streams, and electrolytic processes offer efficient solutions for metal recovery and wastewater treatment. This trend is particularly evident in the electronics industry, where there is a growing need for sustainable methods to recover precious metals from e-waste.
Furthermore, the automotive industry's shift towards electric vehicles has created a new market for electrolytic cells in battery production and recycling. As the demand for lithium-ion batteries continues to rise, there is an increasing need for efficient and environmentally friendly processes for battery material production and end-of-life recycling, where electrolytic techniques play a vital role.
Current State and Challenges of Electrolytic Technology
Electrolytic cells play a crucial role in the pursuit of zero-emission industrial goals, yet their current state and associated challenges present significant hurdles. The technology has made substantial progress in recent years, with improvements in efficiency, durability, and scalability. However, several key challenges remain to be addressed before widespread industrial adoption can be achieved.
One of the primary challenges facing electrolytic technology is energy efficiency. While advancements have been made, the process still requires a considerable amount of electricity to operate effectively. This high energy demand poses a significant barrier to large-scale implementation, particularly in regions where electricity costs are high or where the grid relies heavily on fossil fuels. Improving the energy efficiency of electrolytic cells is crucial for their economic viability and environmental impact.
Material constraints also present a significant challenge. Many current electrolytic cell designs rely on rare and expensive materials, such as platinum group metals, for catalysts and electrodes. The scarcity and cost of these materials limit the scalability of the technology and increase overall production costs. Research into alternative materials that offer similar performance characteristics while being more abundant and cost-effective is ongoing but has yet to yield a definitive solution.
Durability and longevity of electrolytic cells remain areas of concern. The harsh operating conditions, including high temperatures and corrosive environments, can lead to degradation of cell components over time. This degradation not only reduces efficiency but also necessitates frequent maintenance and replacement, increasing operational costs and downtime. Developing more robust materials and designs that can withstand these conditions for extended periods is essential for the long-term viability of the technology.
Scaling up electrolytic technology for industrial applications presents its own set of challenges. While small-scale demonstrations have shown promise, translating this success to large-scale operations introduces complexities in system design, process control, and integration with existing industrial processes. Ensuring consistent performance and reliability at scale is critical for meeting industrial zero-emission goals.
The intermittent nature of renewable energy sources poses another challenge for electrolytic cells in zero-emission applications. As industries aim to power these cells with clean energy, managing the variability in electricity supply becomes crucial. Developing flexible electrolytic systems that can operate efficiently under fluctuating power conditions is an ongoing area of research and development.
Regulatory and safety considerations also play a significant role in the current state of electrolytic technology. Stringent safety standards and regulations, particularly for hydrogen production and storage, can impact the design and implementation of electrolytic systems. Addressing these regulatory requirements while maintaining efficiency and cost-effectiveness is a delicate balance that industry players must navigate.
One of the primary challenges facing electrolytic technology is energy efficiency. While advancements have been made, the process still requires a considerable amount of electricity to operate effectively. This high energy demand poses a significant barrier to large-scale implementation, particularly in regions where electricity costs are high or where the grid relies heavily on fossil fuels. Improving the energy efficiency of electrolytic cells is crucial for their economic viability and environmental impact.
Material constraints also present a significant challenge. Many current electrolytic cell designs rely on rare and expensive materials, such as platinum group metals, for catalysts and electrodes. The scarcity and cost of these materials limit the scalability of the technology and increase overall production costs. Research into alternative materials that offer similar performance characteristics while being more abundant and cost-effective is ongoing but has yet to yield a definitive solution.
Durability and longevity of electrolytic cells remain areas of concern. The harsh operating conditions, including high temperatures and corrosive environments, can lead to degradation of cell components over time. This degradation not only reduces efficiency but also necessitates frequent maintenance and replacement, increasing operational costs and downtime. Developing more robust materials and designs that can withstand these conditions for extended periods is essential for the long-term viability of the technology.
Scaling up electrolytic technology for industrial applications presents its own set of challenges. While small-scale demonstrations have shown promise, translating this success to large-scale operations introduces complexities in system design, process control, and integration with existing industrial processes. Ensuring consistent performance and reliability at scale is critical for meeting industrial zero-emission goals.
