Seamless Integration of Robotic Systems for ICP-MS Automation
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Robotic ICP-MS Automation Background and Objectives
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has evolved significantly since its commercial introduction in the 1980s, becoming an essential analytical technique for elemental analysis across various industries including environmental monitoring, pharmaceuticals, semiconductor manufacturing, and geological research. The technology offers exceptional sensitivity, multi-element detection capabilities, and wide dynamic range, making it the gold standard for trace element analysis.
Traditional ICP-MS workflows involve substantial manual intervention, including sample preparation, calibration, introduction, and post-analysis maintenance. These manual processes introduce variability, increase the risk of contamination, and limit throughput. As analytical demands grow more complex and regulatory requirements become more stringent, there is an increasing need for automation solutions that can enhance reproducibility, accuracy, and operational efficiency.
The integration of robotics with ICP-MS represents a natural technological evolution, building upon earlier laboratory automation efforts. Initial automation attempts focused primarily on autosampling devices, but recent advancements in robotics, artificial intelligence, and laboratory information management systems have created opportunities for comprehensive end-to-end automation solutions.
Current technological trends point toward fully integrated robotic systems capable of handling complex sample preparation workflows, intelligent sample management, adaptive calibration procedures, and predictive maintenance. These systems aim to minimize human intervention while maximizing analytical performance and instrument uptime.
The primary objectives of seamless robotic integration for ICP-MS automation include: reducing human error and variability in analytical results; increasing sample throughput without compromising quality; enabling 24/7 operation capabilities; minimizing analyst exposure to hazardous materials; optimizing resource utilization including reagents and consumables; and generating comprehensive data trails for regulatory compliance.
Additionally, there is growing interest in developing modular automation platforms that can be customized to specific application requirements while maintaining compatibility with existing laboratory infrastructure. This approach allows laboratories to implement automation incrementally, addressing immediate needs while building toward comprehensive solutions.
The convergence of ICP-MS with robotics also aligns with broader industry trends toward smart laboratories and Industry 4.0 principles, where interconnected systems communicate seamlessly to optimize workflows and generate actionable insights from analytical data. Future developments are expected to incorporate machine learning algorithms that can identify patterns in analytical data, predict maintenance needs, and continuously optimize analytical methods.
Traditional ICP-MS workflows involve substantial manual intervention, including sample preparation, calibration, introduction, and post-analysis maintenance. These manual processes introduce variability, increase the risk of contamination, and limit throughput. As analytical demands grow more complex and regulatory requirements become more stringent, there is an increasing need for automation solutions that can enhance reproducibility, accuracy, and operational efficiency.
The integration of robotics with ICP-MS represents a natural technological evolution, building upon earlier laboratory automation efforts. Initial automation attempts focused primarily on autosampling devices, but recent advancements in robotics, artificial intelligence, and laboratory information management systems have created opportunities for comprehensive end-to-end automation solutions.
Current technological trends point toward fully integrated robotic systems capable of handling complex sample preparation workflows, intelligent sample management, adaptive calibration procedures, and predictive maintenance. These systems aim to minimize human intervention while maximizing analytical performance and instrument uptime.
The primary objectives of seamless robotic integration for ICP-MS automation include: reducing human error and variability in analytical results; increasing sample throughput without compromising quality; enabling 24/7 operation capabilities; minimizing analyst exposure to hazardous materials; optimizing resource utilization including reagents and consumables; and generating comprehensive data trails for regulatory compliance.
Additionally, there is growing interest in developing modular automation platforms that can be customized to specific application requirements while maintaining compatibility with existing laboratory infrastructure. This approach allows laboratories to implement automation incrementally, addressing immediate needs while building toward comprehensive solutions.
The convergence of ICP-MS with robotics also aligns with broader industry trends toward smart laboratories and Industry 4.0 principles, where interconnected systems communicate seamlessly to optimize workflows and generate actionable insights from analytical data. Future developments are expected to incorporate machine learning algorithms that can identify patterns in analytical data, predict maintenance needs, and continuously optimize analytical methods.
Market Demand Analysis for Automated Analytical Chemistry
The analytical chemistry market is experiencing a significant shift towards automation, with ICP-MS (Inductively Coupled Plasma Mass Spectrometry) automation representing a particularly high-growth segment. Current market research indicates that the global ICP-MS market is projected to grow at a compound annual growth rate of 7.2% through 2028, driven primarily by increasing demand for high-throughput analytical capabilities in pharmaceutical, environmental, and materials science applications.