The intermittent nature of renewable energy sources poses another challenge for electrolytic cells in zero-emission applications. As industries aim to power these cells with clean energy, managing the variability in electricity supply becomes crucial. Developing flexible electrolytic systems that can operate efficiently under fluctuating power conditions is an ongoing area of research and development.
Regulatory and safety considerations also play a significant role in the current state of electrolytic technology. Stringent safety standards and regulations, particularly for hydrogen production and storage, can impact the design and implementation of electrolytic systems. Addressing these regulatory requirements while maintaining efficiency and cost-effectiveness is a delicate balance that industry players must navigate.
Existing Electrolytic Solutions for Zero-Emission Goals
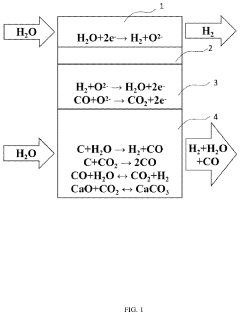
01 Reduction of chlorine emissions in electrolytic cells
Methods and systems for reducing chlorine emissions in electrolytic cells, particularly in chlor-alkali production. This involves optimizing cell design, improving membrane technology, and implementing gas capture and treatment systems to minimize the release of chlorine gas during the electrolysis process.- Reduction of chlorine emissions in electrolytic cells: Methods and systems for reducing chlorine emissions in electrolytic cells, particularly in chlor-alkali production. This involves optimizing cell design, improving membrane technology, and implementing advanced gas collection systems to minimize the release of chlorine gas during the electrolysis process.
- Carbon dioxide capture in electrolytic processes: Techniques for capturing and utilizing carbon dioxide emissions from electrolytic cells. This includes integrating CO2 capture systems with electrolytic processes, converting captured CO2 into valuable products, and developing novel electrode materials that can efficiently reduce CO2 during electrolysis.
- Hydrogen management in electrolytic systems: Strategies for managing hydrogen emissions from electrolytic cells, focusing on safety and efficiency. This involves developing advanced hydrogen collection and storage systems, implementing hydrogen recycling technologies, and optimizing cell parameters to control hydrogen evolution rates.
- Emission control in high-temperature electrolysis: Methods for controlling emissions in high-temperature electrolytic processes, such as molten salt electrolysis. This includes developing specialized materials for cell components, implementing advanced gas handling systems, and optimizing operating conditions to minimize the formation and release of harmful emissions.
- Wastewater treatment and emission reduction in electrolytic processes: Integrated approaches for treating wastewater and reducing emissions from electrolytic cells. This involves developing advanced filtration and purification systems, implementing closed-loop water recycling, and utilizing byproducts from electrolysis for wastewater treatment, thereby minimizing overall environmental impact.
02 Carbon dioxide capture from electrolytic processes
Techniques for capturing and utilizing carbon dioxide emissions from electrolytic cells, particularly in industrial processes. This includes integrating CO2 capture systems, developing novel electrode materials that promote CO2 reduction, and implementing methods to convert captured CO2 into valuable products.Expand Specific Solutions03 Hydrogen management in electrolytic systems
Strategies for managing hydrogen emissions from electrolytic cells, focusing on safety and efficiency. This involves developing advanced gas separation techniques, implementing hydrogen recovery systems, and designing cells to minimize hydrogen evolution or to utilize it effectively in other processes.Expand Specific Solutions04 Emission control in high-temperature electrolysis
Methods for controlling emissions in high-temperature electrolytic processes, such as those used in metal production. This includes developing refractory materials resistant to corrosive emissions, implementing advanced filtration systems, and optimizing cell designs to minimize the generation of harmful byproducts.Expand Specific Solutions05 Wastewater treatment and emission reduction in electrolytic processes
Integrated approaches to treating wastewater and reducing emissions from electrolytic cells. This involves developing closed-loop systems, implementing advanced oxidation processes for contaminant removal, and utilizing electrolytic treatment methods to purify wastewater while minimizing gaseous emissions.Expand Specific Solutions
Key Players in Electrolytic Cell Industry
The electrolytic cell industry for zero emission industrial goals is in a growth phase, driven by increasing demand for clean energy solutions. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the field is advancing but still evolving, with varying levels of maturity across different applications. Key players like Asahi Kasei Corp., ThyssenKrupp Uhde Chlorine Engineers GmbH, and Verdagy Inc. are at the forefront, developing innovative solutions. Research institutions such as the Dalian Institute of Chemical Physics and the Naval Research Laboratory are contributing to technological advancements. Companies like Saudi Aramco and Honda Motor Co. are exploring applications in their respective industries, indicating a broadening interest across sectors.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei has developed advanced chlor-alkali electrolysis technology for zero-emission industrial goals. Their membrane process uses ion-exchange membranes to produce chlorine, caustic soda, and hydrogen with high energy efficiency. The company's latest innovation is the "Zero-Gap" electrolysis cell, which reduces the inter-electrode gap to near zero, minimizing electrical resistance and energy consumption[1]. This technology achieves an energy efficiency of over 97% in industrial-scale plants, significantly contributing to CO2 emission reduction[2]. Asahi Kasei has also integrated renewable energy sources into their electrolysis systems, further enhancing the sustainability of their chlor-alkali production process[3].