Laboratory automation has become a critical need rather than a luxury, as organizations face mounting pressure to increase analytical throughput while maintaining precision and reducing operational costs. A recent survey of analytical laboratory managers revealed that over 65% consider automation integration as a top investment priority for the next three years, with robotic systems for sample preparation and handling ranking as the most sought-after automation solution.
The pharmaceutical and biotechnology sectors represent the largest market segment for ICP-MS automation, accounting for approximately 38% of the total market share. These industries require high-precision elemental analysis for quality control, impurity profiling, and regulatory compliance. Environmental testing laboratories constitute the second-largest market segment at 27%, driven by increasingly stringent regulatory requirements for monitoring heavy metals and other elemental contaminants in various environmental matrices.
Geographically, North America leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to expanding pharmaceutical manufacturing capabilities and increasing environmental monitoring requirements.
Key market drivers include the growing need for multi-element analysis with lower detection limits, increasing sample volumes, laboratory staff shortages, and the push for greater reproducibility in analytical results. End-users specifically demand seamless integration capabilities between robotic systems and ICP-MS instruments, with particular emphasis on flexible automation solutions that can handle variable sample types and preparation protocols.
Return on investment considerations are paramount for potential adopters, with laboratories expecting automation solutions to demonstrate measurable improvements in throughput (minimum 40% increase), reduction in manual errors (at least 60% decrease), and operational cost savings (25-30% reduction in per-sample analysis costs) within 18-24 months of implementation. Additionally, there is growing demand for automation solutions that can be integrated with laboratory information management systems (LIMS) to enable comprehensive data tracking and quality assurance.
Laboratory automation has become a critical need rather than a luxury, as organizations face mounting pressure to increase analytical throughput while maintaining precision and reducing operational costs. A recent survey of analytical laboratory managers revealed that over 65% consider automation integration as a top investment priority for the next three years, with robotic systems for sample preparation and handling ranking as the most sought-after automation solution.
The pharmaceutical and biotechnology sectors represent the largest market segment for ICP-MS automation, accounting for approximately 38% of the total market share. These industries require high-precision elemental analysis for quality control, impurity profiling, and regulatory compliance. Environmental testing laboratories constitute the second-largest market segment at 27%, driven by increasingly stringent regulatory requirements for monitoring heavy metals and other elemental contaminants in various environmental matrices.
Geographically, North America leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to expanding pharmaceutical manufacturing capabilities and increasing environmental monitoring requirements.
Key market drivers include the growing need for multi-element analysis with lower detection limits, increasing sample volumes, laboratory staff shortages, and the push for greater reproducibility in analytical results. End-users specifically demand seamless integration capabilities between robotic systems and ICP-MS instruments, with particular emphasis on flexible automation solutions that can handle variable sample types and preparation protocols.
Return on investment considerations are paramount for potential adopters, with laboratories expecting automation solutions to demonstrate measurable improvements in throughput (minimum 40% increase), reduction in manual errors (at least 60% decrease), and operational cost savings (25-30% reduction in per-sample analysis costs) within 18-24 months of implementation. Additionally, there is growing demand for automation solutions that can be integrated with laboratory information management systems (LIMS) to enable comprehensive data tracking and quality assurance.
Current Challenges in ICP-MS Robotics Integration
The integration of robotic systems with Inductively Coupled Plasma Mass Spectrometry (ICP-MS) faces several significant technical challenges that impede seamless automation. One primary obstacle is the precision requirements for sample handling. ICP-MS analysis demands extremely precise sample preparation and introduction, with tolerances often in the microliter range. Current robotic systems struggle to consistently achieve this level of precision across varied sample types and viscosities, leading to potential measurement errors.
Compatibility issues between robotic platforms and ICP-MS instruments represent another major hurdle. Most ICP-MS systems were not originally designed with full automation in mind, resulting in interface limitations. The proprietary nature of both robotic systems and analytical instruments creates communication barriers, with manufacturers using different protocols and software architectures that resist straightforward integration.