Strengths: High energy efficiency, reduced CO2 emissions, and integration with renewable energy. Weaknesses: High initial investment costs and potential challenges in retrofitting existing plants.
ThyssenKrupp Uhde Chlorine Engineers GmbH
Technical Solution: ThyssenKrupp Uhde Chlorine Engineers has developed the NCS BiTAC electrolysis technology for chlor-alkali production, which plays a crucial role in zero-emission industrial goals. Their system utilizes a zero-gap cell design with an optimized electrode package, reducing energy consumption by up to 30% compared to conventional technologies[4]. The company's electrolyzers are capable of producing green hydrogen when powered by renewable energy sources, contributing to the decarbonization of various industries[5]. ThyssenKrupp has also implemented advanced digital solutions for real-time monitoring and optimization of electrolysis processes, further enhancing efficiency and reducing environmental impact[6].
Strengths: Significant energy savings, versatility in producing green hydrogen, and advanced digital monitoring. Weaknesses: Potential high costs for implementation and the need for specialized maintenance.
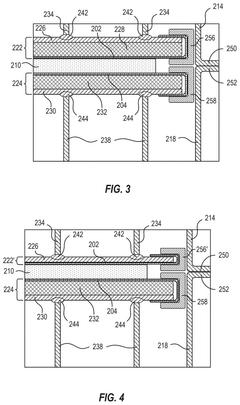
Core Innovations in Electrolytic Cell Design
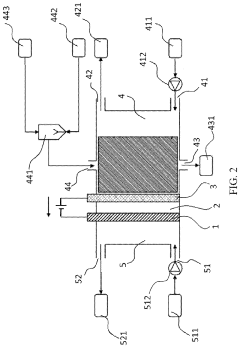
Carbon-assisted solid oxide electrolysis cell
PatentInactiveUS20230167562A1
Innovation
- The introduction of a CO2 absorber in the anode chamber, where water is used as an in situ gasification agent to enhance carbon gasification, promoting steam-carbon gasification and suppressing the Boudouard reaction, thereby increasing carbon gasification rates and generating more hydrogen fuel, which improves electrochemical kinetics and reduces energy consumption.
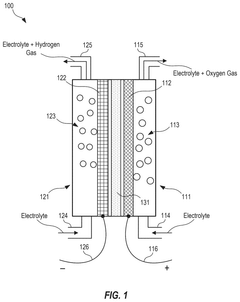
Electrolyzer cell and methods of using and manufacturing the same
PatentPendingUS20250230560A1
Innovation
- An electrolyzer cell design featuring non-welded electrodes and a zero-gap configuration with elastic elements and conductive meshes to minimize resistive losses, allowing for easy electrode replacement without welding, thereby enhancing efficiency and reducing maintenance complexity.
Environmental Impact Assessment of Electrolytic Processes
The environmental impact assessment of electrolytic processes is crucial in evaluating the role of electrolytic cells in achieving zero-emission industrial goals. These processes, while essential for various industries, can have significant environmental implications that need to be carefully considered and mitigated.