Environmental control presents additional complications. ICP-MS requires stringent contamination prevention measures, yet many robotic systems generate particulates during operation or are constructed from materials that may introduce trace contaminants. Creating a robotic system that maintains the necessary clean-room conditions while performing complex manipulations remains technically challenging.
Sample tracking and chain of custody management become increasingly complex in automated environments. Current systems often lack robust error recovery mechanisms, meaning that when a sample transfer fails, the entire analytical sequence may be compromised without clear identification of which samples were affected. This creates significant validation challenges for regulated industries.
The speed mismatch between robotic handling capabilities and ICP-MS analytical cycles creates inefficiencies. While modern ICP-MS instruments can analyze samples in seconds to minutes, robotic systems often require longer periods for sample preparation and transfer, creating bottlenecks that reduce overall throughput advantages of automation.
Calibration and quality control integration pose additional challenges. Automated systems must incorporate regular calibration checks and quality control samples, but current solutions struggle with adaptive scheduling based on analytical results. When calibration drift is detected, many systems lack the intelligence to automatically adjust sampling parameters or initiate recalibration sequences.
Finally, the expertise gap between robotics engineers and analytical chemists creates implementation barriers. Few professionals possess deep knowledge in both domains, resulting in integration solutions that may excel in robotics but fail to address critical analytical chemistry requirements, or vice versa. This knowledge siloing slows development of truly seamless integration solutions for ICP-MS automation.
Compatibility issues between robotic platforms and ICP-MS instruments represent another major hurdle. Most ICP-MS systems were not originally designed with full automation in mind, resulting in interface limitations. The proprietary nature of both robotic systems and analytical instruments creates communication barriers, with manufacturers using different protocols and software architectures that resist straightforward integration.
Environmental control presents additional complications. ICP-MS requires stringent contamination prevention measures, yet many robotic systems generate particulates during operation or are constructed from materials that may introduce trace contaminants. Creating a robotic system that maintains the necessary clean-room conditions while performing complex manipulations remains technically challenging.
Sample tracking and chain of custody management become increasingly complex in automated environments. Current systems often lack robust error recovery mechanisms, meaning that when a sample transfer fails, the entire analytical sequence may be compromised without clear identification of which samples were affected. This creates significant validation challenges for regulated industries.
The speed mismatch between robotic handling capabilities and ICP-MS analytical cycles creates inefficiencies. While modern ICP-MS instruments can analyze samples in seconds to minutes, robotic systems often require longer periods for sample preparation and transfer, creating bottlenecks that reduce overall throughput advantages of automation.
Calibration and quality control integration pose additional challenges. Automated systems must incorporate regular calibration checks and quality control samples, but current solutions struggle with adaptive scheduling based on analytical results. When calibration drift is detected, many systems lack the intelligence to automatically adjust sampling parameters or initiate recalibration sequences.
Finally, the expertise gap between robotics engineers and analytical chemists creates implementation barriers. Few professionals possess deep knowledge in both domains, resulting in integration solutions that may excel in robotics but fail to address critical analytical chemistry requirements, or vice versa. This knowledge siloing slows development of truly seamless integration solutions for ICP-MS automation.
Current Robotic Integration Solutions for ICP-MS
01 Standardized Communication Protocols for Robotic Integration
Standardized communication protocols enable seamless integration between different robotic systems and components. These protocols establish common languages and interfaces that allow robots from various manufacturers to communicate effectively, share data, and coordinate actions. By implementing standardized protocols, organizations can create interoperable robotic ecosystems that reduce integration complexity and enhance system flexibility.- Integration of robotic systems with communication networks: Robotic systems can be seamlessly integrated with communication networks to enable real-time data exchange and remote operation. These networks facilitate the coordination between multiple robots and control systems, allowing for efficient task execution and monitoring. The integration involves protocols that ensure secure and reliable communication, supporting various operational environments from industrial settings to healthcare applications.
- Modular robotic system architecture: Modular architectures enable flexible and scalable integration of robotic components. These systems feature standardized interfaces that allow for plug-and-play functionality, where different modules can be added or removed based on specific requirements. The modular approach simplifies maintenance, upgrades, and customization while ensuring compatibility between various hardware and software components across the robotic ecosystem.
- Human-robot collaboration interfaces: Advanced interfaces facilitate seamless interaction between humans and robotic systems in collaborative environments. These interfaces incorporate intuitive controls, augmented reality overlays, and natural language processing to enable effective communication. Safety mechanisms are integrated to ensure human protection while maximizing productivity. The collaborative approach allows robots to complement human capabilities in complex tasks requiring both precision and adaptability.
- Cloud-based robotic system integration: Cloud platforms provide centralized management and integration services for distributed robotic systems. These platforms enable remote deployment of software updates, task allocation, and data analytics across multiple robots. The cloud infrastructure supports machine learning capabilities that improve robot performance over time through shared learning experiences. This approach reduces local computational requirements while enhancing system scalability and flexibility.
- Standardized integration protocols for heterogeneous robotic systems: Standardized protocols enable seamless integration between different types of robots and control systems from various manufacturers. These protocols define common data formats, communication methods, and operational parameters that ensure interoperability. The standardization simplifies system integration, reduces implementation costs, and supports the creation of complex robotic ecosystems that can perform coordinated tasks across different domains and applications.
02 Modular Robotic System Architecture
Modular architecture in robotic systems allows for plug-and-play integration of different components and subsystems. This approach enables easy reconfiguration, upgrading, and scaling of robotic capabilities without requiring complete system redesigns. Modular designs feature standardized interfaces, self-identifying components, and abstraction layers that separate hardware from software concerns, facilitating seamless integration of new technologies and adaptation to changing requirements.Expand Specific Solutions03 Cloud-Based Integration Platforms for Robotics
Cloud-based platforms provide centralized infrastructure for integrating distributed robotic systems across various locations. These platforms enable remote monitoring, control, and coordination of robotic assets while facilitating data sharing and analytics. Cloud integration solutions offer scalable computing resources, standardized APIs, and service-oriented architectures that simplify the connection of heterogeneous robotic systems and their integration with enterprise applications.Expand Specific Solutions04 AI-Driven Adaptive Integration Systems
Artificial intelligence technologies enable adaptive integration of robotic systems through machine learning algorithms that can automatically configure, optimize, and troubleshoot connections between different robotic components. These systems can identify integration patterns, predict compatibility issues, and suggest optimal configurations. AI-driven approaches reduce manual integration efforts and enable robotic systems to adapt to changing environments and requirements with minimal human intervention.Expand Specific Solutions05 Human-Robot Collaboration Interfaces
Specialized interfaces facilitate seamless integration between human workers and robotic systems in collaborative environments. These interfaces include intuitive control systems, safety mechanisms, and communication tools that enable effective human-robot interaction. By focusing on natural interaction methods, contextual awareness, and adaptive behavior, these interfaces create integrated work environments where humans and robots can complement each other's capabilities and work together efficiently.Expand Specific Solutions
Leading Manufacturers in ICP-MS and Robotics
The ICP-MS automation market is currently in a growth phase, characterized by increasing integration of robotic systems to enhance laboratory efficiency. The global market size for automated analytical instruments is expanding rapidly, driven by demand for higher throughput and precision in analytical chemistry. From a technological maturity perspective, companies like Revvity Health Sciences and Applied Materials are leading with established automation platforms, while innovative players such as Tomahawk Robotics and Chef Robotics are introducing advanced robotic control systems. Traditional analytical instrumentation manufacturers like Jiangsu Skyray Instrument are incorporating robotics into their ICP-MS workflows, while research institutions including University of Electronic Science & Technology of China are developing next-generation integration protocols. The competitive landscape shows a convergence of analytical chemistry expertise and robotics innovation, with varying degrees of seamless integration capabilities across the ecosystem.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has pioneered a comprehensive robotic integration platform for ICP-MS automation called "LabWorks ICP Automation Suite." This system features a modular design that can be tailored to various laboratory workflows and throughput requirements. At its core is a collaborative robotic arm with six degrees of freedom that interfaces seamlessly with their proprietary sample handling system. The platform incorporates advanced scheduling algorithms that optimize sample processing based on priority, complexity, and instrument availability. Revvity's solution includes specialized end-effectors designed specifically for handling different sample containers, from microplates to individual vials. Their system integrates with laboratory information management systems (LIMS) through standardized APIs, enabling full traceability from sample receipt to result reporting. The automation suite also includes intelligent quality control monitoring that automatically schedules and processes QC samples based on predefined rules and detects drift or calibration issues in real-time.