Electrolytic cells are fundamental in many industrial applications, including the production of chemicals, metals, and gases. However, their operation often involves high energy consumption and the potential release of harmful byproducts. The primary environmental concerns associated with electrolytic processes include greenhouse gas emissions, water pollution, and the generation of hazardous waste.
One of the most significant environmental impacts of electrolytic processes is their contribution to carbon dioxide emissions. Many electrolytic cells require substantial electrical power, which, if sourced from fossil fuels, can lead to considerable carbon footprints. This aspect is particularly critical when assessing the technology's role in zero-emission goals. To address this issue, industries are increasingly exploring the integration of renewable energy sources to power electrolytic processes, thereby reducing their carbon intensity.
Water usage and potential contamination are also key environmental factors to consider. Electrolytic processes often require large volumes of water, both as a reactant and for cooling purposes. The discharge of process water can lead to thermal pollution and the release of chemical contaminants into aquatic ecosystems. Advanced water treatment and recycling systems are being developed to minimize these impacts and improve water efficiency in electrolytic operations.
The generation and management of hazardous waste is another critical environmental aspect of electrolytic processes. Many of these processes produce byproducts that can be toxic or corrosive. Proper handling, treatment, and disposal of these materials are essential to prevent soil and groundwater contamination. Industries are investing in innovative waste management techniques and exploring ways to valorize byproducts, turning potential waste into valuable resources.
Air quality is also a concern in some electrolytic processes, particularly those involving the production of chlorine or aluminum. Emissions of chlorine gas or fluoride compounds can have severe local environmental impacts if not properly controlled. State-of-the-art scrubbing and filtration technologies are being employed to minimize these emissions and protect air quality in surrounding areas.
As industries strive towards zero-emission goals, the environmental assessment of electrolytic processes is evolving to include life cycle analyses. These comprehensive assessments consider the environmental impacts from raw material extraction through to product end-of-life, providing a holistic view of the technology's sustainability. Such analyses are instrumental in identifying opportunities for improvement and guiding the development of more environmentally friendly electrolytic technologies.
Electrolytic cells are fundamental in many industrial applications, including the production of chemicals, metals, and gases. However, their operation often involves high energy consumption and the potential release of harmful byproducts. The primary environmental concerns associated with electrolytic processes include greenhouse gas emissions, water pollution, and the generation of hazardous waste.
One of the most significant environmental impacts of electrolytic processes is their contribution to carbon dioxide emissions. Many electrolytic cells require substantial electrical power, which, if sourced from fossil fuels, can lead to considerable carbon footprints. This aspect is particularly critical when assessing the technology's role in zero-emission goals. To address this issue, industries are increasingly exploring the integration of renewable energy sources to power electrolytic processes, thereby reducing their carbon intensity.
Water usage and potential contamination are also key environmental factors to consider. Electrolytic processes often require large volumes of water, both as a reactant and for cooling purposes. The discharge of process water can lead to thermal pollution and the release of chemical contaminants into aquatic ecosystems. Advanced water treatment and recycling systems are being developed to minimize these impacts and improve water efficiency in electrolytic operations.
The generation and management of hazardous waste is another critical environmental aspect of electrolytic processes. Many of these processes produce byproducts that can be toxic or corrosive. Proper handling, treatment, and disposal of these materials are essential to prevent soil and groundwater contamination. Industries are investing in innovative waste management techniques and exploring ways to valorize byproducts, turning potential waste into valuable resources.
Air quality is also a concern in some electrolytic processes, particularly those involving the production of chlorine or aluminum. Emissions of chlorine gas or fluoride compounds can have severe local environmental impacts if not properly controlled. State-of-the-art scrubbing and filtration technologies are being employed to minimize these emissions and protect air quality in surrounding areas.
As industries strive towards zero-emission goals, the environmental assessment of electrolytic processes is evolving to include life cycle analyses. These comprehensive assessments consider the environmental impacts from raw material extraction through to product end-of-life, providing a holistic view of the technology's sustainability. Such analyses are instrumental in identifying opportunities for improvement and guiding the development of more environmentally friendly electrolytic technologies.
Economic Viability of Electrolytic Zero-Emission Solutions
The economic viability of electrolytic zero-emission solutions is a critical factor in determining their widespread adoption in industrial processes. As industries strive to meet increasingly stringent environmental regulations and sustainability goals, the cost-effectiveness of these technologies becomes paramount.