Strengths: Extensive experience in laboratory automation across multiple analytical techniques; strong software integration capabilities that connect with existing laboratory ecosystems; comprehensive validation packages for regulated environments. Weaknesses: Higher initial investment compared to less integrated solutions; requires significant laboratory space for full implementation of the robotic workflow.
Lockheed Martin Corp.
Technical Solution: Lockheed Martin has developed a sophisticated robotic integration system for ICP-MS automation called "Precision Analytics Automation Platform" (PAAP). This military-grade solution leverages technologies originally developed for aerospace applications to deliver exceptional precision and reliability in laboratory environments. The system features a dual-arm robotic configuration that enables simultaneous handling of multiple samples, significantly increasing throughput compared to conventional automation solutions. Lockheed's platform incorporates advanced machine learning algorithms that continuously optimize movement patterns and sample handling procedures based on operational data. Their system includes specialized containment features for handling hazardous or sensitive materials, making it particularly suitable for environmental monitoring and nuclear applications. The PAAP integrates with ICP-MS instruments through a secure, encrypted communication protocol that ensures data integrity throughout the analytical process. Lockheed's solution also includes comprehensive audit trail capabilities that document all system operations, supporting compliance with stringent regulatory requirements in defense and environmental testing applications.
Strengths: Exceptional reliability and precision derived from aerospace engineering standards; advanced security features that protect both physical samples and digital data; robust design suitable for challenging environments including mobile laboratories. Weaknesses: Significantly higher cost compared to commercial laboratory automation solutions; potential export restrictions for certain markets due to dual-use technology classifications.
Key Technologies for Seamless ICP-MS Automation
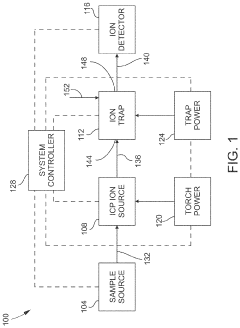
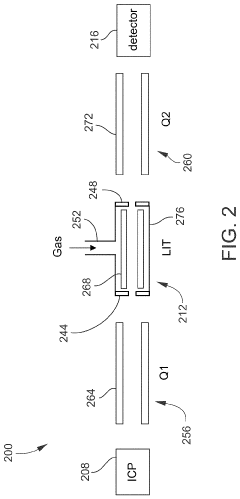
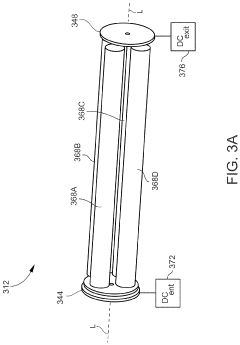
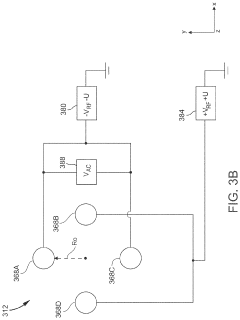
Inductively coupled plasma mass spectrometry (ICP-MS) with ion trapping
PatentActiveUS11443933B1
Innovation
- Incorporating an ion trap, such as a linear ion trap, into the ICP-MS system to confine and mass-selectively eject ions, allowing for the simultaneous analysis of multiple elements from transient signals by preventing ion exit and entry during a confinement period and transmitting selected ions to a detector for measurement.
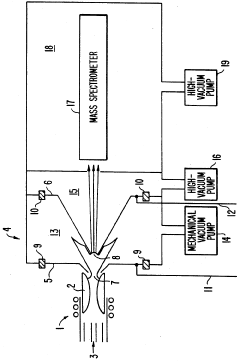
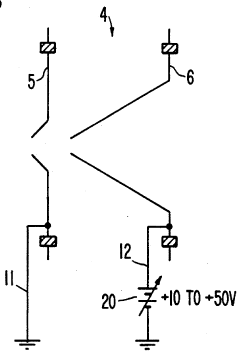
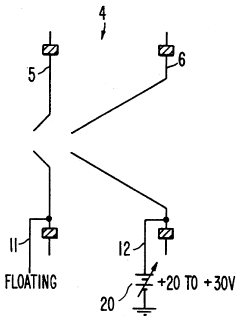
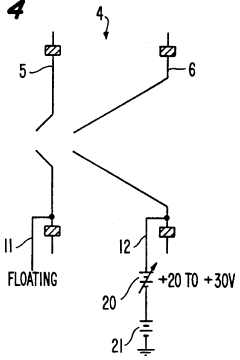
Plasma sampling interface for inductively coupled plasma-mass spectrometry (ICP-MS)
PatentInactiveUS5218204A
Innovation
- A plasma sampling interface with insulating spacers and an adjustable DC bias voltage source applying a DC bias voltage of 10 to 50 V to the skimmer, allowing the sampler to float or grounding it, enhances ion transmission by using a DC offset voltage for mass spectrometers requiring higher initial ion energy.
Laboratory Safety and Compliance Considerations
The integration of robotic systems with ICP-MS technology introduces significant laboratory safety and compliance considerations that must be addressed to ensure worker protection and regulatory adherence. Automated systems handling potentially hazardous samples and reagents require comprehensive safety protocols beyond traditional manual operations. These systems often work with corrosive acids, toxic metals, and other dangerous substances that pose serious health risks if mishandled.
Risk assessment frameworks specifically designed for robotic ICP-MS systems must be implemented, accounting for both chemical hazards and mechanical risks associated with moving robotic components. Facilities must install appropriate engineering controls including ventilation systems, safety barriers, emergency shutdown mechanisms, and containment solutions tailored to automated workflows. These controls should be regularly tested and validated to ensure continued effectiveness.
Personnel training requirements become more complex in automated environments, necessitating dual competency in both analytical chemistry and robotic system operation. Staff must understand not only traditional laboratory safety but also automation-specific hazards, including pinch points, unexpected robot movements, and system malfunctions. Regular certification and refresher training should be documented to demonstrate compliance with occupational safety standards.
Regulatory compliance presents unique challenges for automated ICP-MS systems. Organizations must navigate requirements from multiple authorities including OSHA, EPA, and industry-specific regulators. Documentation systems must be established to track automated processes, maintenance schedules, and safety incidents. Many jurisdictions require specific validation protocols for automated analytical systems, particularly those handling regulated substances or producing results for regulatory submission.
Emergency response procedures require significant modification when implementing robotic systems. Standard operating procedures must include robot-specific emergency shutdown sequences, containment protocols for automated spills, and clear delineation of human versus automated intervention points. Facilities should conduct regular drills simulating automation-specific emergencies to ensure preparedness.
Waste management considerations also evolve with automation, as robotic systems may generate different waste streams or volumes compared to manual processes. Automated waste handling must comply with hazardous waste regulations while maintaining sample integrity and preventing cross-contamination. Systems should incorporate automated tracking of waste generation to simplify regulatory reporting requirements.
Long-term safety monitoring programs should be established to collect data on system performance, near-miss incidents, and potential exposure events. This information proves invaluable for continuous improvement of safety protocols and demonstrating due diligence to regulatory authorities. Regular third-party safety audits specifically addressing the robotic-analytical interface provide additional compliance assurance.
Risk assessment frameworks specifically designed for robotic ICP-MS systems must be implemented, accounting for both chemical hazards and mechanical risks associated with moving robotic components. Facilities must install appropriate engineering controls including ventilation systems, safety barriers, emergency shutdown mechanisms, and containment solutions tailored to automated workflows. These controls should be regularly tested and validated to ensure continued effectiveness.
Personnel training requirements become more complex in automated environments, necessitating dual competency in both analytical chemistry and robotic system operation. Staff must understand not only traditional laboratory safety but also automation-specific hazards, including pinch points, unexpected robot movements, and system malfunctions. Regular certification and refresher training should be documented to demonstrate compliance with occupational safety standards.
Regulatory compliance presents unique challenges for automated ICP-MS systems. Organizations must navigate requirements from multiple authorities including OSHA, EPA, and industry-specific regulators. Documentation systems must be established to track automated processes, maintenance schedules, and safety incidents. Many jurisdictions require specific validation protocols for automated analytical systems, particularly those handling regulated substances or producing results for regulatory submission.
Emergency response procedures require significant modification when implementing robotic systems. Standard operating procedures must include robot-specific emergency shutdown sequences, containment protocols for automated spills, and clear delineation of human versus automated intervention points. Facilities should conduct regular drills simulating automation-specific emergencies to ensure preparedness.