Electrolytic cells offer a promising pathway to zero-emission production, particularly in sectors such as chemical manufacturing, metal refining, and energy storage. However, their economic feasibility depends on several key factors. The initial capital investment for electrolytic systems can be substantial, often requiring significant modifications to existing industrial infrastructure. This upfront cost can be a barrier for many companies, especially small and medium-sized enterprises.
Operating costs are another crucial consideration. Electrolytic processes typically require large amounts of electricity, which can lead to high energy expenses. The economic viability of these solutions is therefore closely tied to electricity prices and the availability of low-cost, renewable energy sources. In regions with abundant and cheap renewable energy, electrolytic zero-emission solutions become more attractive from an economic standpoint.
Maintenance and operational requirements also play a role in the overall cost structure. While electrolytic cells generally have fewer moving parts compared to traditional industrial equipment, they may require specialized maintenance and periodic replacement of components such as electrodes and membranes. The longevity and reliability of these systems directly impact their long-term economic viability.
The scale of production is another critical factor. Larger-scale operations can often achieve better economies of scale, reducing the per-unit cost of production. This can make electrolytic solutions more economically viable for large industrial players but may pose challenges for smaller operations.
Government policies and incentives significantly influence the economic landscape for zero-emission technologies. Carbon pricing mechanisms, tax incentives, and subsidies for clean energy technologies can tilt the balance in favor of electrolytic solutions. As global efforts to combat climate change intensify, such supportive policies are likely to become more prevalent, enhancing the economic attractiveness of these technologies.
Market demand for zero-emission products is also a key driver. As consumers and businesses increasingly prioritize sustainability, products manufactured using clean technologies may command premium prices or gain preferential market access. This trend can offset higher production costs associated with electrolytic processes, improving their overall economic viability.
In conclusion, while electrolytic zero-emission solutions face economic challenges, their viability is improving as technology advances, energy costs evolve, and environmental regulations tighten. The long-term economic benefits of these technologies, including reduced environmental liabilities and improved corporate image, are increasingly being recognized as valuable assets in a carbon-constrained world.
Electrolytic cells offer a promising pathway to zero-emission production, particularly in sectors such as chemical manufacturing, metal refining, and energy storage. However, their economic feasibility depends on several key factors. The initial capital investment for electrolytic systems can be substantial, often requiring significant modifications to existing industrial infrastructure. This upfront cost can be a barrier for many companies, especially small and medium-sized enterprises.
Operating costs are another crucial consideration. Electrolytic processes typically require large amounts of electricity, which can lead to high energy expenses. The economic viability of these solutions is therefore closely tied to electricity prices and the availability of low-cost, renewable energy sources. In regions with abundant and cheap renewable energy, electrolytic zero-emission solutions become more attractive from an economic standpoint.
Maintenance and operational requirements also play a role in the overall cost structure. While electrolytic cells generally have fewer moving parts compared to traditional industrial equipment, they may require specialized maintenance and periodic replacement of components such as electrodes and membranes. The longevity and reliability of these systems directly impact their long-term economic viability.
The scale of production is another critical factor. Larger-scale operations can often achieve better economies of scale, reducing the per-unit cost of production. This can make electrolytic solutions more economically viable for large industrial players but may pose challenges for smaller operations.
Government policies and incentives significantly influence the economic landscape for zero-emission technologies. Carbon pricing mechanisms, tax incentives, and subsidies for clean energy technologies can tilt the balance in favor of electrolytic solutions. As global efforts to combat climate change intensify, such supportive policies are likely to become more prevalent, enhancing the economic attractiveness of these technologies.
Market demand for zero-emission products is also a key driver. As consumers and businesses increasingly prioritize sustainability, products manufactured using clean technologies may command premium prices or gain preferential market access. This trend can offset higher production costs associated with electrolytic processes, improving their overall economic viability.
In conclusion, while electrolytic zero-emission solutions face economic challenges, their viability is improving as technology advances, energy costs evolve, and environmental regulations tighten. The long-term economic benefits of these technologies, including reduced environmental liabilities and improved corporate image, are increasingly being recognized as valuable assets in a carbon-constrained world.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!