Waste management considerations also evolve with automation, as robotic systems may generate different waste streams or volumes compared to manual processes. Automated waste handling must comply with hazardous waste regulations while maintaining sample integrity and preventing cross-contamination. Systems should incorporate automated tracking of waste generation to simplify regulatory reporting requirements.
Long-term safety monitoring programs should be established to collect data on system performance, near-miss incidents, and potential exposure events. This information proves invaluable for continuous improvement of safety protocols and demonstrating due diligence to regulatory authorities. Regular third-party safety audits specifically addressing the robotic-analytical interface provide additional compliance assurance.
ROI Analysis for Automated ICP-MS Implementation
Implementing automated ICP-MS systems requires significant capital investment, making a comprehensive return on investment analysis essential for decision-makers. The initial investment typically ranges from $150,000 to $500,000 depending on the level of automation and integration complexity, encompassing robotic hardware, software interfaces, sample preparation equipment, and system integration costs.
Labor cost reduction represents the most substantial financial benefit, with automated systems reducing operator hours by 60-80% compared to manual operations. A typical laboratory running 50 samples daily can save approximately $45,000-$70,000 annually in direct labor costs, with additional savings from reduced training requirements and staff turnover expenses.
Throughput improvements deliver significant economic advantages, with automated systems processing 30-50% more samples within the same timeframe. This increased capacity translates to either higher revenue potential or reduced cost per sample, depending on the laboratory's business model. For commercial testing facilities, this can generate an additional $100,000-$200,000 in annual revenue without proportional cost increases.
Quality improvements from automation yield substantial cost avoidance benefits. Error reduction decreases repeat testing requirements by 15-25%, saving reagent costs and instrument time. More consistent results reduce investigation time for anomalous findings, estimated at $10,000-$30,000 annually for mid-sized laboratories.
Operational efficiency gains extend beyond direct testing activities. Automated systems enable 24/7 operation with minimal supervision, increasing instrument utilization by 40-60%. This maximizes the return on the ICP-MS instrument investment, effectively reducing the amortized cost per test.
The payback period for automated ICP-MS systems typically ranges from 18-36 months, depending on sample volume and laboratory-specific factors. Organizations processing over 100 samples daily often achieve ROI within 18 months, while those with lower volumes may require 30-36 months to reach the breakeven point.
Long-term financial benefits include reduced instrument maintenance costs due to more consistent operation, extended consumable life through precise reagent handling, and improved space utilization in laboratory facilities. These factors contribute an additional 5-10% to the overall ROI calculation beyond the immediately quantifiable benefits.
Labor cost reduction represents the most substantial financial benefit, with automated systems reducing operator hours by 60-80% compared to manual operations. A typical laboratory running 50 samples daily can save approximately $45,000-$70,000 annually in direct labor costs, with additional savings from reduced training requirements and staff turnover expenses.
Throughput improvements deliver significant economic advantages, with automated systems processing 30-50% more samples within the same timeframe. This increased capacity translates to either higher revenue potential or reduced cost per sample, depending on the laboratory's business model. For commercial testing facilities, this can generate an additional $100,000-$200,000 in annual revenue without proportional cost increases.
Quality improvements from automation yield substantial cost avoidance benefits. Error reduction decreases repeat testing requirements by 15-25%, saving reagent costs and instrument time. More consistent results reduce investigation time for anomalous findings, estimated at $10,000-$30,000 annually for mid-sized laboratories.
Operational efficiency gains extend beyond direct testing activities. Automated systems enable 24/7 operation with minimal supervision, increasing instrument utilization by 40-60%. This maximizes the return on the ICP-MS instrument investment, effectively reducing the amortized cost per test.
The payback period for automated ICP-MS systems typically ranges from 18-36 months, depending on sample volume and laboratory-specific factors. Organizations processing over 100 samples daily often achieve ROI within 18 months, while those with lower volumes may require 30-36 months to reach the breakeven point.
Long-term financial benefits include reduced instrument maintenance costs due to more consistent operation, extended consumable life through precise reagent handling, and improved space utilization in laboratory facilities. These factors contribute an additional 5-10% to the overall ROI calculation beyond the immediately quantifiable benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!